Abstract
The prevalence of hypertension in African Americans in the USA is among the highest in the world and increasing. The identification of genes and pathways regulating blood pressure in African Americans has been challenging. An early predictor of hypertension is arterial stiffness. The prevalence of arterial stiffness is significantly higher in African Americans compared to Caucasians. Approximately 20 % of the variance in arterial stiffness is estimated to be heritable. Identifying genes and biological pathways regulating arterial stiffness may provide insight into the genetics underlying the increased risk of hypertension in African Americans. This paper reviews the genetic findings to date in the area of arterial stiffness and blood pressure in African Americans with an emphasis on the current limitations and new efforts to move the field forward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of hypertension in African Americans in the USA is among the highest in the world and increasing [1]. According to the 2005–2006 National Health and Nutrition Examination Survey (NHANES), 27 % of African American adults are hypertensive compared to 17 % of Caucasians [2]. This racial disparity in blood pressure exists after adjusting for demographics, socioeconomic status, clinical characteristics, and modifiable health behaviors [2]. This difference in hypertension prevalence suggests that other factors, including genetics, may play a significant contributing role in blood pressure.
Hypertension is the most common risk factor associated with cardiovascular disease and is associated with a threefold increase in age-adjusted death rate [3]. Thus, developing effective therapies to decrease hypertension would significantly lower the prevalence of cardiovascular disease. Identifying genes and pathways that predispose African Americans to high blood pressure would represent a major first step in developing new effective therapies to treat hypertension.
Impaired arterial elasticity is an early predictor of hypertension in asymptomatic individuals [4–9]. The prevalence of impaired arterial elasticity (as well as hypertension) is significantly higher in African Americans compared to Caucasians [10–14]. Approximately 20 % of the variance in arterial elasticity is estimated to be heritable [15, 16]. Previous studies have identified common variants associated with arterial elasticity across different ethnicities [15–56]. Despite these investigations, relatively little of the phenotypic variance in arterial elasticity has been identified through common genetic variants using either candidate gene or genome-wide association scan (GWAS) approaches.
Impaired Arterial Elasticity in African Americans
Evidence to date suggests that the prevalence of impaired arterial elasticity and hypertension is significantly higher in African American men compared to Caucasian men [11, 12, 14, 57–60] (Table 1). Duprez and colleagues recently demonstrated that small artery elasticity is an early predictor of hypertension [6]. In addition, Duprez showed that small artery elasticity is impaired in African Americans compared to Caucasian and Asian American participants in the Multi-Ethnic Study of Atherosclerosis (MESA) [14]. The MESA cohort consists of ~6,800 men and women ages 45–84 years with no clinical evidence of cardiovascular disease. These findings are similar to previously published work in the Bogalusa Heart Study showing decreased pulse wave velocity and decreased small and large artery elasticity in African Americans compared to Whites [61]. Shah and colleagues showed that arterial stiffness (as measured by pulse wave velocity and Augmentation index) is significantly increased in adolescent African Americans with type 2 diabetes compared to adolescent Whites with type 2 diabetes [59]. A meta-analysis with five cohorts by Chirinos et al. showed that the central augmentation index, defined as a ratio calculated from a blood pressure waveform, is increased in African Americans compared to British Whites [57]. Heffernan showed that racial differences in low arterial compliance in African Americans compared to Whites are unchanged with exercise [58]. A lower arterial compliance in African Americans had previously been shown by Zion et al. in 2003, Ferreira et al., in 1999 and Din-Dzietham et al. in 2004 [60].
Over the last decade, different measurements including augmentation index, pulse wave velocity, pulse pressure, and small and large artery elasticity have been utilized by different research groups and separate cohorts to measure arterial stiffness. The overwhelming conclusion is that African Americans exhibit impaired arterial stiffness, starting at a younger age, even after controlling for height, weight, LDL-C, diabetes, and blood pressure and other risk factors compared to Caucasians [14, 61–63].
Genetics of Pulse Pressure
To date, there has been little accomplished in defining the role of genetics in arterial stiffness in any ethnic group, but in particular, African Americans. A long-term goal of genetic studies in the area of arterial stiffness is to identify genes and biological pathways that contribute to early hypertension. A genome-wide linkage scan was performed as part of the Family Blood Pressure Program (FBPP) for loci affecting pulse pressure on 10,798 participants in 3,320 families [64]. The Family Blood Pressure Program, FBPP, was established in 1995 to identify the role of DNA variants in hypertension in African Americans, Hispanic, Asian, and non-Hispanic White populations. Four separate networks are combined to form the FBPP: GenNet, Genetic Epidemiology Network of Arteriopathy (GENOA), Hypertension Genetics, Epidemiology Network (HyperGen), and the Stanford Asian Pacific Program in Hypertension and Insulin Resistance (SAPPHIRE) [65]. All ascertained families in the network include individuals with hypertension or genetic predisposition towards hypertension [64].
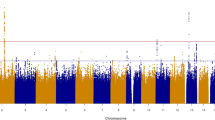
Pulse pressure (the difference between systolic and diastolic blood pressure) is not a direct method of measuring aortic stiffness; yet, pulse pressure has been used as a surrogate marker of arterial compliance. The FBPP linkage scan identified a region on chromosome 7 at 75 cM with a LOD = 3.1 in African Americans (LOD > 3 typically suggests a significant region of linkage), a region on chromosome 19 at 0 cM with a LOD = 3.1 in a combined sample of Whites and African Americans, and a region on chromosome 18 at 71 cM with LOD = 3.2 in a combined racial sample (Whites, African Americans, and Hispanics) [64]. Simino et al. reported more recent results from the FBPP using an overall meta-analysis and four race-specific meta-analyses of genome-wide blood pressure linkage scans. These analyses used data from 13,044 participants and identified one locus associated with pulse pressure (chromosome 6, position 42.27 cM, LOD = 3.23) from the meta-analysis and a second locus (chromosome 1, 212.44 cM, LOD = 3.16) that associated with pulse pressure in one of the FBPP cohorts (HyperGEN) [66]. The locus for pulse pressure on chromosome 6p22.3 was later identified as containing the most associated SNP (rs16877320) with systolic blood pressure in a meta-analysis of normotensive African Americans [67].
The HyperGEN (Hypertension Genetic Epidemiology Network) investigators (a component of the FBPP) performed a genome-wide linkage scan on 1,251 African Americans [16]. This study identified two regions of linkage with pulse pressure on chromosome 1 at 215 cM (LOD = 3.08) and another locus linked to pulse pressure on chromosome 14 at 85 cM with LOD = 2.42 [16]. These loci contain many genes, but the ones closest to the linkage peaks were GPR25 and SMOC1. These regions were not sequenced and there has been no follow-up studies as of yet to replicate or confirm these findings.
Genetics of Systolic and Diastolic Blood Pressure/Hypertension
The first GWAS to focus on blood pressure in African Americans was conducted by Adeyemo et al. in 2009 in 1,017 individuals (509 cases of hypertension and 508 normotensive controls) from the Howard University Family Study [67]. No locus met genome-wide significance (typically P < 5 × 10−8) for association with hypertension. Six SNPs met genome-wide significance for association with systolic blood pressure (see Table 1). One of these SNPs (rs3751664) was in a non-synonymous coding region of the gene CACNA1H (calcium channel, voltage-dependent, T-type, alpha 1H subunit) that encodes a member of the alpha-1 subunit family in the voltage-dependent calcium channel complex. No SNPs were found to significantly associate with diastolic blood pressure. The SNPs most associated with systolic blood were used for replication in a cohort of non-diabetic West Africans (n = 980). Of the six most associated SNPs with systolic blood pressure in the Howard Family study, the non-synonymous coding SNP in the calcium channel protein (rs3751664) was monomorphic in the West African cohort, and the remaining five were not significantly associated with systolic blood pressure [67]. Moreover, for the SNPs tested, the direction of the genotypic effect on blood pressure was not always similar in the original and replication study [67]. One explanation for the difference in results is admixture between the discovery cohort (African Americans) and the replication cohort (West Africa), with its effects on LD structure, allelic frequency distributions, and risk factor profiles [68, 69]. There was no overlap in the genes identified in this study [67] with the loci associated with impaired arterial elasticity or pulse pressure reported earlier [16, 64]. Associations with pulse pressure were not reported in this study by Adeyemo [67].
An admixture mapping study has implicated two regions, on 6q24 and 21q21, that may contain genes that influence risk of hypertension in African Americans [70]. An admixture mapping approach used in the Dallas Heart Study Cohort implicated the gene, vanin 1 (VNN1) in the region on 6q24, (rs2272996) as associated with the risk for hypertension in African Americans [71]. The VNN1 SNP increased risk for hypertension in African Americans yet conferred protection in European Americans (although it did not reach statistical significance in European Americans)[71]. Recent transcription profiling work by Blangero and colleagues in lymphocytes suggests that sequence variants in VNN1 are related to HDL cholesterol concentrations [72].
Fox et al. [73] examined both genome-wide and candidate gene associations with systolic and diastolic blood pressure in 8,591 African Americans in the NHLBI Candidate Gene Association Resource (CARe) consortium. None of the SNPs tested using either the Affymetrix 6.0 GWAS panel or the ITMAT-Broad_CARe custom SNP array were replicated in an additional 11,882 African Americans or European Americans (69,899) [73]. Previously identified blood pressure SNPs in European Americans were identified in African Americans although with weak significance (SH2B3, TBX3-5, DBP) [73]. This study highlights many issues currently challenging the field of blood pressure, African Americans and genetics including the complexity of the phenotype, and the admixture effects in African Americans. An admixture approach using the CARe consortium in 6,303 African Americans followed by replication in four different African American populations and one native Nigerian African sample (total n = 11,882) identified a novel SNP, rs7726475, associated with systolic and diastolic blood pressure between genes SUB1 and NPR3 [74].
A recent study by the International Consortium for Blood Pressure Genome-Wide Association Studies in multiple ancestries including a cohort of 19,775 African Americans identified multiple novel loci associated with blood pressure [75]. These loci were associated with systolic and diastolic blood pressure in 200,000 Europeans, ~19,000 African Americans, ~29,000 East Asians, and ~24,000 South Asians. In African Americans, 22 loci were significantly associated with systolic or diastolic blood pressure (P < 1 × 10−8) [75] (see Table 1). Many of these SNPs were also significant in other race/ethnicities. Genes that had been implicated in previous studies but may have not achieved statistical significance were replicated in this study including (but not limited to) NPR3, SLC4A7, and SH2B3. A new gene SLC39A8, identified to be associated with both systolic and diastolic blood pressure in African Americans, European Americans, and South Asians, is also associated with HDL cholesterol levels (similar to VNN1, but not replicated in this analysis). NPR3 encodes the clearance receptor for natriuretic peptide and mice lacking this receptor have reduced blood pressure [76]. SLC4A7 is expressed in vascular smooth muscle and the nephron and regulates sodium bicarbonate [77]. Knockout of this gene induces mild hypertension in mice. SH2B3 has been implicated in multiple associations with immune and inflammatory diseases as well as hypertension [78, 79]; however its function remains unknown.
Conclusions, Limitations, and Future Directions
In sum, the strength of the genetic studies for associations in systolic and diastolic blood pressure in African Americans is at a very early stage and will continue to improve with increased sample size as well as understanding of the complexities in uniqueness between ancestral genomes. The genetic associations between arterial stiffness in African Americans are also at a very early stage. Utilizing pulse pressure to estimate arterial stiffness may be a weakness of the early studies and the small sample size also represents a major hurdle of the early work done in this field. The potential importance of pairing genetics with phenotypic measurements of arterial stiffness includes the identification of new pathways for drug development to prevent or inhibit cardiovascular disease.
References
Lloyd-Jones, D., Adams, R. J., Brown, T. M., et al. (2010). Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation, 121, e46–e215.
Redmond, N., Baer, H. J., & Hicks, L. S. (2011). Health behaviors and racial disparity in blood pressure control in the national health and nutrition examination survey. Hypertension, 57, 383–389.
Heron, M., Hoyert, D. L., Murphy, S. L., Xu, J., Kochanek, K. D., & Tejada-Vera, B. (2009). Deaths: final data for 2006. National Vital Statistics Reports, 57, 1–134.
Dernellis, J., & Panaretou, M. (2005). Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension, 45, 426–431.
Liao, D., Arnett, D. K., Tyroler, H. A., et al. (1999). Arterial stiffness and the development of hypertension. The ARIC study. Hypertension, 34, 201–206.
Peralta, C. A., Adeney, K. L., Shlipak, M. G., et al. (2010). Structural and functional vascular alterations and incident hypertension in normotensive adults: the multi-ethnic study of atherosclerosis. American Journal of Epidemiology, 171, 63–71.
Blacher, J., Asmar, R., Djane, S., London, G. M., & Safar, M. E. (1999). Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension, 33, 1111–1117.
Femia, R., Kozakova, M., Nannipieri, M., et al. (2007). Carotid intima-media thickness in confirmed prehypertensive subjects: predictors and progression. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 2244–2249.
Najjar, S. S., Scuteri, A., Shetty, V., et al. (2008). Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology, 51, 1377–1383.
Heffernan, K. S., Jae, S. Y., Wilund, K. R., Woods, J. A., & Fernhall, B. (2008). Racial differences in central blood pressure and vascular function in young men. American Journal of Physiology. Heart and Circulatory Physiology, 295, H2380–H2387.
Din-Dzietham, R., Couper, D., Evans, G., Arnett, D. K., & Jones, D. W. (2004). Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. American Journal of Hypertension, 17, 304–313.
Ferreira, A. V., Viana, M. C., Mill, J. G., Asmar, R. G., & Cunha, R. S. (1999). Racial differences in aortic stiffness in normotensive and hypertensive adults. Journal of Hypertension, 17, 631–637.
Heffernan, K. S., Jae, S. Y., Lee, M., Woods, J. A., & Fernhall, B. (2008). Arterial wave reflection and vascular autonomic modulation in young and older men. Aging Clinical and Experimental Research, 20, 1–7.
Duprez, D. A., Jacobs, D. R., Jr., Lutsey, P. L., et al. (2009). Race/ethnic and sex differences in large and small artery elasticity—results of the multi-ethnic study of atherosclerosis (MESA). Ethnicity & Disease, 19, 243–250.
Ge, D., Young, T. W., Wang, X., Kapuku, G. K., Treiber, F. A., & Snieder, H. (2007). Heritability of arterial stiffness in black and white American youth and young adults. American Journal of Hypertension, 20, 1065–1072.
Sherva, R., Miller, M. B., Lynch, A. I., et al. (2007). A whole genome scan for pulse pressure/stroke volume ratio in African Americans: the HyperGEN study. American Journal of Hypertension, 20, 398–402.
Akasaka, H., Katsuya, T., Saitoh, S., et al. (2009). A promoter polymorphism of lamin A/C gene is an independent genetic predisposition to arterial stiffness in a Japanese general population (the Tanno and Sobetsu study). Journal of Atherosclerosis and Thrombosis, 16, 404–409.
Baker, M., Rahman, T., Hall, D., et al. (2007). The C-532 T polymorphism of the angiotensinogen gene is associated with pulse pressure: a possible explanation for heterogeneity in genetic association studies of AGT and hypertension. International Journal of Epidemiology, 36, 1356–1362.
Bjorck, H. M., Lanne, T., Alehagen, U., et al. (2009). Association of genetic variation on chromosome 9p21.3 and arterial stiffness. Journal of Internal Medicine, 265, 373–381.
Chen, W., Srinivasan, S. R., Boerwinkle, E., & Berenson, G. S. (2007). Beta-adrenergic receptor genes are associated with arterial stiffness in black and white adults: the Bogalusa Heart Study. American Journal of Hypertension, 20, 1251–1257.
Chen, W., Srinivasan, S. R., Li, S., Boerwinkle, E., & Berenson, G. S. (2004). Gender-specific influence of NO synthase gene on blood pressure since childhood: the Bogalusa Heart Study. Hypertension, 44, 668–673.
Dima, I., Vlachopoulos, C., Alexopoulos, N., et al. (2008). Association of arterial stiffness with the angiotensin-converting enzyme gene polymorphism in healthy individuals. American Journal of Hypertension, 21, 1354–1358.
Durier, S., Fassot, C., Laurent, S., et al. (2003). Physiological genomics of human arteries: quantitative relationship between gene expression and arterial stiffness. Circulation, 108, 1845–1851.
Engelen, L., Ferreira, I., Gaens, K. H., et al. (2010). The association between the -374 T/A polymorphism of the receptor for advanced glycation endproducts gene and blood pressure and arterial stiffness is modified by glucose metabolism status: the Hoorn and CoDAM studies. Journal of Hypertension, 28, 285–293.
Fava, C., Ricci, M. S., Burri, P., Minuz, P., & Melander, O. (2008). Heritability of the ambulatory arterial stiffness index in Swedish families. Journal of Human Hypertension, 22, 298–300.
Franceschini, N., MacCluer, J. W., Rose, K. M., et al. (2008). Genome-wide linkage analysis of pulse pressure in American Indians: the Strong Heart Study. American Journal of Hypertension, 21, 194–199.
Gardier, S., Vincent, M., Lantelme, P., Rial, M. O., Bricca, G., & Milon, H. (2004). A1166C polymorphism of angiotensin II type 1 receptor, blood pressure and arterial stiffness in hypertension. Journal of Hypertension, 22, 2135–2142.
Greenwald, S. E. (2007). Ageing of the conduit arteries. The Journal of Pathology, 211, 157–172.
Iemitsu, M., Maeda, S., Otsuki, T., et al. (2008). Arterial stiffness, physical activity, and atrial natriuretic peptide gene polymorphism in older subjects. Hypertension Research, 31, 767–774.
Iemitsu, M., Maeda, S., Otsuki, T., et al. (2006). Polymorphism in endothelin-related genes limits exercise-induced decreases in arterial stiffness in older subjects. Hypertension, 47, 928–936.
Kelley-Hedgepeth, A., Peter, I., Montefusco, M. C., et al. (2009). The KCNMB1 E65K variant is associated with reduced central pulse pressure in the community-based Framingham Offspring Cohort. Journal of Hypertension, 27, 55–60.
Lajemi, M., Labat, C., Gautier, S., et al. (2001). Angiotensin II type 1 receptor-153A/G and 1166A/C gene polymorphisms and increase in aortic stiffness with age in hypertensive subjects. Journal of Hypertension, 19, 407–413.
Levy, D., Larson, M. G., Benjamin, E. J., et al. (2007). Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Medical Genetics, 8(Suppl 1), S3.
Mattace-Raso, F. U., van der Cammen, T. J., Sayed-Tabatabaei, F. A., et al. (2004). Angiotensin-converting enzyme gene polymorphism and common carotid stiffness. The Rotterdam study. Atherosclerosis, 174, 121–126.
Mayer, O., Jr., Filipovsky, J., Pesta, M., Cifkova, R., Dolejsova, M., & Simon, J. (2008). Synergistic effect of angiotensin II type 1 receptor and endothelial nitric oxide synthase gene polymorphisms on arterial stiffness. Journal of Human Hypertension, 22, 111–118.
Mitchell, G. F., DeStefano, A. L., Larson, M. G., et al. (2005). Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation, 112, 194–199.
Mitchell, G. F., Guo, C. Y., Kathiresan, S., et al. (2007). Vascular stiffness and genetic variation at the endothelial nitric oxide synthase locus: the Framingham Heart study. Hypertension, 49, 1285–1290.
North, K. E., MacCluer, J. W., Devereux, R. B., et al. (2002). Heritability of carotid artery structure and function: the Strong Heart Family Study. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 1698–1703.
Peter, I., Kelley-Hedgepeth, A., Huggins, G. S., et al. (2009). Association between arterial stiffness and variations in oestrogen-related genes. Journal of Human Hypertension, 23, 636–644.
Plat, A. W., Stoffers, H. E., de Leeuw, P. W., et al. (2009). The association between arterial stiffness and the angiotensin II type 1 receptor (A1166C) polymorphism is influenced by the use of cardiovascular medication. Journal of Hypertension, 27, 69–75.
Rehman, A., Ismail, S. B., Naing, L., Roshan, T. M., & Rahman, A. R. (2007). Reduction in arterial stiffness with angiotensin II antagonism and converting enzyme inhibition. A comparative study among malay hypertensive subjects with a known genetic profile. American Journal of Hypertension, 20, 184–189.
Safar, M. E., Cattan, V., Lacolley, P., et al. (2005). Aldosterone synthase gene polymorphism, stroke volume and age-related changes in aortic pulse wave velocity in subjects with hypertension. Journal of Hypertension, 23, 1159–1166.
Schnabel, R., Larson, M. G., Dupuis, J., et al. (2008). Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension, 51, 1651–1657.
Seidlerova, J., Bochud, M., Staessen, J. A., et al. (2008). Heritability and intrafamilial aggregation of arterial characteristics. Journal of Hypertension, 26, 721–728.
Sie, M. P., Isaacs, A., de Maat, M. P., et al. (2009). Genetic variation in the fibrinogen-alpha and fibrinogen-gamma genes in relation to arterial stiffness: the Rotterdam Study. Journal of Hypertension, 27, 1392–1398.
Sie, M. P., Mattace-Raso, F. U., Uitterlinden, A. G., et al. (2007). TGF-beta1 polymorphisms and arterial stiffness; the Rotterdam Study. Journal of Human Hypertension, 21, 431–437.
Sie, M. P., Yazdanpanah, M., Mattace-Raso, F. U., et al. (2009). Genetic variation in the renin-angiotensin system and arterial stiffness. The Rotterdam Study. Clinical and Experimental Hypertension, 31, 389–399.
Tarasov, K. V., Sanna, S., Scuteri, A., et al. (2009). COL4A1 is associated with arterial stiffness by genome-wide association scan. Circulation. Cardiovascular Genetics, 2, 151–158.
Vlachopoulos, C., Terentes-Printzios, D., Dima, I., Aznaouridis, K., & Stefanadis, C. (2009). Polymorphisms of inflammatory markers/mediators and arterial stiffness. Hypertension, 53, e39. author reply e40.
Wojciechowska, W., Staessen, J. A., Stolarz, K., et al. (2004). Association of peripheral and central arterial wave reflections with the CYP11B2–344 C allele and sodium excretion. Journal of Hypertension, 22, 2311–2319.
Yasmin, Falzone, R., & Brown, M. J. (2004). Determinants of arterial stiffness in offspring of families with essential hypertension. American Journal of Hypertension, 17, 292–298.
Yasmin, McEniery, C. M., O’Shaughnessy, K. M., et al. (2006). Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1799–1805.
Yasmin, O’Shaughnessy, K. M., McEniery, C. M., Cockcroft, J. R., & Wilkinson, I. B. (2006). Genetic variation in fibrillin-1 gene is not associated with arterial stiffness in apparently healthy individuals. Journal of Hypertension, 24, 499–502.
Ye, S. (2006). Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovascular Research, 69, 636–645.
Yuan, M., Ohishi, M., Ito, N., et al. (2006). Genetic influences of beta-adrenoceptor polymorphisms on arterial functional changes and cardiac remodeling in hypertensive patients. Hypertension Research, 29, 875–881.
Zhou, S., Feely, J., Spiers, J. P., & Mahmud, A. (2007). Matrix metalloproteinase-9 polymorphism contributes to blood pressure and arterial stiffness in essential hypertension. Journal of Human Hypertension, 21, 861–867.
Chirinos, J. A., Kips, J. G., Roman, M. J., et al. (2011). Ethnic differences in arterial wave reflections and normative equations for augmentation index. Hypertension, 57, 1108–1116.
Heffernan, K. S., Jae, S. Y., & Fernhall, B. (2007). Racial differences in arterial stiffness after exercise in young men. American Journal of Hypertension, 20, 840–845.
Shah, A. S., Dolan, L. M., Gao, Z., Kimball, T. R., & Urbina, E. M. (2011). Racial differences in arterial stiffness among adolescents and young adults with type 2 diabetes. Pediatric Diabetes, 13, 170–175.
Zion, A. S., Bond, V., Adams, R. G., et al. (2003). Low arterial compliance in young African-American males. American Journal of Physiology. Heart and Circulatory Physiology, 285, H457–H462.
Bhuiyan, A. R., Li, S., Li, H., Chen, W., Srinivasan, S. R., & Berenson, G. S. (2005). Distribution and correlates of arterial compliance measures in asymptomatic young adults: the Bogalusa Heart Study. American Journal of Hypertension, 18, 684–691.
Gardner, A. W., Montgomery, P. S., Blevins, S. M., & Parker, D. E. (2010). Gender and ethnic differences in arterial compliance in patients with intermittent claudication. Journal of Vascular Surgery, 51, 610–615.
Wassel, C. L., Jacobs, D. R., Jr., Duprez, D. A., et al. (2011). Association of self-reported race/ethnicity and genetic ancestry with arterial elasticity: the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of the American Society of Hypertension, 5, 463–472.
Bielinski, S. J., Lynch, A. I., Miller, M. B., et al. (2005). Genome-wide linkage analysis for loci affecting pulse pressure: the Family Blood Pressure Program. Hypertension, 46, 1286–1293.
Multi-center genetic study of hypertension. (2002). The Family Blood Pressure Program (FBPP). Hypertension, 39, 3–9.
Simino, J., Shi, G., Kume, R., et al. (2011). Five blood pressure loci identified by an updated genome-wide linkage scan: meta-analysis of the Family Blood Pressure Program. American Journal of Hypertension, 24, 347–354.
Adeyemo, A., Gerry, N., Chen, G., et al. (2009). A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genetics, 5, e1000564.
Parra, E. J., Marcini, A., Akey, J., et al. (1998). Estimating African American admixture proportions by use of population-specific alleles. American Journal of Human Genetics, 63, 1839–1851.
Xu, S., Huang, W., Wang, H., et al. (2007). Dissecting linkage disequilibrium in African-American genomes: roles of markers and individuals. Molecular Biology and Evolution, 24, 2049–2058.
Zhu, X., Luke, A., Cooper, R. S., et al. (2005). Admixture mapping for hypertension loci with genome-scan markers. Nature Genetics, 37, 177–181.
Zhu, X., & Cooper, R. S. (2007). Admixture mapping provides evidence of association of the VNN1 gene with hypertension. PLoS One, 2, e1244.
Goring, H. H., Curran, J. E., Johnson, M. P., et al. (2007). Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nature Genetics, 39, 1208–1216.
Fox, E. R., Young, J. H., Li, Y., et al. (2011). Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Human Molecular Genetics, 20, 2273–2284.
Zhu, X., Young, J. H., Fox, E., et al. (2011). Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Human Molecular Genetics, 20, 2285–2295.
Ehret, G. B., Munroe, P. B., Rice, K. M., et al. (2011). Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature, 478, 103–109.
Matsukawa, N., Grzesik, W. J., Takahashi, N., et al. (1999). The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proceedings of the National Academy of Sciences of the United States of America, 96, 7403–7408.
Boedtkjer, E., Praetorius, J., Matchkov, V. V., et al. (2011). Disruption of Na+, HCO cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca(2) sensitivity, and hypertension development in mice. Circulation, 124, 1819–1829.
Hunt, K. A., Zhernakova, A., Turner, G., et al. (2008). Newly identified genetic risk variants for celiac disease related to the immune response. Nature Genetics, 40, 395–402.
Smyth, D. J., Plagnol, V., Walker, N. M., et al. (2008). Shared and distinct genetic variants in type 1 diabetes and celiac disease. The New England Journal of Medicine, 359, 2767–2777.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, J.L., Duprez, D.A., Barac, A. et al. A Review of Genetics, Arterial Stiffness, and Blood Pressure in African Americans. J. of Cardiovasc. Trans. Res. 5, 302–308 (2012). https://doi.org/10.1007/s12265-012-9362-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-012-9362-y