Abstract
In recent years, stem cell treatment of myocardial infarction has elicited great enthusiasm upon scientists and physicians alike, thus making the finding of a suitable cell a compulsory subject for modern medicine. Due to its potential, accessibility and efficiency of harvesting, adipose tissue has become one of the most attractive sources of stem cells for regenerative therapies. The differentiation capacity and the paracrine activity of these cells has made them an optimal candidate for the treatment of a diverse range of diseases from immunological disorders as graft versus host disease to cardiovascular pathologies like peripheral ischemia. In this review, we will focus on the use of stem cells derived from adipose tissue for treatment of myocardial infarction, with special attention to their putative in vivo mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past several years, increasing awareness of the shortcomings of heart transplantation and left ventricular assist system has led cardiovascular surgeons to consider alternative means of treating heart disease. Although the use of reperfusion and pharmacological therapies have increased not only the rate of survival but also the quantity of salvaged tissue in myocardial infarction (MI) patients, MI is still a degenerative disease with a peak of cell death at the onset of the infarction but continuing thereafter (reviewed in [1]).
Regenerative therapies aim at restoring organ functionality by replenishing the cell loss caused by the ischemia either by stimulating the remaining cells or by providing a new pool of them. Among the diversity of sources (reviewed in [2, 3]), adipose tissue has become one of the most attractive not only due to its regenerative potential but also for other more practical reasons. Free of ethical, oncological, and immunological concerns present in pluripotent cells (embryonic stem cells or induced pluripotent stem cells (iPS)), adipose tissue stem cell obtention lacks the painful and time-consuming process associated with cells from other sources as skeletal muscle, bone marrow, or heart. Fat is abundant in most individuals, allowing a simpler and more efficient harvesting, as adipose tissue has a higher stem cell yield than bone marrow [4], and diminishing the need of in vitro expansion. In this review, we will focus on adipose-derived stem cells, highlighting their characteristics, potentials, and their application to MI, ranging from animal models up to clinical trials.
Adipose Tissue: Origin and Characteristics
Almost every animal species has a way to store excess energy in the form of fat. In the majority, this is done in a mesodermal tissue termed white adipose tissue (WAT), in which under hormonal influence mass triglyceride storage takes place. However, WAT not only performs functions as a reservoir tissue but is also responsible for mechanical support and thermal insulation and works as an efficient endocrine organ, releasing molecules capable of regulating immune response, blood pressure, angiogenesis, or bone mass among others [5]. At the histological level, WAT is characterized by being composed mostly of adipocytes, big cells with a peripheral nucleus and a cytoplasm occupied by a single drop of lipid. Aside from this population is the so-called stromal vascular fraction (SVF) composed of endothelial and smooth muscle cells, leukocytes, mast cell precursors, cells with hematopoietic progenitor activity, and adipose-derived stem cells (ASC) [4, 6, 7].
On the other hand, adipocytes in brown adipose tissue (BAT) present medium-sized lipid droplets usually surrounded by mitochondria, which give its characteristic brown color. BAT is mainly involved in producing non-shivering thermogenesis through expression of uncoupling protein-1 (UCP-1), which allows the dispersion of the proton electrochemical gradient generated by respiration in the form of heat [8]. With some exceptions, BAT has been traditionally considered insignificant in adult humans. However, recent studies have demonstrated expression of UCP-1 within WAT of normal individuals [9], thus suggesting some mixture of BAT in WAT depots.
Both adipose tissues are considered to be of mesodermal origin. In humans, first signs of adipogenesis are detected in the second trimester of gestation, with aggregation of mesenchymal stem cells (MSC) and formation of adjacent vessels [10]. In mice, lineage-tracing experiments have shown that brown adipocytes are derived from Myf5-positive progenitors, which also give rise to myocytes, while white adipocytes are Myf5-negative [11]. However, brown adipocytes detected in WAT after cold exposure or adrenergic stimulation do not originate from the Myf5-positive progenitor pool, having a clearly distinct origin from brown adipocytes in BAT [12]. The fact that different WAT depots respond differently to some hormones (sex hormones for adipose tissue in breasts and thighs or glucocortocoids for fat in the neck and the upper back) also points towards a distinct embryonic development, although suitable lineage-tracing experiments have not been performed so far.
Adipose Stem Cells: Differentiation Capacity
After a simple harvest, adipose tissue can be collagenase-digested, thus segregating mature adipocytes from the SVF by the floating ability of the formers. SVF displays a phenotype characteristic of a mixture of several populations [13] as stated above. These include not only multipotent stem cells (CD44-, CD73-, and CD90-positive) but also hematopoietic (CD11b-, CD34-, and CD45-expressing cells), endothelial (CD31- and CD133-positive; EC), smooth muscle (smooth muscle actin-expressing; SMC), or mast (c-kit-positive) [7, 13] cells. Consequently, SVF has shown its ability to reconstitute lethally irradiated mice [14] and support differentiation of bone marrow-hematopoietic progenitors [15]. Moreover, CD34+/CD31− endothelial procursors can be isolated from SVF, being able to derive into EC both in vitro and when injected in a hind limb ischemia model [16]. Additionally, SVF cells cultured on methylcellulose give rise to cells with angiogenic potential [17] and are able to develop into cardiomyocytes (CM) [18]. This group has recently published a method of obtaining and expanding this cardiomyogenic population from mouse SVF [19], termed adipose-derived cardiomyogenic cells (AD-CMG).
Upon adherent culture, the multipotent fraction of SVF is enriched and homogenized [13]. Although a large body of acronyms has been employed to name these cells, the International Fat Applied Technology Society standardized the nomenclature in 2004 by adopting the term adipose-derived stem cells to identify them [6]. This population depicts a close similarity with bone marrow MSC in either plastic adherence, phenotype, or multipotency (see [20] for a review). Not only do these cells display a mesenchymal-differentiation potential, being able to give rise to mesodermal lineages as chondrocytes, osteoblasts, or adipocytes [13], but other progenies have also been described. Thus, human ASC either from biopsy [21] or lipoaspirate [22] has shown its capacity to give rise to SMC and EC [23, 24]. In vitro differentiation of ASC towards CM has also been reported by either using demethylating agents [25] or coculture with CM protein extracts [26]. Moreover, ASC seem to retain the potential to develop into cardiac cells due to the harboring of a progenitor subset characterized by expression of Nkx2.5 and Mcl2v [27] and whose differentiation is dependent on the autocrine/paracrine action of vascular endothelial growth factor (VEGF) [28]. Whether this subset is lost or not during passage homogenization of ASC remains unknown.
Therapeutic Potential of ASC in MI
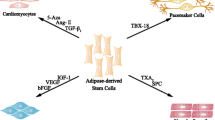
After MI, cardiac tissue remains chronically scarred. Even in the case of an early reperfusion, myocardium is rarely completely rescued. Physiological processes (reviewed in [1]) try to heal the damage, but although they exert a compensatory and positive action during the first stages, the scar is never repopulated, leaving a less compliant tissue that inexorably drives the patient towards cardiac failure. Thus, regenerating an infarcted heart encompasses a triple goal: (1) the regeneration of a new myocardial mass, (2) the creation of a functional vascular network, and (3) the return of the ventricle to its proper geometry. All of these processes may be accomplished by two ways, both of them achievable for cell therapy: direct differentiation of transplanted cells and paracrine action upon damaged tissue (Fig. 1).
Adipose-derived cells are capable of direct differentiation and paracrine actions upon damaged tissue. Both SVF and ASC are able to give rise to the needed phenotypes for cardiac regeneration (SMC, EC, and CM). Moreover, they secrete cytokines and chemokines capable of fibroblast regulation, angiogenesis, or cell homing. ASC have also demonstrated to be susceptible to reprogramming into iPS and, due to their immunoregulatory actions, to be allotransplanted
At first glance, differentiation into the desired lineages (mainly cardiac and smooth muscle and endothelium) is the best option to definitively heal the scar. As already pointed out, both cells from SVF or ASC have the potential to turn into any of these phenotypes [17, 18, 21, 23, 27, 29], although their capacity to retain it in elderly/diseased patients is under debate [30, 31]. However, one of the major drawbacks of cell therapy is the low rate of engraftment of transplanted cells (see [32] for a review), and though this can be improved through several approaches [33, 34], it is important to take into account the magnitude of the catastrophe. Myocardium hosts on average 20 million CM per gram of tissue. Considering that the human left ventricle weights about 400 g, this makes 4 billion CM. Heart failure can affect about 25% of the left ventricle, which means that in some cases about 1 billion CM need replacement [35]. Since cell engraftment is usually about 0.1–5% of transplanted cells and the percentage of the cells that differentiate into the desired types is also quite low, it is plausible that this mechanism of action, although conceptually important, is thus far therapeutically inefficient.
On the other hand, paracrine activity has demonstrated that even a few engrafted cells can exert a beneficial effect on cardiac tissue (reviewed in [36]). In general, transplanted cells can act upon the damaged heart in several ways, of which the leading are increasing myocardial perfusion, enhancing endogenous cell survival, attracting progenitors, and regulating tissue fibrosis.
Sengenès et al. showed that CD34+/CD31− from SVF are able to migrate towards EC [37]. SVF cells expressed CXCR4, the cognate receptor of the chemokine SDF-1, that was shown to be secreted by EC. This would make stem cells susceptible of chemotaxis towards the damaged heart, due to its early expression of SDF-1 after MI [38], and possibly increasing the homing of transplanted cells into the injured organ. Kondo et al. additionally demonstrated that ASC also express SDF-1 and that this molecule is at least partially responsible of the benefits found when treating a periferal ischemia model [39]. Moreover, CD34+/CD31− cells have been shown to be able to interact with EC and increase neovessel stability [29]. These cells of pericytic nature have demonstrated, when injected subcutaneously in a NOD/SCID mouse in a mixture with endothelial progenitors, that they increase vessel density and the complexity of the newly formed vascular network [40]. This group also analyzed the secretome of human ASC, showing high levels of the cytokines TGFβ1, VEGF, and hepatocyte growth factor (HGF) [41], a set of growth factors with important implications for therapy. TGFβ1 regulates the end of the inflammatory phase during the healing of MI as well as the function of cardiac fibroblasts [42, 43]. VEGF is a potent inductor of angiogenesis at the capillary level [44]. It has repeatedly been reported to be expressed by adipose-derived cells [28, 45], and its expression is enhanced by hypoxia [41], a process that could be mimicked in vivo, given the fact that the myocardial scar is an hypoxic tissue [46]. Finally, HGF is also a potent inductor of angiogenesis, and its effects have been shown to be additive to those of VEGF [47]. More importantly, HGF has demonstrated a capacity to alleviate the adverse effects of MI through increased angiogenesis, diminished apoptosis, cardiac hypertrophy and fibrosis, to improve pump function [48, 49] and to antagonize the pro-fibrotic activity of TGFβ1 [50]. Selective inhibition of HGF with small hairpin RNA significantly decreases the capacity of ASC to promote endothelial and endothelial progenitor cell survival, migration, and proliferation in vitro and to elicit a benefit in tissue perfusion in a mouse model of hind limb ischemia [51]. Taken together, these examples stress the fact that adipose-derived cells are potent paracrine mediators and can have an important impact on the evolution of the ischemic tissue.
Similarly to what has been described to MSC, ASC are able to regulate immunological reaction. McIntosh et al. showed that ASC beyond passage one failed to elicit a response from allogenic T cells [52]. Moreover, this immune-suppressive activity is enhanced under inflammatory stimuli [53] in vitro. Consequently, ASC are opened as an allogenic therapeutic option, thus broadening the possibilities for their application. Eventually, mouse and human ASC have been shown to be amenable to reprogramming towards iPS [54, 55], showing a higher efficiency than fibroblasts even without the use of the oncogene c-myc [56] or in a feeder-free condition [57], thus broadening the way to future cardiovascular applications.
ASC in Animal Models of MI
As a consequence of the previous described studies, ASC are a therapeutic option for MI, and therefore animal models of MI have extensively been employed as a means to test their suitability (Table 1). We foresee three main settings in which cells can be transplanted. The first is the acute model of MI, in which the procedure will take place immediately after the induction of the infarction. Thus, cells will have to cope with the development of an inflammatory reaction and the release of death signals from necrotic and apoptotic cells within the tissue [58, 59]. However, an inflammatory milieu can exert a positive action on cell activity [53]. Even more, the presence of an anti-fibrotic microenvironment [60] as well as homing signals [61] may enhance therapy outcome. In this sense, a plethora of studies have been published in which adipose cells were transplanted in this acute setting [19, 51, 62–67]. With the exception of the report by van der Bogt et al. [66], all of them have shown a consistent and significant benefit of transplanted cells upon cardiac function either by echocardiography [19, 51, 63, 67] or magnetic resonance imaging (MRI) [62, 64, 68]. Although ASC have homogeneously been shown to engraft and give rise to EC, SMC, and even CM and to remain within infarcted hearts up to 12 weeks, their direct contribution to those phenotypes has been minor not only because of the low percentage of cells that remain in the tissue [19, 63] but also due to the limited rate of differentiation [68]. As a consequence, as already mentioned it is conceivable that paracrine actions of transplanted cells account for most of the benefit. Again, consistent and significant effect of injected cells has been dependent on their actions upon damaged tissue, mostly by increasing tissue perfusion and diminishing infarct size and fibrosis. This has also been related to their in vitro expression of cytokines as VEGF, HGF [19], bFGF, TNFα, PDGFBB [69], or to the in vivo decrease in the level of profibrotic molecules [67].
However, the use of ASC in the acute setting of MI has an impending hindrance, namely, the impossibility to expand sufficient number of cells for an optimal treatment. In all the studies mentioned in the previous paragraph (with the exception of [63] in which fresh uncultured cells were employed), cells were subjected to two to five in vitro passages, which translates to at least 2–5 weeks, and would not allow to turn any of those protocols to a bedside application. Nevertheless, an allogenic therapy could be envisioned in view of the immunological properties of adipose cells [52].
In clear opposition to the acute, the chronic stage is characterized by collagen deposition, maturation of the scar, and stabilization of cardiac function (reviewed in [70]), although insufficient perfusion and a disorganized vascular network [71] can impair cell transplantation at this stage. However, it is a clinically significant setting in which therapeutic options are limited, and thus cell therapy provides an approach that warrants investigation. Only two reports have explored the effects of ASC in chronically infarcted hearts. Miyahara and coworkers merged ASC with tissue engineering [72]. They seeded ASC at passage 4 onto temperature-responsive dishes, thus maintaining cell-to-cell contact and intact adhesive proteins upon transplantation. Four weeks after MI induction in rats, approximately 1 million ASC were transferred into the infarction. The treatment elicited a significant benefit in cardiac function both by echocardiography and hemodynamic assessment, and this was related to an increase in wall thickness and cell release of growth factors (VEGF, HGF). No effect was found in rats treated with dermal fibroblasts. Also, ASC differentiated to cardiac and vascular lineages in vivo. Our group compared the effect of ASC with AD-CMG and bone marrow-mononuclear cells in a xenogenic model of chronic MI [73]. Surprisingly, only ASC elicited a positive response, with a significant increase in left ventricular ejection fraction and on tissue metabolism. Importantly, although AD-CMG cells also increased 18F-FDG uptake, they were unable to survive even for 1 week. Accordingly, Léobon et al. reported that AD-CMG transplanted in the acute phase of MI were also unable to survive unless co-injected in a mixture that contains stromal cells [19].
Finally, the intermediate situation is also possible. The so-called sub-acute setting would be characterized by an incomplete deposition of the scar but also by a strong revascularization activity either by the concourse of endothelial progenitors [74] or reparative monocytes [75]. Wang and collaborators transplanted rat ASC 1 week after MI and found that treatment induced a significant benefit on cardiac contractility by MRI, as well as increased the density of capillaries and decreased infarct size [68]. Transplanted cells remained at least for 4 weeks within the tissue and were able to differentiate towards CM, albeit in low numbers (0.5% of engrafted ASC). Recently, the group of Dr Ispizúa-Belmonte described the isolation, culture, and characterization of a subset of cells termed cardiac adipose tissue-derived progenitor cells [69]. When injected either in a mouse or rat model of sub-acute MI (1 week post-ligation), these cells induced a positive response at the functional and histological level and were able to differentiate along vascular and cadiomyocytic lineages. Rigol and coworkers, in a pig model of ischemia reperfusion, injected passage 3 ASC either via a transendocardial catheter or through intracoronary infusion 1 week after induction of MI [76]. Transplanted cells engrafted, differentiated to SMC, and increased the density of arterioles to a similar degree by either approach, although they were not able to demonstrate a significant benefit on cardiac function.
Conclusion
ASC have raised increasing interest in the cardiovascular field in recent years due to their suitable properties. In preclinical studies with either small or big animal models of MI, adipose cells have performed well, inducing significant and beneficial changes in cardiac function and anatomy. On this basis, key clinical trials have been initiated: APOLLO (3D adipose-derived stem-cell transplant in the treatment of patients with an acute ST-elevation MI) by Serryus and colleagues and PRECISE (3D adipose-derived stem cells in the treatment of patients with non-revascularizable ischemic myocardium) headed by Dr Fernández-Avilés. Their outcome will shed more light on the clinical capacity of ASC as treatment for MI. However, disparity in isolation, selection, characterization, and use are issues that still remain to be solved.
References
Mazo, M., Pelacho, B., & Prosper, F. (2010). Stem cell therapy for chronic myocardial infarction. Journal of Cardiovascular Translational Research, 3(2), 79–88. doi:10.1007/s12265-009-9159-9.
Pelacho, B., & Prosper, F. (2008). Stem cells and cardiac disease: where are we going? Current Stem Cell Research & Therapy, 3(4), 265–276.
Segers, V. F., & Lee, R. T. (2008). Stem-cell therapy for cardiac disease. Nature, 451(7181), 937–942.
Fraser, J. K., Wulur, I., Alfonso, Z., & Hedrick, M. H. (2006). Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology, 24(4), 150–154.
Trayhurn, P. (2005). Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiologica Scandinavica, 184(4), 285–293. doi:10.1111/j.1365-201X.2005.01468.x.
Daher, S. R., Johnstone, B. H., Phinney, D. G., & March, K. L. (2008). Adipose stromal/stem cells: basic and translational advances: the IFATS collection. Stem Cells, 26(10), 2664–2665. doi:10.1634/stemcells.2008-0927.
Poglio, S., De Toni-Costes, F., Arnaud, E., Laharrague, P., Espinosa, E., Casteilla, L., et al. (2010). Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells, 28(11), 2065–2072. doi:10.1002/stem.523.
Cannon, B., & Nedergaard, J. (2004). Brown adipose tissue: function and physiological significance. Physiological Reviews, 84(1), 277–359. doi:10.1152/physrev.00015.2003.
Nedergaard, J., Bengtsson, T., & Cannon, B. (2007). Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology: Endocrinology and Metabolism, 293(2), E444–E452. doi:10.1152/ajpendo.00691.2006.
Ailhaud, G., & Hauner, H. (1998). Development of white adipose tissue. In G. A. Bray, C. Bouchard, & W. P. T. James (Eds.), Handbook of obesity. New York: Marcel Dekker Inc.
Seale, P., Bjork, B., Yang, W., Kajimura, S., Chin, S., Kuang, S., et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature, 454(7207), 961–967. doi:10.1038/nature07182.
Guerra, C., Koza, R. A., Yamashita, H., Walsh, K., & Kozak, L. P. (1998). Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. Journal of Clinical Investigation, 102(2), 412–420. doi:10.1172/JCI3155.
Mitchell, J. B., McIntosh, K., Zvonic, S., Garrett, S., Floyd, Z. E., Kloster, A., et al. (2006). Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells, 24(2), 376–385.
Cousin, B., Andre, M., Arnaud, E., Penicaud, L., & Casteilla, L. (2003). Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochemical and Biophysical Research Communications, 301(4), 1016–1022.
Corre, J., Barreau, C., Cousin, B., Chavoin, J. P., Caton, D., Fournial, G., et al. (2006). Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. Journal of Cellular Physiology, 208(2), 282–288.
Miranville, A., Heeschen, C., Sengenes, C., Curat, C. A., Busse, R., & Bouloumie, A. (2004). Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation, 110(3), 349–355.
Planat-Benard, V., Silvestre, J. S., Cousin, B., Andre, M., Nibbelink, M., Tamarat, R., et al. (2004). Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation, 109(5), 656–663.
Planat-Benard, V., Menard, C., Andre, M., Puceat, M., Perez, A., Garcia-Verdugo, J. M., et al. (2004). Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circulation Research, 94(2), 223–229.
Leobon, B., Roncalli, J., Joffre, C., Mazo, M., Boisson, M., Barreau, C., et al. (2009). Adipose-derived cardiomyogenic cells: in vitro expansion and functional improvement in a mouse model of myocardial infarction. Cardiovascular Research, 83(4), 757–767.
Gimble, J. M., Katz, A. J., & Bunnell, B. A. (2007). Adipose-derived stem cells for regenerative medicine. Circulation Research, 100(9), 1249–1260. doi:10.1161/01.RES.0000265074.83288.09.
Kim, Y. M., Jeon, E. S., Kim, M. R., Jho, S. K., Ryu, S. W., & Kim, J. H. (2008). Angiotensin II-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. International Journal of Biochemistry & Cell Biology, 40(11), 2482–2491.
Rodriguez, L. V., Alfonso, Z., Zhang, R., Leung, J., Wu, B., & Ignarro, L. J. (2006). Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America, 103(32), 12167–12172.
Fischer, L. J., McIlhenny, S., Tulenko, T., Golesorkhi, N., Zhang, P., Larson, R., et al. (2009). Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. Journal of Surgical Research, 152(1), 157–166.
Martinez-Estrada, O. M., Munoz-Santos, Y., Julve, J., Reina, M., & Vilaro, S. (2005). Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovascular Research, 65(2), 328–333. doi:10.1016/j.cardiores.2004.11.015.
van Dijk, A., Niessen, H. W., Zandieh Doulabi, B., Visser, F. C., & van Milligen, F. J. (2008). Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell & Tissue Research, 334, 457–467.
Gaustad, K. G., Boquest, A. C., Anderson, B. E., Gerdes, A. M., & Collas, P. (2004). Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochemical and Biophysical Research Communications, 314(2), 420–427.
Bai, X., Pinkernell, K., Song, Y. H., Nabzdyk, C., Reiser, J., & Alt, E. (2007). Genetically selected stem cells from human adipose tissue express cardiac markers. Biochemical and Biophysical Research Communications, 353(3), 665–671.
Song, Y. H., Gehmert, S., Sadat, S., Pinkernell, K., Bai, X., Matthias, N., et al. (2007). VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochemical and Biophysical Research Communications, 354(4), 999–1003.
Traktuev, D. O., Merfeld-Clauss, S., Li, J., Kolonin, M., Arap, W., Pasqualini, R., et al. (2008). A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation Research, 102(1), 77–85.
Madonna, R., Renna, F. V., Cellini, C., Cotellese, R., Picardi, N., Francomano, F., et al. (2010). Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. European Journal of Clinical Investigation. doi:10.1111/j.1365-2362.2010.02384.x.
Zhang, P., Moudgill, N., Hager, E., Tarola, N., Dimatteo, C., McIlhenny, S., et al. (2010). Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells and Development. doi:10.1089/scd.2010.0152.
Haider, H. K., & Ashraf, M. (2008). Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. Journal of Molecular and Cellular Cardiology, 45, 554–666.
Penn, M. S., & Mangi, A. A. (2008). Genetic enhancement of stem cell engraftment, survival, and efficacy. Circulation Research, 102(12), 1471–1482.
Suuronen, E. J., Kuraitis, D., & Ruel, M. (2008). Improving cell engraftment with tissue engineering. Seminars in Thoracic and Cardiovascular Surgery, 20(2), 110–114.
Robey, T. E., Saiget, M. K., Reinecke, H., & Murry, C. E. (2008). Systems approaches to preventing transplanted cell death in cardiac repair. Journal of Molecular and Cellular Cardiology, 45, 567–581.
Fedak, P. W. (2008). Paracrine effects of cell transplantation: modifying ventricular remodeling in the failing heart. Seminars in Thoracic and Cardiovascular Surgery, 20(2), 87–93.
Sengenes, C., Miranville, A., Maumus, M., de Barros, S., Busse, R., & Bouloumie, A. (2007). Chemotaxis and differentiation of human adipose tissue CD34+/CD31− progenitor cells: role of stromal derived factor-1 released by adipose tissue capillary endothelial cells. Stem Cells, 25(9), 2269–2276.
Ma, J., Ge, J., Zhang, S., Sun, A., Shen, J., Chen, L., et al. (2005). Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Research in Cardiology, 100(3), 217–223.
Kondo, K., Shintani, S., Shibata, R., Murakami, H., Murakami, R., Imaizumi, M., et al. (2009). Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 29(1), 61–66. doi:10.1161/ATVBAHA.108.166496.
Traktuev, D. O., Prater, D. N., Merfeld-Clauss, S., Sanjeevaiah, A. R., Saadatzadeh, M. R., Murphy, M., et al. (2009). Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circulation Research, 104(12), 1410–1420.
Rehman, J., Traktuev, D., Li, J., Merfeld-Clauss, S., Temm-Grove, C. J., Bovenkerk, J. E., et al. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation, 109(10), 1292–1298.
Frangogiannis, N. G. (2004). Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflammation Research, 53(11), 585–595.
Wynn, T. (2008). Cellular and molecular mechanisms of fibrosis. Journal of Pathology, 214(2), 199–210.
Ferrara, N., Gerber, H. P., & LeCouter, J. (2003). The biology of VEGF and its receptors. Nature Medicine, 9(6), 669–676. doi:10.1038/nm0603.
Li, B., Zeng, Q., Wang, H., Shao, S., Mao, X., Zhang, F., et al. (2007). Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coronary Artery Disease, 18(3), 221–227.
Chacko, S. M., Khan, M., Kuppusamy, M. L., Pandian, R. P., Varadharaj, S., Selvendiran, K., et al. (2009). Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. American Journal of Physiology. Heart and Circulatory Physiology, 296, H1263–H1273.
Van Belle, E., Witzenbichler, B., Chen, D., Silver, M., Chang, L., Schwall, R., et al. (1998). Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation, 97(4), 381–390.
Nakamura, T., Matsumoto, K., Mizuno, S., Sawa, Y., Matsuda, H., & Nakamura, T. (2005). Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. American Journal of Physiology. Heart and Circulatory Physiology, 288, H2131–H2139.
Jayasankar, V., Woo, Y. J., Pirolli, T. J., Bish, L. T., Berry, M. F., Burdick, J., et al. (2005). Induction of angiogenesis and inhibition of apoptosis by hepatocyte growth factor effectively treats postischemic heart failure. Journal of Cardiac Surgery, 20(1), 93–101. doi:10.1111/j.0886-0440.2005.200373.x.
Dai, C., & Liu, Y. (2004). Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. Journal of the American Society of Nephrology, 15(6), 1402–1412.
Cai, L., Johnstone, B. H., Cook, T. G., Tan, J., Fishbein, M. C., Chen, P. S., et al. (2008). IFATS Series: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells, 27(1), 230–237.
McIntosh, K., Zvonic, S., Garrett, S., Mitchell, J. B., Floyd, Z. E., Hammill, L., et al. (2006). The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells, 24(5), 1246–1253.
Crop, M. J., Baan, C. C., Korevaar, S. S., Ijzermans, J. N., Pescatori, M., Stubbs, A. P., et al. (2010). Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clinical and Experimental Immunology, 162(3), 474–486. doi:10.1111/j.1365-2249.2010.04256.x.
Sun, N., Panetta, N. J., Gupta, D. M., Wilson, K. D., Lee, A., Jia, F., et al. (2009). Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proceedings of the National Academy of Sciences of the United States of America, 106(37), 15720–15725.
Tat, P. A., Sumer, H., Jones, K. L., Upton, K., & Verma, P. J. (2010). The efficient generation of induced pluripotent stem (iPS) cells from adult mouse adipose tissue-derived and neural stem cells. Cell Transplantation, 19(5), 525–536. doi:10.3727/096368910X491374.
Aoki, T., Ohnishi, H., Oda, Y., Tadokoro, M., Sasao, M., Kato, H., et al. (2010). Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-MYC. Tissue Eng Part A, 16(7), 2197–2206. doi:10.1089/ten.TEA.2009.0747.
Sugii, S., Kida, Y., Kawamura, T., Suzuki, J., Vassena, R., Yin, Y. Q., et al. (2010). Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(8), 3558–3563. doi:10.1073/pnas.0910172106.
Mann, D. L. (1999). Mechanisms and models in heart failure: a combinatorial approach. Circulation, 100(9), 999–1008.
Nian, M., Lee, P., Khaper, N., & Liu, P. (2004). Inflammatory cytokines and postmyocardial infarction remodeling. Circulation Research, 94(12), 1543–1553.
Cleutjens, J. P., Kandala, J. C., Guarda, E., Guntaka, R. V., & Weber, K. T. (1995). Regulation of collagen degradation in the rat myocardium after infarction. Journal of Molecular and Cellular Cardiology, 27(6), 1281–1292.
Penn, M. S. (2009). Importance of the SDF-1:CXCR4 axis in myocardial repair. Circulation Research, 104(10), 1133–1135.
Valina, C., Pinkernell, K., Song, Y. H., Bai, X., Sadat, S., Campeau, R. J., et al. (2007). Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European Heart Journal, 28(21), 2667–2677.
Schenke-Layland, K., Strem, B. M., Jordan, M. C., Deemedio, M. T., Hedrick, M. H., Roos, K. P., et al. (2008). Adipose tissue-derived cells improve cardiac function following myocardial infarction. Journal of Surgical Research, 153(2), 217–223.
Bai, X., Yan, Y., Song, Y. H., Seidensticker, M., Rabinovich, B., Metzele, R., et al. (2009). Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European Heart Journal, 31(4), 489–501.
Jumabay, M., Matsumoto, T., Yokoyama, S., Kano, K., Kusumi, Y., Masuko, T., et al. (2009). Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. Journal of Molecular and Cellular Cardiology, 47(5), 565–575.
van der Bogt, K. E., Schrepfer, S., Yu, J., Sheikh, A. Y., Hoyt, G., Govaert, J. A., et al. (2009). Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation, 87(5), 642–652.
Zhu, X. Y., Zhang, X. Z., Xu, L., Zhong, X. Y., Ding, Q., & Chen, Y. X. (2009). Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochemical and Biophysical Research Communications, 379(4), 1084–1090. doi:10.1016/j.bbrc.2009.01.019.
Wang, L., Deng, J., Tian, W., Xiang, B., Yang, T., Li, G., et al. (2009). Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: an MR imaging study of rat hearts. American Journal of Physiology. Heart and Circulatory Physiology, 297(3), H1020–H1031.
Bayes-Genis, A., Soler-Botija, C., Farre, J., Sepulveda, P., Raya, A., Roura, S., et al. (2010). Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. Journal of Molecular and Cellular Cardiology, 49(5), 771–780. doi:10.1016/j.yjmcc.2010.08.010.
Sun, Y., Kiani, M. F., Postlethwaite, A. E., & Weber, K. T. (2002). Infarct scar as living tissue. Basic Research in Cardiology, 97(5), 343–347.
Virag, J. I., & Murry, C. E. (2003). Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. American Journal of Pathology, 163(6), 2433–2440.
Miyahara, Y., Nagaya, N., Kataoka, M., Yanagawa, B., Tanaka, K., Hao, H., et al. (2006). Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine, 12(4), 459–465.
Mazo, M., Planat-Benard, V., Abizanda, G., Pelacho, B., Leobon, B., Gavira, J. J., et al. (2008). Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. European Journal of Heart Failure, 10(5), 454–462.
Jujo, K., Ii, M., & Losordo, D. W. (2008). Endothelial progenitor cells in neovascularization of infarcted myocardium. Journal of Molecular and Cellular Cardiology, 45(4), 530–544.
Nahrendorf, M., Swirski, F. K., Aikawa, E., Stangenberg, L., Wurdinger, T., Figueiredo, J. L., et al. (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. Journal of Experimental Medicine, 204(12), 3037–3047.
Rigol, M., Solanes, N., Farre, J., Roura, S., Roque, M., Berruezo, A., et al. (2010). Effects of adipose tissue-derived stem cell therapy after myocardial infarction: impact of the route of administration. Journal of Cardiac Failure, 16(4), 357–366. doi:10.1016/j.cardfail.2009.12.006.
Acknowledgements
This work was supported in part by ISCIII PI050168, PI070474, CP09/00333, and ISCIII-RETIC RD06/0014, MICCIN PLE2009-0116, and PSE SINBAD, Gobierno de Navarra (Departamento de Educación), Comunidad de Trabajo de los Pirineos (CTP), European Union Framework Project VII (INELPY), Caja de Ahorros de Navarra (Programa Tu Eliges: Tu Decides), and the “UTE project CIMA”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazo, M., Gavira, J.J., Pelacho, B. et al. Adipose-derived Stem Cells for Myocardial Infarction. J. of Cardiovasc. Trans. Res. 4, 145–153 (2011). https://doi.org/10.1007/s12265-010-9246-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-010-9246-y