Abstract
While human embryonic stem cells (hESCs) can differentiate into functional cardiomyocytes, their immature phenotypes limit their therapeutic application for myocardial regeneration. We sought to determine whether electrical stimulation could enhance the differentiation and maturation of hESC-derived cardiomyocytes. Cardiac differentiation was induced in a HES3 hESC line via embryoid bodies formation treated with a p38 MAP kinase inhibitor. Detailed molecular and functional analysis were performed in those hESC-derived cardiomyocytes cultured for 4 days in the absence or presence of electrical field stimulation (6.6 V/cm, 1 Hz, and 2 ms pulses) using an eight-channel C-Pace stimulator (Ion-Optics Co., MA). Upon electrical stimulation, quantitative polymerase chain reaction demonstrated significant upregulation of cardiac-specific gene expression including HCN1, MLC2V, SCN5A, SERCA, Kv4.3, and GATA4; immunostaining and flow cytometry analysis revealed cellular elongation and an increased proportion of troponin-T positive cells (6.3 ± 1.2 % vs. 15.8 ± 2.1 %; n = 3, P < 0.01). Electrophysiological studies showed an increase in the proportion of ventricular-like hESC-derived cardiomyocytes (48 vs. 29 %, P < 0.05) with lengthening of their action potential duration at 90 % repolarization (387.7 ± 35.35; n = 11 vs. 291.8 ± 20.82; n = 10, P < 0.05) and 50 % repolarization (313.9 ± 27.94; n = 11 vs. 234.0 ± 16.10; n = 10, P < 0.05) after electrical stimulation. Nonetheless, the membrane diastolic potentials and action potential upstrokes of different hESC-derived cardiomyocyte phenotypes, and the overall beating rate remained unchanged (all P > 0.05). Fluorescence confocal imaging revealed that electrical stimulation significantly increased both spontaneous and caffeine-induced calcium flux in the hESC-derived cardiomyocytes (approximately 1.6-fold for both cases; P < 0.01). In conclusion, electrical field stimulation increased the expression of cardiac-specific genes and the yield of differentiation, promoted ventricular-like phenotypes, and improved the calcium handling of hESC-derived cardiomyocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adult mammalian heart has a very limited self-regeneration capacity by pre-existing cardiomyocytes [1], thus various therapeutic techniques of cardiac regeneration using different types of stem cell have been developed [2]. Human embryonic stem cells (ESC) that are pluripotent and able to differentiate into functional cardiomyocytes in vitro are considered a potential cell source for cardiac regeneration. Nevertheless the immature phenotypes of ESC-derived cardiomyocytes can induce proarrhythmias, so limiting their therapeutic application [3, 4]. Our previous studies have demonstrated that genetic [5] or chemical [6] modulation of the hypoxia-inducible factor-1 pathway can enhance differentiation and maturation of ESC-derived cardiomyocytes. Unfortunately, the introduction of gene vectors or chemical compounds may lead to unwanted toxicities for ESCs.
Electrical signals are known to play an important role during normal fetal development. In vivo, direct current electric fields are involved in embryonic development [7], and disturbance of this endogenous electric field by environmental electric fields will result in abnormal embryonic development [7, 8]. Prior studies have shown that exogenous electrical stimulation of ESCs can enhance cardiac differentiation [9–11]. Nevertheless, it remains unclear how electrical stimulation affects cardiac differentiation of human ESCs. In addition, there are no data on the effects of electrical stimulation on the maturation of ESC-derived cardiomyocytes.
In this study, we performed detailed molecular and functional analysis of hESC-derived cardiomyocytes (at day 23 post-differentiation) subjected to appropriate electrical field stimulation. We demonstrated that electrical stimulation increased the expression of cardiac-specific genes and the yield of differentiation, promoted ventricular-like phenotypes and improved the calcium handling of hESC-derived cardiomyocytes.
Materials and Methods
Cell Culture and Cardiomyocyte Differentiation of HES3 Line
Briefly, human ESC HES3 cells were cultured on mitotically inactivated human fibroblasts CCD919 (ATCC, Manassas, VA, USA). The hESC culture medium comprised KO-DMEM supplemented with 20 % KO-SR, 0.1 mM non-essential amino acids, 2 mM l-glutamine and 0.5 % v/v penicillin/streptomycin (all from Invitrogen, Carlsbad, CA, USA) [12]. For routine passaging, HES3 cells were split by manually cutting the colonies and transferring them to a new dish of human feeders. Human ESC cultures were washed using phosphate-buffered saline (PBS) with Ca2+/Mg2+ (Invitrogen), cut into small clumps (EZ-passage tool; Invitrogen), and seeded at 0.75–1 × 106 cells/mL as embryoid bodies (EBs) on ultra-low attachment 12-well plates (Nunc) or 6-well plates (Costar) [13]. The plates were agitated for 1 h and then cultured in static conditions at 37 °C in a humidified atmosphere with 5 % CO2. The differentiation medium comprised Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 2 mM l-glutamine (Invitrogen), 0.182 mM sodium pyruvate (Invitrogen), 1 % non-essential amino acids (Invitrogen), 0.1 mM 2-mercaptoethanol, 5.6 mg/L transferrin (Invitrogen), and 20 mg/L sodium selenite (Sigma). A solution of 5 mM of p38-MAPK inhibitor (SB203580; Sigma), dissolved in dimethylsulfoxide (Sigma), was added to the medium at a concentration of 5 μM. The medium was refreshed every 2 days [13, 14]. To determine whether the differentiated cardiomyocytes were ventricular-, atrial- or nodal-like, the ratios of action potential duration at 50 % repolarization (APD50) to action potential duration at 90 % repolarization (APD90) of individual cells were recorded. In this study, ESC-derived cardiomyocytes dissociated from EBs were defined as ventricular-like when the APD50/APD90 ratio is ≧0.8 [4, 15]. While cardiomyocytes with nodal-like phenotype, which could not be easily distinguished by the similar APD50/APD90 ratio as atrial-like cells, were instead defined by the mean value of APD90 < 230 ms. The cardiomyocytes excluded by these two criteria were considered as cells with atrial-like phenotype.
Electrical Stimulation
Beating EBs were seeded on a 0.1 % gelatin-coated six-well plate filled with corresponding medium. Electrical stimulation was then delivered to the cultured EBs for 4 days with carbon electrodes using an eight-channel C-Pace cell culture stimulator (Ion-Optics Co., Milton, MA, USA) with alternating polarity in a humidified incubator (5 % CO2). Various stimulation voltages, frequencies and pulse durations were tested (data not shown) to define the optimal electrical stimulation parameter at 6.6 V/cm, 1 Hz and 2 ms that could maintain the culture with >90 % cell viability [16]. After electrical stimulation, the EBs were immediately harvested for molecular and electrophysiological studies.
Quantitative Polymerase Chain Reaction Analysis
To determine the effect of electrical stimulation on gene expression, quantitative real-time polymerase chain reaction (PCR) analysis was performed on HES3-derived beating EBs in the absence (control) and presence of electrical stimulation. Total RNA from HES3-derived EBs was extracted using the Illustra RNAspin Mini kit (GE Healthcare, Buckinghamshire, UK). Reverse transcription was then performed using 0.5 μg RNA in a final volume of 20 μl, using QuantiTect® reverse transcription kit (Qiagen, Hilden, Germany, http://www.qiagen.com) according to the manufacturer’s instructions. Quantitative PCR analysis was performed with real-time PCR Detector (Opticon 2 DNA Engine, MJ Research, Minnesota, USA) using the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). For amplification, after initial holds for 5 min at 95 °C, 50 cycles of 95 °C for 15 s followed by corresponding annealing temperature for 30 s and 72 °C for 30 s, melt curve analysis was performed. The relative quantification of PCR products was performed according to the 2−ΔΔCt method, using a mouse GAPDH as internal control. Where ΔΔCt = [(Cttarget gene − CtGAPDH)E Stim group–(Cttarget gene − CtGAPDH)Control group].
Immunostaining Analysis
The HES3-derived cardiomyocytes were fixed with 2 % paraformaldehyde for 15 min at 4 °C followed by two washes with PBS. Prior to overnight incubation with murine monoclonal anti-cardiac troponin-T antibody (NeoMarker, Fremont, CA) and rabbit monoclonal anti-sarcomeric actinin antibody (A7811, Sigma, St Louis, MO, USA) at 4 °C, fixed cells were blocked in 2 % BSA, PBS, 0.1 % Triton-X100 for 2 h. After removal of unbound antibodies with washing buffer, cells were incubated with Alexa-594-conjugated anti-mouse and Alexa-488-conjugated anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA) (1:100 dilution in washing buffer) for 1 h at room temperature. Cells were then mounted on glass slides with a mounting medium containing DAPI. The presence of the immune-complex was examined by fluorescence microscopy.
Flow Cytometry Analysis
The percentage of HES3-derived cardiomyocytes in the beating EBs was quantified by fluorescence-activated cell sorter (FACS) analysis. In brief, beating EBs were first dissociated with 1 mg/ml collagenase B (Roche Applied Sciences Penzberg, Germany). Cells were then fixed and permeabilized using a Cytofix/Cytoperm permeabilization kit (BD Biosciences, San Diego, CA). Thereafter, cells were stained with monoclonal anti-troponin-T antibody (dilution 1:100; NeoMarker, Fremont, CA) or monoclonal anti-α-actinin (sarcomeric) antibody (A7811, dilution 1:100; Sigma) followed by a secondary antibody, anti-mouse IgG H+L-PE (dilution 1:100; Beckman Coulter, Fullerton, CA) to stain for cardiac structural protein–troponin-T or α-sarcomeric actinin. Analysis was performed using a Beckman Coulter FC500 flow cytometer. The primary antibody, IgG1, was used as an isotypic control to determine background signal [5, 6].
Cellular Electrophysiological Recording
Standard whole-cell patch-clamp recordings were performed at 37 ± 0.5 °C to record the action potential phenotypes (HEKA Instruments Inc. Southboro, MA) of individual HES3-derived cardiomyocytes as previously described [3, 4]. Patch pipettes were prepared from 1.5-mm thin-walled borosilicate glass tubes using a Sutter micropipette puller P-97 and had typical resistances of 3–4 MΩ when filled with an internal solution containing (in millimolar): 110 K+ aspartate, 20 KCl, 1 MgCl2, 0.1 Na-GTP, 5 Mg-ATP, 5 Na2-phosphocreatine, 5 EGTA, 10 HEPES, and pH adjusted to 7.3 with KOH. The external Tyrode’s bath solution consisted of (in millimolar): 140 NaCl, 5 KCl, 1 MgCl2, 0.4 KH2PO4, 1.8 CaCl2, 10 Glucose, 5 HEPES, with pH adjusted to 7.4 with NaOH. Twenty consecutive action potentials from spontaneously firing HES3-derived cardiomyocytes dissociated from EBs were recorded per cell to ensure stable waveforms for analysis. For electrically quiescent cardiomyocytes, a stimulation of 0.1–1 nA for 5 ms was given to elicit an action potential. The sampling frequency was 2.00 kHz and data were corrected for the liquid junction potentials of +15.9 mV. Maximal diastolic potential (MDP) as well as action potential duration at 90 % (APD90) and 50 % repolarization level (APD50) were measured.
Confocal Calcium Imaging
In brief, beating cardiomyocyte clusters or individual cardiomyocytes digested from the beating EBs were placed onto glass coverslips for confocal calcium imaging [5, 6]. They were incubated with 5 μM Fluo-3 AM (Invitrogen) for 25 min at 37 °C in Tyrode’s solution containing (in millimolar): 140 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose and 10 HEPES at pH 7.4. The spontaneous calcium transient of single or clusters of ESC-derived cardiomyocytes was recorded with a confocal imaging system (Olympus Fluoview System version 4.2 FV300 TIEMPO) mounted on an upright Olympus microscope (IX71). To record the caffeine-induced calcium transient, 20 μl of 10 mM caffeine was applied to the cell surface during measurement. The fluorescence intensity was converted to the calcium concentration referring to the calibration performed using calcium calibration buffer kits (Invitrogen). To examine the spontaneous beating rates of the EBs, basal calcium transients were recorded for 50–100 s. For data analysis, raw recordings were quantified as the background-subtracted fluorescence intensity changes normalized to the background-subtracted baseline fluorescence using Felix32 fluorescence analysis software (Photon Technology International, Birmingham, NJ).
Statistical Analysis
All data are expressed as mean ± SEM. Statistical significance was determined for all individual data points and fitting parameters using Student’s t test and one-way ANOVA with Bonferroni’s post-test, as appropriate. Calculations were performed using OriginPro 7.5 software (OriginLab Corporation, Northampton, MA). A P value < 0.05 was considered statistically significant.
Results
Cell viability of hESC-derived cardiomyocytes was tested against by Trypan Blue (Life Technologies Inc., Cat# 15250–061) exclusion test. It was shown that the percentage of viable cardiomyocytes cultured for days in the absence (control) or presence of electrical stimulation was similar (>95 %, P > 0.05) (data not shown).
Gene Expression Profiles
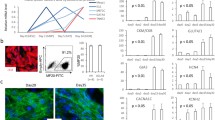
The expression of cardiac-specific and ion channel genes in human ESC-derived cardiomyocytes with or without electrical stimulation was compared. As shown in Fig. 1, quantitative real-time PCR analysis revealed that early cardiac marker—GATA-binding protein 4 (GATA4, 1.2-fold); and late cardiac markers—myosin light chain-2 ventricular (MLC2V, 1.6-fold) and sarcoplasmic reticulum Ca2+-ATPase (SERCA, 1.9-fold) were upregulated in hESC-derived cardiomyocytes following electrical stimulation compared with the control. These observations suggest that electrical stimulation increased the expression of both early and late cardiac-specific genes.
In addition, expression of several genes on ion channels was modulated following electrical stimulation. The expression of hyperpolarization-activated cyclic-nucleotide-gated channel 1(HCN1, 1.2-fold); sodium channel, voltage-gated, type V, alpha subunit (SCN5A, 1.7-fold); and voltage-gated potassium channel subunit (Kv4.3, 1.8-fold) was increased in hESC-derived cardiomyocytes following electrical stimulation. Contrary to this, the expression of hyperpolarization-activated cyclic-nucleotide-gated channel 3 (HCN3, 0.6-fold); potassium voltage-gated channel, KQT-like subfamily, member 1 (KCNQ1, 0.7-fold) and potassium voltage-gated channel, subfamily H (eag-related), member 2 (KCNH2, 0.7-fold) was decreased in hESC-derived cardiomyocytes following electrical stimulation.
Cardiac Differentiation
Figure 2 shows the representative immunocytochemical pattern of the cardiac-specific proteins, troponin-T and α-sarcomeric actinin, in ESC-derived cardiomyocytes with or without electrical stimulation. Morphological assessment of the ESC-derived cardiomyocytes revealed that electrical stimulation contributed to not only a well-organized cytoskeleton network (Fig. 2a), but also cell elongation as reflected by the significantly larger length/width ratio upon electrical stimulation (3.66 ± 0.22, n = 132) than the control (1.90 ± 0.10, n = 137) (P < 0.001) (Fig. 2b). Flow cytometry analysis also demonstrated that the percentage of troponin-T positive cells was significantly increased following electrical stimulation compared with the control (15.8 ± 2.1 % vs. 6.3 ± 1.2 % n = 3, P < 0.01) (Fig. 3). This result indicates that electrical stimulation more than doubled the yield of cardiac differentiation from hESCs.
Effect of electrical stimulation on the morphology of the HES3-derived cardiomyocytes. a Cardiomyocytes subjected to electrical stimulation (E Stim) and the control group (Control) were revealed by immunostaining with antibody specific to human cardiac troponin-T (cTnT) and sarcomeric alpha-actinin (sACT); nuclei (blue) were revealed by DAPI staining; scale bar 25 μm. b The length-to-width ratio of the cTnT-positive cells was found significantly increased upon electrical stimulation from 1.90 ± 0.10, n = 137 (Control) to 3.66 ± 0.22, n = 132 (E Stim); P < 0.001
Cellular Electrophysiological Properties
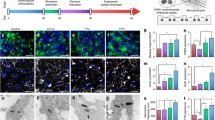
Detailed electrophysiological properties of hESC-derived cardiomyocytes with or without electrical stimulation were further characterized by a whole-cell patch-clamping experiment. For both groups, the hESC-derived cardiomyocytes dissociated from EBs exhibited all the three major types of cardiomyocytes: atrial-, nodal-, and ventricular-like phenotypes. Figure 4a depicts the representative single action potential waveforms recorded of atrial-, nodal-, and ventricular-like phenotypes from both control and electrically stimulated (E Stim) cardiomyocytes. Following electrical stimulation, the percentage of hESC-derived cardiomyocytes with ventricular-like phenotype significantly increased (48 vs. 29 %; P < 0.05) and the percentage of nodal-like cells decreased (39 vs. 59 %; P < 0.05) compared with controls, while the percentage of cells exhibiting atrial-like phenotype remained unchanged (13 vs. 12 %; P > 0.05). This finding indicates that electrical stimulation enhanced the differentiation of ventricular-like cardiomyocytes from human ESCs.
a–f Electrophysiological study by patch-clamping technique on action potential of both control and the electrically stimulated individual HES3-derived cardiomyocytes. a Cells from both groups displayed all the three major cardiac phenotypes: ventricular-, atrial-, and nodal-like; b spontaneous beating rate; c maximal diastolic potentials (MDPs); d upstroke velocity; e action potential durations at 90 % repolarization (APD90); f action potential durations at 50 % repolarization (APD50). *P < 0.05; ***P < 0.001
Nevertheless, there was no significant difference in the spontaneous beating rate of hESC-derived cardiomyocytes with or without electrical stimulation (Fig. 4b). There was also no significant difference in the MDPs (Fig. 4c) and action potential upstroke (Fig. 4d) of the corresponding spontaneous beating cardiomyocytes from both groups. Nonetheless, the action potential duration of cells at 90 % repolarization (APD90) (Fig. 4e) and 50 % repolarization (APD50) (Fig. 4f) were longer in those ventricular- and atrial-like phenotypes following electrical stimulation compared with the control (Table 1).
Calcium Handling
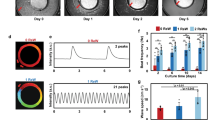
Spontaneous intracellular calcium ([Ca2+]i) transients in the hESC-derived cardiomyocytes were recorded using confocal laser microscopy. As shown in Fig. 5a, b, the amplitude of [Ca2+]i transients measured in cardiomyocytes following electrical stimulation was approximately 1.6-fold higher than that in the control group (P < 0.01). Similarly, kinetic analysis revealed that cardiomyocytes displayed faster calcium release and removal rates following electrical stimulation than the controls, as indicated by the significant increase in the upstroke (maximal [Ca2+]i release rate) and decay (maximal [Ca2+]i removal rate) velocities (Fig. 5c, d, n = 10, P < 0.01).
Spontaneous intracellular calcium transients of the HES3-derived cardiomyocytes. a Representative tracings of rhythmic spontaneous intracellular calcium ([Ca2+]i) transients in HES3-derived cardiomyocytes developed under normal (Control) and electrical stimulation (E Stim) conditions; b amplitude; c maximal calcium release rate; d maximal calcium removal rate of the spontaneous [Ca2+]i transients in the HES3-derived cardiomyocytes. Data shown as mean ± SEM from the recordings of 10–15 cells from three to five independent experiments. **P < 0.01 comparing to control group
To evaluate the sarcoplasmic reticulum performance, the caffeine-induced [Ca2+]i transient was also recorded. As shown in Fig. 6a, brief exposure to 10 mM caffeine induced a surge in [Ca2+]i that subsequently decayed back to baseline in the cardiomyocytes derived from both the control and electrically stimulated groups. Similar to the observations in spontaneous [Ca2+]i transient, the amplitude of the caffeine-induced [Ca2+]i transient from the electrically stimulated group was significantly higher than the control (Fig. 6b, approximately 1.6-fold increase, P < 0.01). This finding suggests that sarcoplasmic reticulum [Ca2+]i storage was increased in cardiomyocytes upon electrical stimulation. In the kinetic analysis, hESC-derived cardiomyocytes also showed faster upstroke (Fig. 6c, n = 10, P < 0.01) and decay (Fig. 6d, n = 10, P < 0.05) velocities during caffeine-induced [Ca2+]i transient following electrical stimulation compared with the control. Interestingly, despite the significantly increased calcium removal rates during spontaneous calcium transient in the electrically stimulated group (about 3.5-fold, P < 0.01) (Fig. 5d), the caffeine-induced calcium transient was increased by only 1.7-fold in the electrically stimulated group (Fig. 6d). This suggests that the enhanced calcium removal ability following electrical stimulation may in part also be due to the increased non-sarcoplasmic reticulum-mediated calcium removal mechanism.
Caffeine-induced intracellular calcium transients of the HES3-derived cardiomyocytes. a Representative tracings of caffeine-induced [Ca2+]i release from sarcoplasmic reticulum in the HES3-derived cardiomyocytes developed under normal (Control) or electrical stimulation (E Stim) conditions; b amount of calcium released; c maximal calcium release rate; d maximal calcium removal rate of caffeine-induced [Ca2+]i transients in the HES3-derived cardiomyocytes. Data shown as mean ± SEM from the recordings of 10–15 cells from three to five independent experiments, *P < 0.05; **P < 0.01 comparing to control group
Taken together, these results demonstrate improved calcium handing of hESC-derived cardiomyocytes following electrical stimulation.
Discussion
In this study, we investigated the effects of electrical stimulation on cardiac differentiation and maturation of human ESCs. The principal findings of this study are that electrical stimulation results in significant upregulation of cardiac-specific gene expression including HCN1, MLC2V, SCN5A, SERCA, Kv4.3, and GATA4; an increased proportion of troponin-T positive cells, and promotion of ventricular-like phenotypes, and maturation of the electrophysiological properties and the calcium handling of human ESC-derived cardiomyocytes.
Upregulated Expression of Cardiac-Specific Genes upon Electrical Stimulation
Our real-time PCR data demonstrated significant upregulation of early transcription factor (GATA4) following electrical stimulation that contributes to cardiac development [17, 18]; and late cardiac-specific genes that are involved in calcium handling (SERCA) and develop into ventricular phenotypes (MLC2V). These findings concur with an increased yield in cardiomyocyte differentiation with a higher percentage of troponin-T positive cells and ventricular-like phenotype as demonstrated by FACS and patch-clamping analysis, respectively; and improved calcium handling in the hESC-derived cardiomyocytes. Morphological analysis also showed that hESC-derived cardiomyocytes have a more elongated and organized cytoskeletal structure following electrical stimulation.
In addition, expression of genes on several ion channels including HCN1, SCN5A, and Kv4.3 that respectively encode for I f (hyperpolarization-activated cyclic-nucleotide-modulated channel current), I Na (voltage-gated sodium current), and I to (transient-outward potassium current) were increased, while HCN3, KCNQ1, and KCNH2 that respectively encode for I f, IKs (slowly activating delayed rectifier potassium current) and IKr (rapid activating delayed rectifier potassium current) were decreased following electrical stimulation. These changes in ion channel gene expression did not affect either the maximal diastolic potential or the action potential upstroke but increased the action potential duration of ventricular- and atrial-like cardiomyocytes. This is likely due to the more prominent effects of decreased IKs and IKr on the overall action potential duration than increased I Na and I to [19, 20]. Moreover, the increase in action potential duration may also be attributed to the improved calcium handling of the hESC-derived cardiomyocytes due to increased systolic Ca2+ entry into the cells [21].
Possible Underlying Pathways that Govern Stem Cell Differentiation Towards a Cardiac Lineage Upon Electrical Stimulation
The present FACS analysis showed that the percentage of troponin-T positive cells was significantly increased following electrical stimulation of the HES3-derived cardiomyocytes compared with the control. This result indicates that electrical stimulation more than doubled the yield of cardiac differentiation from human ESCs. Previous reports revealed that electrical stimulation induced cardiomyocyte pre-commitment of fibroblasts in vitro and is an effective alternative to cytokine-induced differentiation [22, 23]. In a study by Genovese et al., the effects of long term electrical stimulation on human mesenchymal stem cells were studied. Electrical stimulation induced both morphological and biochemical changes in human mesenchymal stem cells that resulted in a shift toward a striated muscle cell phenotype expressing cardiac-specific markers [22]. In another study, Genovese et al. also demonstrated the induction of cardiac-specific gap junction proteins, cardiomyogenic nuclear transcription factors and cardiomyogenic cytoplasmic filaments following in vitro electrical stimulation of fibroblasts [23].
Contrary to these findings, electrical stimulation of mouse ESCs has been found to promote cardiomyogenic [10] and angiogenic [24, 25] differentiation. Nonetheless, the means by which electrical stimulation activates cell differentiating pathways is poorly understood. One possible mechanism involves the generation of reactive oxygen species (ROS) within the cell [10, 26]. Sauer et al. [10] demonstrated that stimulation with an exogenous electric field increased intracellular ROS production in mouse EBs. ROS are highly reactive molecules generated during the normal metabolism of oxygen by NADPH oxidases or as side products of several enzymatic systems (e.g., cyclooxygenases, nitric oxide synthases, and mitochondrial cytochromes). Although excessive concentration of ROS, such as superoxide anions (O2−) and hydrogen peroxide (H2O2), is considered destructive and results in inhibition of gene expression [27, 28], small amounts of ROS function as intracellular second messengers and activate signaling cascades involved in growth and differentiation of many cell types [29–31]. Taken together, these previous studies suggest that electrical stimulation could mediate hESC differentiation towards cardiac lineages, probably due to the effect of the small amounts of ROS generated upon electrical stimulation [11].
Organization of HES3-derived Cardiomyocytes upon Electrical Stimulation
The morphological analysis of the present study showed that hESC-derived cardiomyocytes have a more elongated and organized cytoskeletal structure following electrical stimulation. Typically, stimulation methods often rely on producing a homogenous electrical potential between two large electrodes over a small volume. It has been shown that such electrical field stimulation over an 8-day period increases the amplitude of synchronous contractions in a tissue construct of cardiac cells, and promotes structure (presence of striations, ordered gap junctions) in otherwise disorganized cardiomyocytes [32]. With mouse ESCs, the application of a single 90-s DC pulse over an EB had, in certain cases, doubled the yield of beating EBs [10].
Calcium Handling Enhancement upon Electrical Stimulation
Sarcoplasmic reticulum Ca2+ release through ryanodine receptor-2 (RYR2) and Ca2+ uptake via sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) play a major role in the Ca2+-induced calcium release mechanism mediating excitation–contraction coupling [33]. As shown by calcium imaging experiments, the HES3-derived cardiomyocytes exhibited significant increases in the amplitude, upstroke rate, and decay rates of the calcium transient upon electrical stimulation compared with the control group, indicating more effective handling of the release and removal of intracellular Ca2+ during excitation–contraction coupling. To confirm whether there is any improvement in the sarcoplasmic reticulum load, caffeine-induced calcium transients were measured. In the electrically stimulated group, though the calcium removal rates during spontaneous calcium transient were significantly increased approximately 3.5-fold (P < 0.01), that of the caffeine-induced calcium transient was merely raised approximately 1.7-fold. This implies the possible involvement of the non-sarcoplasmic reticulum-mediated calcium removal mechanism in enhancing the calcium removal ability and deserves further study.
Clinical Applications and Limitations
Our previous studies have demonstrated that genetic [5] or chemical [6] modulation of the hypoxia-inducible factor-1 pathway can enhance differentiation and maturation of ESC-derived cardiomyocytes. Unfortunately, the introduction of gene vectors or chemical compounds may lead to unwanted toxicities for ESCs. This may explain the potential advantages of using electrical stimulation in driving cardiomyocyte differentiation as compared with other common methods.
To date, differentiation methods that use either small molecules or growth factors have very little control on the phenotype of the cardiomyocytes produced [34]. Specifically, the differentiation methods cannot specify cardiomyocytes into a ventricular, atrial, or pacemaker phenotype. Therefore, the advantage of electrical stimulation is the ability to further mature cardiomyocytes into a specific and desired phenotype.
Previous work has demonstrated the importance of electrical factors, that electrical field stimulation over an 8-day period increased the amplitude of synchronous contractions in a tissue construct of cardiac cells [32]. Stimulation appears to be helpful in establishing physiological structure and function, as shown by the presence of striations, gap junctions, and cell coupling [35].
It has been shown that monolayers of cells in DC fields respond with higher sensitivity towards DC fields than the sparse populations of cells [36]. This was attributed to the fact that the latter experienced a smaller voltage drop across the width of each individual cell than the former that came across a larger voltage drop across the barely adjacent cells [37]. Nonetheless, in the myocardium, cells are depolarized by local currents propagating in a wave-like pattern but not by synchronous field stimulation. Such stimulation techniques would thus not adequately mimic the electrical micro-environment that stem cells may be subjected to in a graft. An alternative method is a local “point-source” stimulation approach using a controlled current. By using a current source, stimulation thresholds stay relatively constant even with drifting electrode impedance [35]. Voltage drop across the load can readily be measured to ensure that voltages stay within a safe margin, thus preventing electrode corrosion and water electrolysis [38].
Conclusion
Our results showed that electrical stimulation not only increased the expression of cardiac-specific genes and the yield of differentiation, but also promoted ventricular-like phenotypes and improved the calcium handling of hESC-derived cardiomyocytes. To enhance the future clinical application, electrical stimulation of the graft micro-environment to drive differentiation and maturation of transplanted hESC-derived cardiomyocytes for myocardial regeneration deserve further development and investigation.
References
Senyo, S. E., Steinhauser, M. L., Pizzimenti, C. L., Yang, V. K., Cai, L., Wang, M., et al. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature, 493(7432), 433–436.
Siu, C. W., & Tse, H. F. (2012). Cardiac regeneration: messages from CADUCEUS. Lancet, 379(9819), 870–871.
Liao, S. Y., Liu, Y., Siu, C. W., Zhang, Y., Lai, W. H., Au, K. W., et al. (2010). Proarrhythmic risk of embryonic stem cell-derived cardiomyocyte transplantation in infarcted myocardium. Heart Rhythm, 7(12), 1852–1859.
Liao, S. Y., Tse, H. F., Chan, Y. C., Mei-Chu Yip, P., Zhang, Y., Liu, Y., et al. (2013). Overexpression of Kir2.1 channel in embryonic stem cell-derived cardiomyocytes attenuates posttransplantation proarrhythmic risk in myocardial infarction. Heart Rhythm, 10(2), 273–282.
Ng, K. M., Lee, Y. K., Chan, Y. C., Lai, W. H., Fung, M. L., Li, R. A., et al. (2010). Exogenous expression of HIF-1 alpha promotes cardiac differentiation of embryonic stem cells. Journal of Molecular and Cellular Cardiology, 48(6), 1129–1137.
Ng, K. M., Chan, Y. C., Lee, Y. K., Lai, W. H., Au, K. W., Fung, M. L., et al. (2011). Cobalt chloride pretreatment promotes cardiac differentiation of human embryonic stem cells under atmospheric oxygen level. Cellular Reprogramming, 13(6), 527–537.
Cameron, I. L., Hardman, W. E., Winters, W. D., Zimmerman, S., & Zimmerman, A. M. (1993). Environmental magnetic fields: influences on early embryogenesis. Journal of Cellular Biochemistry, 51(4), 417–425.
Robinson, K. R. (1985). The responses of cells to electrical fields: a review. The Journal of Cell Biology, 101(6), 2023–2027.
Chen, M. Q., Xie, X., Hollis Whittington, R., Kovacs, G. T., Wu, J. C., & Giovangrandi, L. (2008). Cardiac differentiation of embryonic stem cells with point-source electrical stimulation. Conference Proceedings, IEEE Engineering in Medicine and Biology Society, 2008, 1729–1732.
Sauer, H., Rahimi, G., Hescheler, J., & Wartenberg, M. (1999). Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. Journal of Cellular Biochemistry, 75(4), 710–723.
Serena, E., Figallo, E., Tandon, N., Cannizzaro, C., Gerecht, S., Elvassore, N., et al. (2009). Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Experimental Cell Research, 315(20), 3611–3619.
Choo, A., Padmanabhan, J., Chin, A., Fong, W. J., & Oh, S. K. (2006). Immortalized feeders for the scale-up of human embryonic stem cells in feeder and feeder-free conditions. Journal of Biotechnology, 122(1), 130–141.
Ting, S., Lecina, M., Reuveny, S., & Oh, S. (2012). Differentiation of human embryonic stem cells to cardiomyocytes on microcarrier cultures. Current Protocols in Stem Cell Biology, Chapter 1, Unit1D 7.
Lecina, M., Ting, S., Choo, A., Reuveny, S., & Oh, S. (2010). Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Engineering. Part C, Methods, 16(6), 1609–1619.
Chan, Y. C., Siu, C. W., Lau, Y. M., Lau, C. P., Li, R. A., & Tse, H. F. (2009). Synergistic effects of inward rectifier (I) and pacemaker (I) currents on the induction of bioengineered cardiac automaticity. Journal of Cardiovascular Electrophysiology, 20(9), 1048–1054.
Tandon, N., Cannizzaro, C., Chao, P. H., Maidhof, R., Marsano, A., Au, H. T., et al. (2009). Electrical stimulation systems for cardiac tissue engineering. Nature Protocols, 4(2), 155–173.
Pu, W. T., Ishiwata, T., Juraszek, A. L., Ma, Q., & Izumo, S. (2004). GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Biology, 275(1), 235–244.
Zeisberg, E. M., Ma, Q., Juraszek, A. L., Moses, K., Schwartz, R. J., Izumo, S., et al. (2005). Morphogenesis of the right ventricle requires myocardial expression of Gata4. Journal of Clinical Investigation, 115(6), 1522–1531.
Charpentier, F., Merot, J., Loussouarn, G., & Baro, I. (2010). Delayed rectifier K(+) currents and cardiac repolarization. Journal of Molecular and Cellular Cardiology, 48(1), 37–44.
Ravens, U., & Wettwer, E. (1998). Electrophysiological aspects of changes in heart rate. Basic Research in Cardiology, 93(Suppl 1), 60–65.
Qu, Z., & Chung, D. (2012). Mechanisms and determinants of ultralong action potential duration and slow rate-dependence in cardiac myocytes. PLoS One, 7(8), e43587.
Genovese, J. A., Spadaccio, C., Chachques, E., Schussler, O., Carpentier, A., Chachques, J. C., et al. (2009). Cardiac pre-differentiation of human mesenchymal stem cells by electrostimulation. Frontiers in Bioscience, 14, 2996–3002.
Genovese, J. A., Spadaccio, C., Langer, J., Habe, J., Jackson, J., & Patel, A. N. (2008). Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts. Biochemical and Biophysical Research Communications, 370(3), 450–455.
Gamry Instruments. (2005). Electrochemical impedance spectroscopy theory: a primer.
Setsukinai, K., Urano, Y., Kakinuma, K., Majima, H. J., & Nagano, T. (2003). Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. Journal of Biological Chemistry, 278(5), 3170–3175.
Sauer, H., & Wartenberg, M. (2005). Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxidants and Redox Signaling, 7(11–12), 1423–1434.
Puceat, M., Travo, P., Quinn, M. T., & Fort, P. (2003). A dual role of the GTPase Rac in cardiac differentiation of stem cells. Molecular Biology of the Cell, 14(7), 2781–2792.
Puceat, M. (2005). Role of Rac-GTPase and reactive oxygen species in cardiac differentiation of stem cells. Antioxidants and Redox Signaling, 7(11–12), 1435–1439.
Cannizzaro, C., Tandon, N., Figallo, E., Park, H., Gerecht, S., Radisic, M., et al. (2007). Practical aspects of cardiac tissue engineering with electrical stimulation. Methods in Molecular Medicine, 140, 291–307.
Huang, J. Z. C., Zhang, W., & Zhou, X. (1997). Application of a platinum dual-disk microelectrode to measurement of the electron transfer number of dioxygen reduction. Journal of Electroanalytical Chemistry, 433, 33–39.
Li, J., Stouffs, M., Serrander, L., Banfi, B., Bettiol, E., Charnay, Y., et al. (2006). The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Molecular Biology of the Cell, 17(9), 3978–3988.
Radisic, M., Park, H., Shing, H., Consi, T., Schoen, F. J., Langer, R., et al. (2004). Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America, 101(52), 18129–18134.
Satin, J., Itzhaki, I., Rapoport, S., Schroder, E. A., Izu, L., Arbel, G., et al. (2008). Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells, 26(8), 1961–1972.
Burridge, P. W., Keller, G., Gold, J. D., & Wu, J. C. (2012). Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell, 10(1), 16–28.
Loeb, G. E., Zamin, C. J., Schulman, J. H., & Troyk, P. R. (1991). Injectable microstimulator for functional electrical stimulation. Medical & Biological Engineering & Computing, 29(6), NS13–19.
Tandon, N., Goh, B., Marsano, A., Chao, P. H., Montouri-Sorrentino, C., Gimble, J., et al. (2009). Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation. Conference Proceedings, IEEE Engineering in Medicine and Biology Society, 2009, 6517–6521.
Levin, M. (2003). Motor protein control of ion flux is an early step in embryonic left-right asymmetry. Bioessays, 25(10), 1002–1010.
Donaldson, N. D., & Donaldson, P. E. (1986). When are actively balanced biphasic (‘lilly’) stimulating pulses necessary in a neurological prosthesis? I. Historical background; Pt resting potential; Q studies. Medical & Biological Engineering & Computing, 24, 41–49.
Acknowledgments
This study was supported by Hong Kong Research Grant Council (HKU 8/CRF/09, HKU 8/CRF/10, HKU 780110 M to H.F.T), Theme-based Research Scheme (T12-705/11 to C.W.S and H.F.T), and CRCG Small Project Funding of University of Hong Kong (Y.C.C); Agency for Science Technology and Research (S.T. and S.K.O).
Disclosure Statement
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer L. Hall oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Chan, YC., Ting, S., Lee, YK. et al. Electrical Stimulation Promotes Maturation of Cardiomyocytes Derived from Human Embryonic Stem Cells. J. of Cardiovasc. Trans. Res. 6, 989–999 (2013). https://doi.org/10.1007/s12265-013-9510-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9510-z