Abstract
Stem cell therapy has emerged as a promising approach to improve healing of the infarcted myocardium, to treat or prevent cardiac failure, and to restore lost cardiac function. Despite initial excitement, recent clinical trials using nonhomogenous human stem cells preparations showed variable results, raising concerns about the best cell type to transplant. Selected CD133+ hematopoietic stem cells are promising candidate cells with great potential. COMPARE-acute myocardial infarction (AMI) study is a phase II, randomized, double-blind, placebo-controlled trial evaluating the safety and effectiveness of intracoronary CD133+-enriched hematopoietic bone marrow stem cells in patients with acute myocardial infarction and persistent left ventricular dysfunction. Patients who underwent successful percutaneous coronary intervention and present a persistent left ventricular ejection fraction <50% will be eligible to have bone marrow aspiration and randomized for intracoronary injection of selected CD 133+ bone marrow cells vs placebo. The primary end point is a composite of a safety and efficacy end points evaluating the change at 4 months in the coronary atherosclerotic burden progression proximal and distal to the coronary stent in the infarct related artery; and the change in global left ventricular ejection fraction at 4 months relative to baseline as measured by magnetic resonance imaging. The secondary end point will be the occurrence of a major adverse cardiac event. To date, 14 patients were successfully randomized and treated without any protocol-related complication. COMPARE-AMI trial will help identify the effect of a selected population of the bone marrow stem cells on cardiac recovery of infarcted myocardium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of postinfarction congestive heart failure is caused by myocardial cell loss in the area supplied by the infarct-related artery and the subsequent formation of a scar. In patients with large myocardial infarction (MI), and especially when the necrotic zone is weakly supplied by collaterals, the loss of cardiomyocytes results in the formation of fibrous tissue and subsequently, in left ventricular (LV) remodeling, aneurysm formation, and progression of congestive heart failure, which remains despite improved pharmacological therapy the leading cause of cardiovascular mortality in North America and Europe [1–3]. The ability to repair and regenerate ischemic or damaged myocardium presents a major challenge in the treatment of cardiovascular disease.
Cellular transplantation is now emerging as a potential therapeutical approach to improve healing of infarcted heart. Several cell candidates have been recently investigated for cellular therapy such as embryonic stem cells, cardiac resident stem cells, or skeletal myoblasts [4–8]. Alternative sources are the bone marrow stem cells (BMSC) [9–11]. The ability of the latter cells to mobilize from the bone marrow to the injured myocardium and to participate in the healing process has encouraged several groups to investigate the potential benefit of these stem cells for the cardiac repair [12–15]. Although data supporting significant cardiomyocytic differentiation in the preclinical studies have not been uniform [16, 17], other mechanisms such as cellular fusion [18] or paracrine effect [19, 20] have been suggested.
The concept of autologous bone marrow (BM)-derived stem cell therapy for cardiac repair has been recently extended to humans. Several clinical trials using autologous bone marrow-derived mononuclear cells (BMMNCs) demonstrated safety of the therapy and improvement of cardiac performance ([21–28]). Meta-analyses of acute myocardial infarction (AMI) stem cell trial [29–31] confirmed that BMMNCs administration is safe over several years of follow-up and results in a small but statistically significant improvement in LV ejection fraction (LVEF). In addition, it appears that cell therapy may attenuate LV remodeling providing hope that further improvements in this therapy could eventually reduce the incidence of heart failure. Recently, the BALANCE study showed that, in addition to the improvement in the LV performance, BMSC therapy leads to significant and longstanding improvements of the quality of life and survival of patients after AMI [32].
There is currently uncertainty as to the optimal stem cell population to use clinically for cardiac repair. It appears that not “one cell fits all” but that the selection of the cell type should be tailored to the primary clinical indication and expected outcome [33]. Despite recent significant progress, answer to basic questions such as the best cell type has not been addressed so far. Different cell type may compete for the engraftment in the injured myocardium. Hematopoietic progenitor stem cells have been shown to uniformly induce neovascularization and cardiomyogenesis and to inhibit apoptosis in acutely infarcted myocardium, which are fundamentals for successful functional repair [12]. These hematopoietic stem cells are characterized by the presence of the surface marker CD34. In addition, CD133 has been identified as a marker that is present on the stem cells that coexpress not only CD34 but also other markers such as c-kit. It may be hypothesized that CD133+ cells are a larger or more primitive group of stem cells that the cells expressing only CD34+ marker. As a matter of fact, the hematopoietic CD133+ cells possess high engraftment, pluripotent, and angiogenic capacity and appear to be critical for cardiac repair in experimental myocardial infarction [34–37]. In addition, a phase I pilot study showed that the intracoronary administration of selected autologous CD133+ bone marrow progenitors in patients with recent myocardial infarction was safe and associated with a significant increase of 7% in the LVEF of the treated group as compared to 3% in controls at 4 months of follow-up. They also showed that, in patients treated with intracoronary CD133+ cells, the improvement of the global and regional LV function was paralleled with increased myocardial perfusion and viability [38].
Design and Objectives: COMPARE-AMI Study
COMPARE-AMI study is a single-center, randomized, prospective, double-arm, double-blind, placebo-controlled phase II clinical trial. In this study, we are investigating the feasibility, safety, and efficacy of the intracoronary (IC) administration of selected (CD133+) enriched hematopoietic bone marrow stem cells versus placebo for the cardiac repair following acute MI in patients with persistent dysfunctional myocardium despite successful catheter revascularization.
The Study End Points
The primary safety end point of the study is to determine the change in the coronary atherosclerotic burden progression proximal and distal to the stented segment of the culprit artery at 4 months as compared to baseline. The primary efficacy endpoint is to determine the change in LVEF at 4 months relative to baseline as measured by magnetic resonance imaging (MRI).
Secondary end points include the occurrence of a major adverse cardiac event defined as cardiac death, myocardial infarction, coronary bypass grafting, or a repeat percutaneous intervention (PCI) of the culprit lesion and the occurrence of major arrhythmias defined as sustained ventricular tachycardia or survived sudden death. Patients will be followed up for 2 years.
Hypothesis of the Trial
The primary hypotheses of the COMPARE-AMI study are that, as compared with placebo therapy, (1) administration of CD133+ bone marrow stem cell therapy will improve global and regional LV function; and (2) this improvement will not depend on modification of the coronary atherosclerotic burden in the infarct-related artery. The secondary hypotheses are that, in comparison with control therapy, the administration of cell therapy will result in a lower incidence of the composite adverse events of death, reinfarction, repeat revascularization, and hospitalization for congestive heart failure.
Study Population
Inclusion criteria of our study are: age between 30 and 75 years, chest pain for more than 30 min and less than 24 h, electrocardiography ST segment elevation >1 mm in two consecutive leads in the limb leads or >2 mm in the precordial leads, and an increase in creatine kinase (CK) or CK-muscle and brain types (CK-MB). Patients need to be <7 days of admission for their acute ST elevation MI, to be clinically and hemodynamically stable, to have successful stenting of the culprit coronary stenosis, to have single vessel disease, an LVEF <50 but >25%, and significant regional wall motion abnormalities in at least two adjacent segments on the resting echocardiography.
Patients with known previous MI, those who presented with cardiogenic shock, chronic cardiomyopathy, liver disease, renal failure, concomitant disease with a life expectancy of less than 1 year, alcohol or drug dependency, and contraindication for bone marrow aspiration are excluded from our trial. Other exclusion criteria include: blood transfusion in the previous 24 h, hematopoietic disease, chronic inflammatory disease, malignancy, stroke in the previous 3 months or transient ischemic attack in the previous 24 h, or extended ongoing myocardial infarction as evidenced by a new episode of chest pain with new ST segment elevations and a new CK/CK-MB peak.
Bone Marrow Aspiration and Cell Processing
After a written informed consent has been obtained, a bone marrow aspiration is performed between the third and the seventh day after PCI from the posterior iliac crest by an experienced operator under appropriate sedation and local anesthesia. A total volume of 100 ml of the bone marrow is obtained and transferred immediately to the cell processing laboratory. Bone marrow-derived CD133+ autologous progenitor cells for this trial are immunomagnetically selected using the CliniMACS® system (Miltenyi Biotec GmbH) at the HMR Cellular Therapy Laboratory. Briefly, immunomagnetic particles composed of iron oxide and dextran conjugated to monoclonal antibodies bind to target cells expressing the CD133+ cell surface antigen. When exposed to a magnetic field, labeled cells are retained within the column and separated from unlabeled cells. Removing the magnetic field from the separation column releases CD133+ cells. Eluted cells are characterized using flow cytometry analysis. All procedures are performed with strict adherence to all applicable regulations regarding the processing and use of human stem cells. Cellular preparations used in this study undergo endotoxin and sterility evaluations. Subjects in the active group receive a maximum of 10 million autologous CD133+ cells or resuspension medium alone. The placebo solution consists of normal saline containing 10% autologous plasma from which the cells have been eliminated. The placebo solution is prepared in a way that it is indistinguishable from a true cellular suspension. The product is provided in 20-ml syringes.
Randomization and Cell Injection
Patients are randomized either to the CD133+ cells treatment or placebo group. With the exception of the technicians at the Cellular Therapy Laboratory who dispatched the cell products, neither the patients nor treating physicians nor data managers have access to the randomization code for the duration of the study. Intracoronary injection of either cells or placebo is performed within 12 h of the bone marrow aspiration. Following heparinization to an activated clotting time of >200 s, the infusate is delivered via an over-the-wire percutaneous transluminal coronary angioplasty catheter over 3 min of balloon inflation within the previously placed intracoronary stent. Three minutes of reperfusion are incorporated after each cycle of cell infusion. Patients are routinely discharged 48 h following the infusion procedure with the usual post-AMI care medications.
Statistical Analysis
Baseline demographic and clinical variables are collected for each randomized arm of the study. All invasive and noninvasive analyses will be performed by operators blinded to all clinical and other functional data. Data are presented as mean ± standard error of the mean. Analyses of variance followed by paired Student's t test are used for group comparisons. Statistical significance was set at P < 0.05.
Preliminary Results
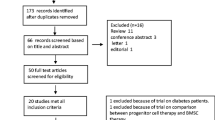
Herein, we are presenting the analysis of our ongoing clinical trial of our first fourteen patients that were successfully randomized and treated in the COMPARE-AMI trial. The study will be unblinded only after recruitment of the last patient with 12-month follow-up. The clinical characteristics are showed in Table 1. The mean age was 50.5 ± 9.1 years with a predominance of males (93%); culprit lesion was located on the left anterior descending artery in 100%, and peak troponin and CK-MB were 8.4 ± 6.1 Ug/L and 322.6 ± 225.6 U/L, respectively. To date, there is no protocol-related complication to report such as death, MI, stroke, or sustained ventricular arrhythmia. Re-PCI was necessary at 4 months of follow-up in two patients to treat bare-metal stent restenosis. These two patients were asymptomatic; however, silent ischemia was documented in the target territory. Baseline fractional flow reserve (FFR) was significantly lower in the stented culprit artery compared to the nonculprit artery at baseline: 0.88 ± 0.03 vs 0.96 ± 0.03, P < 0.001. However, at 4 months of follow-up (n = 14), no significant difference was found in the delta FFR compared to baseline in the culprit vs nonculprit artery (−3.8% ± 7.4 vs −1.1% ± 4.4, respectively, P = 0.296), suggesting no acceleration of the atherosclerosis by the treatment. Finally, at 4 months of follow-up, MRI assessment of the LVEF (n = 14 patients) showed a significant improvement compared to baseline with LVEF 52.1% ± 7.1 vs 41.3% ± 5.3, respectively (P < 0.001; Table 1 and Fig. 1). Finally, Figs. 2 and 3 illustrate a significant improvement in the LV function and myocardial perfusion of a 54-year-old man 4 months after his randomization in COMPARE-AMI trial.
Representative left heart ventriculography. A 54-year-old man presented acute anterior MI treated successfully with a proximal LAD stenting. Following MI, his left ventricular ejection fraction (LEVF) was 43.2% and his left ventriculography showed a significant hypokinesia of the anterior wall and apex (left). Four months later, we observed normalization of the LVEF (66.1%) with normal motion of the anterior wall and apex (arrows, right)
Technetium-99 m Sestamibi single-photon emission computed tomography (MIBI SPECT) scintigraphy looking at myocardial perfusion. The same patient as in Fig. 1, 54-year-old man with acute anterior MI, presented an important perfusion defect of the anteroseptal and apical territories (top left). The MIBI bull's eye image (bottom left) combining the 17 segments revealed an important ischemic territory with summed stress score (SSS) of 21 and a summed rest score (SRS) of 8. The summed difference (SDS = SSS–SRS) was 13 (not shown), indicating a significant redistribution (reversible perfusion defect). Four months later (rights), perfusion defect and ischemia were significantly reduced as revealed by stress and rest MIBI SPECT
Discussion
Preclinical studies have demonstrated that stem cell therapy can significantly reduce the development of LV dysfunction after MI [12, 13] which has rapidly translated into clinical trials using a variety of nonselected bone marrow stem cells [23–25, 27, 28]. Importantly, these trials have demonstrated that cell delivery after AMI is safe over several years of follow-up, and meta-analyses review of these trials have found a small but significant improvement in LV function [30, 31]. However, despite these encouraging findings on infarct size reduction and improvement in contractile function, many fundamental questions have not been addressed, including the important questions of dose, the timing of cell delivery, and optimal cell type delivered post-AMI [39, 40]. Another issue is the very low rate of cell retention by the recipient myocardial tissue [41]. The mechanisms underlying cell survival, engraftment, and ultimately their fate in the ischemic myocardium are multifactorial and interrelated.
In the published randomized clinical trials [23–25, 27, 28], BMMNCs were administered between 1 and 7 days post-AMI. In a subgroup analysis, the REPAIR-AMI trial [27] suggested that cell administration between 5 and 7 days was optimal in regard to recovery of LVEF. The number of infused cells administered post-AMI has varied significantly between the randomized trials, with differences up to several orders of magnitude [23–25, 27, 28]. However, a recent meta-analysis found no effect between the number of cells infused and recovery of LVEF [30].
The COMPARE-AMI trial is addressing one of these unresolved issues focusing on the important question regarding the best cell to be delivered post-AMI. The majority of clinical trials used nonhomogenous precursors with several different fractions of mononuclear BMSC. Different cell types may compete for the engraftment in the injured myocardium, which could account for variable results of previous studies. The group of Drexler showed that selected bone marrow MNCs display a sevenfold higher retention in the infarcted myocardium as compared to unfractionated BMSC [42]. Furthermore, Stamm et al. used CD133+ cells, a more specified subpopulation of BM-derived cells, for intramyocardial injections performed during an open-chest procedure and observed increased left ventricular ejection fraction and improved tissue perfusion during follow-up [43]. Experimental studies demonstrated that selected, well-defined hematopoietic stem cells contribute to cardiac repair of the acutely infarcted myocardium by inducing neovascularization, inhibition of apoptosis, and cardiomyogenesis. Indeed, CD133+ cells possess high engraftment, pluripotent, and angiogenic capacity and appear to be critical for cardiac repair in experimental myocardial infarction [34–37]. The team of Menasche showed that in the setting of postinfarction scars, the transplantation of bone marrow-derived CD133+ progenitors improves cardiac function, and this benefit was similar to that afforded by myogenic cells [44].
In a phase I pilot study, Bartunek et al. tested the feasibility, safety, and functional effects of intracoronary administration of selected autologous CD133+ bone marrow progenitors in patients with recent myocardial infarction. Compared to a matched nonrandomized control group, they noted a significant increase of 7% in the LVEF of the treated group as compared to 3% in controls at 4 months of follow-up. We also showed that, in patients treated with intracoronary CD133+ cells, the improvement of the global and regional LV function was paralleled with increased myocardial perfusion and viability [38].
In our COMPARE-AMI randomized trial, phase II, double-blind, controlled study, a well-defined and translatable cell product and dose will be used in a relatively high-risk population. The safety and functional effect of the IC administration of selected CD133+ BMSCs after AMI will be addressed using time frames that are consistent with clinical applicability and emerging safety profile. To date, 14 patients were successfully randomized and treated without any serious adverse event. In addition, the LVEF of these patients showed a significant improvement higher than what we observed in the control group in Bartunek et al. trial [38] or in the placebo group of the REPAIR-AMI [27] trial suggesting a potential effect on the LV recovery of the CD133+ injected patients. We will remain blinded in this study until the last patient has be randomized and followed up for 12 months. The presented preliminary results proved mainly the safety of our ongoing protocol and may suggest a functional benefit. Additional human investigation in this and other clinical studies will provide a framework to complement ongoing basic science while further clarifying the therapeutic potential of CD133+ cell delivery for the treatment of ischemic cardiovascular disease.
References
Ertl, G., Gaudron, P., & Hu, K. (1993). Ventricular remodeling after myocardial infarction: experimental and clinical studies. Basic Research in Cardiology, 88, 125–137.
Pfeffer, M. A. & Braunwald, E. (1990). Ventricular remodeling after myocardial infarction: experimental observation and clinical implications. Circulation, 81, 1161–1172.
Towbin, J. A. & Bowles, N. E. (2002). The failing heart. Nature, 415, 227–233.
Leor, J., Patterson, M., Quinones, M. J., Kedes, L. H., Kloner, R. A., et al. (1996). Transplantation of fetal myocardial tissue into infarcted myocardium of rat: a potential method for repair of infracted myocardium? Circulation, 94(Suppl II), 332–336.
Menasche, P., Hagege, A. A., Scorsin, M., et al. (2001). Myoblast transplantation for heart failure. Lancet, 357, 279–280.
Menasche, P., Hgege, A. A., Vilquin, J.-T., Pouzet, B., Desnos, M., Duboc, D., et al. (2003). Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. Journal of the American College of Cardiology, 41, 1078–1083.
Murry, C. E., Wiseman, R. W., Schwartz, S. M., Hauschka, S. D., et al. (1996). Skeletal myoblast transplantation for repair of myocardial necrosis. Journal of Clinical Investigation, 98, 2512–2523.
Taylor, D. A., Atkins, B. Z., Hungspreugs, P., Jones, T. R., Reedy, M. C., Hutcheson, K. A., et al. (1998). Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nature Medicine, 4, 929–933.
Perin, E. C., Geng, Y. J., & Willerson, J. T. (2003). Adult stem cell therapy in perspective. Circulation, 107, 935–938.
Siminiak, T. & Kurpisz, M. (2003). Myocardial replacement therapy. Circulation, 108, 1167–1171.
Tomita, S., Li, R.-K., Weisel, R. D., Micjke, D. A. G., Kim, E. J., & Sakai, T. (1999). Autologous transplantation of bone marrow cells improve damaged heart function. Circulation, 100(Suppl 1), II 247–II 256.
Kocher, A. A., Schuster, M. D., Szabolcs, M. J., Takuma, S., Burkhoff, D., Wang, J., et al. (2001). Neovascularization of ischemic myocardium by human bone marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Medicine, 7, 430–436.
Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S. M., Li, B., et al. (2001). Bone marrow cells regenerate infarcted myocardium. Nature, 410, 701–705.
Orlic, D., Hill, J. M., & Arai, A. E. (2002). Stem cells for cardiac regeneration. Circulation Research, 91, 1092–1102.
Shintani, S., Murohara, T., Ikeda, H., Ueno, T., Honma, T., Katoh, A., et al. (2001). Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation, 103, 2776–2779.
Balsam, L. B., Wagers, A. J., Christensen, J. L., Kofidis, T., Weissman, I. L., Robbins, R. C., et al. (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischemic myocardium. Nature, 428, 668–673.
Murry, C. E., Soopa, M. H., Reinecke, H., et al. (2004). Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature, 428, 664–668.
Zhang, S., Wang, D., Estrov, Z., Raj, S., Willerson, J. T., Yeh, E. T., et al. (2004). Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation, 110, 3803–3807.
Kinnaird, T., Stabile, E., Burnett, S. E., Shou, M., Lee, C. W., Barr, S., et al. (2004). Local delivery of marrow derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation, 109, 1543–1549.
Noiseux, N., Gnecchi, M., Lopez-Ilasaca, M., Zhang, L., Solomon, S. D., Deb, A., et al. (2006). Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Molecular Therapy, 14(6), 840–850.
Assmus, B., Scharinger, V., Teupe, C., Britten, M., Lehmann, R., Dobert, N., et al. (2002). Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation, 106, 3009–3017.
Britten, M., Abolmaali, N. D., Assmus, B., Lehmann, R., Honold, J., Schmitt, J., et al. (2003). Infarct remodeling following intracoronary progenitor cells treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation, 108, 2212–2218.
Fernandez-Aviles, F., San Roman, J. A., Garcia-Frade, J., Fernandez, M. E., Penarrubia, M. J., de la Fuente, L., et al. (2004). Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circulation Research, 95, 742–748.
Lunde, K., Solheim, S., Aakhus, S., Arnesen, H., Abdelnoor, M., Egeland, T., et al. (2006). Intracoronary injection of mononuclear cells in acute myocardial infarction. New England Journal of Medicine, 355, 1199–1209.
Meyer, G. P., Wollert, K. C., Lotz, J., Pirr, J., Rager, U., Lippolt, P., Hahn, A., Fichtner, S., Schaefer, A., Arseniev, L., Ganser, A., & Drexler, H. (2009). Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. European Heart Journal, (Sep 22).
Schächinger, V., Assmuss, B., Britten, M. B., Honold, J., Lehmann, R., Teupe, C., et al. (2004). Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) 1 year follow-up. JACC, 44(8).
Schächinger, V., Erbs, S., Elsässer, A., Haberbosch, W., Hambrecht, R., Holschermann, H., et al. (2006). Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. New England Journal of Medicine, 355, 1210–1221.
Wollert, K. C., Meyer, G. P., Lotz, J., Ringes-Lichtenberg, S., Lippolt, P., Breidenbach, C., et al. (2004). Intracoronary autologous bone-marrow transfer after myocardial infarction: the BOOST randomized controlled clinical trial. Lancet, 364, 141–148.
Abdel-Latif, A., Bolli, R., Tleyjeh, I. M., Montori, V. M., Perin, E. C., Hornung, C. A., et al. (2007). Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of Internal Medicine, 167(10), 989–997.
Lipinski, M. J., Biondi-Zoccai, G. G. L., Abbate, A., Khianey, R., Sheiban, I., Bartunek, J., et al. (2007). Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction. JACC, 50, 1761–1767.
Martin-Rendon, E., Brunskill, S. J., Hyde, C. J., Stanworth, S. J., Mathur, A., Watt, S. M., et al. (2008). Autologous bone marrow stem cells to treat acute myocardial infarction: a systemic review. European Heart Journal, 29, 1807–1818.
Yousef, M., Schannwell, C. M., Köstering, M., Zeus, T., Brehm, M., Strauer, B. E., et al. (2009). The BALANCE study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. Journal of the American College of Cardiology, 53(24), 2262–2269.
Menasche, P. (2009). Cell-based therapy for heart disease: A clinically oriented perspective. Molecular Therapy, 17(5), 758–766.
Bhatia, M. (2001). AC133 expression in human stem cells. Leukemia, 15, 1685–1688.
DeWynter, E. A., Buck, D., Hart, C., Heywood, R., Coutinho, L. H., Clayton, A., et al. (1998). CD34+AC133+ cells isolated from cord blood are highly enriched in long term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells, 16, 387–396.
Kuci, S., Wessels, J. T., Buhring, H. J., Schilbach, K., Schumm, M., Seitz, G., et al. (2003). Identification of a novel class of human adherent CD34- stem cells that give rise to SCID-repopulating cells. Blood, 101, 869–876.
Quirici, N., Soligo, D., Caneva, L., Servida, F., Bossolasco, P., Deliliers, G. L., et al. (2001). Differentiation and expansion of endothelial cells from human bone marrow CD133+ cells. British Journal of Hematology, 115, 180–194.
Bartunek, J., Vanderheyden, M., Vandekerckhove, B., Mansour, S., De Bruyne, B., De Bondt, P., et al. (2005). Selected intracoronary CD 133+ bone marrow cells promote cardiac regeneration after acute myocardial infarction. Circulation, 112(Suppl I), I-178–I-183.
Dimmeler, S. & Zeiher, A. M. (2009). Cell therapy of acute myocardial infarction: open questions. Cardiology, 113, 155–160.
Traverse, J. H. & Henry, T. D. (2008). Cell therapy for acute myocardial infarction—where do we go from here? Journal of Cardiovascular Translational Research, 1, 64–70.
Bartunek, J., Sherman, W., Vanderheyden, M., Fernandez-Aviles, F., Wijns, W., & Terzic, A. (2009). Delivery of biologics in cardiovascular regenerative medicine. Clinical Pharmacology and Therapeutics, 85(5), 548–552.
Hofmann, M., Wollert, K. C., Meyer, G. P., Menke, A., Arseniev, L., Hertenstein, B., et al. (2005). Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation, 111(17), 2198–2202.
Stamm, C., Westphal, B., Kleine, H. D., Petzsch, M., Kittner, C., Klinge, H., et al. (2003). Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet, 361(9351), 45–46.
Agbulut, O., Vandervelde, S., Al Attar, N., Larghero, J., Ghostine, S., Leobon, B., et al. (2004). Comparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardium. Journal of the American College of Cardiology, 44(2), 458–463.
Acknowledgments
Dr Lemieux Bernard, Hematologist, CHUM; Dr Ouellette Caroline, Anesthesiologist, CHUM; research coordinators in cardiology Mrs Duclos Renée and Lemay Carole, technicians of the catheterization laboratory in the CHUM, technicians of the Cellular Therapy Laboratory at Maisonneuve-Rosemeont Hospital, Research Center of the CHUM (CRCHUM), Fonds de la recherche en santé du Québec, Miltenyi Biotec, and Boston Scientific in Canada.
Ethical standards
The study has been approved by Health Canada and by the IRB/Ethical committee of the research center in the CHUM.
Conflict of interest
No conflict to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mansour, S., Roy, DC., Bouchard, V. et al. COMPARE-AMI Trial: Comparison of Intracoronary Injection of CD133+ Bone Marrow Stem Cells to Placebo in Patients After Acute Myocardial Infarction and Left Ventricular Dysfunction: Study Rationale and Design. J. of Cardiovasc. Trans. Res. 3, 153–159 (2010). https://doi.org/10.1007/s12265-009-9145-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-009-9145-2