Abstract
Because of its role in maintaining bone density, vitamin D has long been recognized as critical to the health of women, a group at disproportionate risk of osteoporosis. Recent data from epidemiologic and laboratory studies suggest that vitamin D may also protect against the development of cardiovascular and other chronic diseases. Because three quarters of US women (and men) have suboptimal vitamin D status, many experts advocate increasing daily recommended intakes from 200–600 IU to at least 1,000 IU, which may indeed be a prudent strategy. However, data from large randomized clinical trials testing sufficiently high doses of this vitamin for cardiovascular disease prevention—as well as to assess the overall balance of benefits and risks of such supplementation—are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Because of its role in maintaining bone density, vitamin D has long been recognized as critical to the health of women, who are far more likely to develop osteoporosis than are men [48]. Recent data suggest that this vitamin may also help to prevent cancer [11, 19, 34, 37, 47], autoimmune disorders [3,12], cognitive [6] and physical decline [14], and—the focus here—cardiovascular disease (CVD).
A direct association between osteoporosis and vascular disease is well known [25], but reasons for the link remain unclear. Sex hormones, inflammatory factors, and other biomarkers do not completely account for the association [29]. The inverse relation between skeletal calcification and vascular calcification demonstrated in rats [56] and humans [9, 29] suggests that calcium migration from the bone to the blood vessels might be a key mechanism [25]. Because vitamin D reduces parathyroid hormone levels, thus slowing bone resorption and release of calcium into the bloodstream, this nutrient may account at least in part for the correlation between bone and heart health.
In the USA, about three quarters of both women and men have suboptimal vitamin D levels (serum 25-hydroxyvitamin D [25(OH)D] <75 nmol/L); however, a higher percentage of women (~36%) than men (~27%) have vitamin D insufficiency (25(OH)D <50 nmol/L) [39]. At particular risk are individuals with little sun exposure (conversion of 7-dehydrocholesterol in the skin by ultraviolet B [UV-B] radiation from sunlight is a major vitamin D source); blacks (primarly because darker skin synthesizes vitamin D less efficiently than lighter skin but also because of low dietary and supplemental intakes [22]); obese persons (likely because vitamin D is fat soluble and thus less bioavailable but perhaps also because of low sun exposure [23]); and those with liver or kidney disease or fat-absorption disorders such as Crohn’s or celiac disease [50]. Given rising rates of obesity and sun avoidance in the USA, low vitamin D status has become increasingly prevalent in recent years [39].
Evidence for Vasculoprotective Effect of Vitamin D
Although definitive data from randomized clinical trials of sufficiently high doses of vitamin D are lacking, recent epidemiologic investigations have found a strong inverse relation between vitamin D status and subsequent risk for cardiovascular events. In a 5-year follow-up of 1,739 women and men in the Framingham Offspring Study, those with low serum 25(OH)D (<37.5 nmol/L) were 62% more likely to develop CVD than other participants [73]. Among >18,000 US male health professionals followed for 10 years, low 25(OH)D (≤37.5 nmol/L), as compared with high 25(OH)D (≥75), predicted a doubling in coronary heart disease incidence [18]. In a German cohort of ~3,300 coronary angiography patients followed for 7.7 years, persons in the bottom two 25(OH)D quartiles had higher total mortality and cardiovascular mortality than those in the top quartile [15]. Individuals with 25(OH)D < 25 nmol/L were more than twice as likely to die from heart failure and five times as likely to experience sudden cardiac death as those with 25(OH)D ≥ 75 [52]. Among >13,000 US adults in the Third National Health and Nutrition Examination Survey, those in the bottom 25(OH)D quartile (<44.5 nmol/L) were 26% more likely to die than those in the top quartile (≥80) during a 10-year follow-up; the relation was apparent only in women but not men [45]. To date, this study is the only one to report sex-stratified results.
Ecologic studies show higher cardiovascular mortality during the winter and in regions with less exposure to UV-B radiation from sunlight [78]. Clinically, low 25(OH)D has been observed in patients with vascular calcification [74], greater carotid intima-media thickness [63], total CVD [31], myocardial infarction [59], stroke [55], heart failure [77], and peripheral arterial disease [46]. Available data also support a benefit for vitamin D on vascular risk factors, including hypertension [16, 33, 51, 72], impaired glucose tolerance or type 2 diabetes [38, 44, 53, 54], and inflammation [58, 61, 65, 68], as well as cardiovascular and total mortality in patients with kidney disease [32, 62, 64].
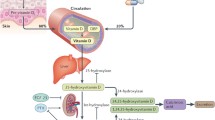
Laboratory studies also suggest that vitamin D confers vascular protection. Many cell types, including vascular smooth muscle cells, endothelial cells, cardiomyocytes, and immune-system cells, synthesize 1α-hydroxylase—which converts 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D], the natural ligand of the vitamin D receptor (VDR)—or express VDR [10, 54, 60, 76]. As shown in Fig. 1, 1,25(OH)2D appears to inhibit vascular smooth muscle cell proliferation [75] and vascular calcification [40], control volume homeostasis and blood pressure via regulation of the renin-angiotensin-aldosterone system [35, 36], and exert anti-inflammatory effects [8, 65, 69]. In animal studies, administration of 1,25(OH)2D or its analogs improves insulin sensitivity and secretion [7, 49] and prevents type 1 diabetes [20, 21, 43], and a lack of vitamin D action leads to hypertension [36] and heightened thrombogenicity [1].
Mechanisms by which vitamin D may lower CVD risk. From [41]. CRP C-reactive protein, IL-6 interleukin-6, IL-10 interleukin-10, MMP-9 matrix metalloproteinase-9, RAAS renin-angiotensin-aldosterone system, TNFα tumor necrosis factor-α
In a 2007 meta-analysis of data from 18 randomized clinical trials of vitamin D supplementation among 57,311 individuals followed for an average of nearly 6 years, the intervention lowered mortality by a statistically significant 7% [4]. However, most trials tested modest doses (mean dose, 528 IU/day) and only two had a sufficient number of cardiovascular events to examine this outcome. A trial that randomized 2,686 British adults aged 65–85 to 100,000 IU of vitamin D3 or placebo (one capsule every 4 months) for up to 5 years reported suggestive though nonsignificant reductions in CVD incidence and CVD mortality [67]. On the other hand, the Women’s Health Initiative (WHI), in which >36,000 postmenopausal women were randomized to daily calcium (1,000 mg) plus a modest dose of vitamin D3 (400 IU) or to placebo and followed for a mean of 7 years, found no reduction in coronary heart disease or stroke risk [28]. However, the intervention was estimated to raise median plasma 25(OH)D from 42 to only 54 nmol/L [17, 71]. Higher doses may be required for measurable health benefits. A recent review of 25(OH)D levels in relation to multiple health outcomes found that advantageous levels began at 75 nmol/L, and optimal levels ranged from 90 to 100 [5].
Risks of Vitamin D Supplementation
The above findings notwithstanding, there is a paucity of data on health effects of 25(OH)D levels above ~90 nmol/L, so it is unclear whether there would be additional benefit, a neutral effect, or actual harm with vitamin D supplementation to achieve higher levels. Too much vitamin D may lead to hypercalcemia, hyperphosphatemia, vascular calcification, and kidney stones. Animal studies show a biphasic dose–response relation between vitamin D and vascular calcification, with deleterious effects of very high as well as very low vitamin D intakes or levels [42, 79]. Furthermore, the WHI found a significant 17% increase in the risk for kidney stones even with a modest dose of vitamin D [71]. (Whether this resulted from concurrent administration of calcium is of limited relevance from a policy standpoint because many older women in the USA take calcium-containing supplements [57].) An additional concern—one not yet adequately addressed in the literature—is that if vitamin D is sequestered in fat tissue and thus is not bioavailable, would there be any risk of vitamin D toxicity with supplementation when weight is lost (e.g., as a consequence of disease, bariatric surgery, or other interventions)? More research on these issues is warranted.
Clinical Guidelines
Current dietary recommendations for US adults call for daily vitamin D intakes of 200 IU to age 50, 400 IU between ages 51 and 70, and 600 IU after age 70 [30]. Many experts have argued for increasing these intakes to at least 1,000 IU [70], which is the dose needed to raise 25(OH)D levels in at least 50% of adults to 75 nmol/L [5]. Sales of vitamin D supplements nearly doubled between 2006 and 2007 (C. Reider, Pharmavite LLC, personal communication). Nonetheless, as noted above, definitive data on the balance of benefits and risks of high-dose supplementation are lacking. To address this knowledge gap, large-scale randomized trials—initiated before supplement use becomes so widespread as to preclude recruitment of participants and testing of hypotheses—are needed [13]. Our research team has proposed a trial of moderate-to-high-dose vitamin D for the primary prevention of CVD, cancer, and other chronic diseases in 20,000 US adults.
The results of future trials will refine clinical guidelines. Until then, some experts recommend that clinicians consider testing for low 25(OH)D status in all patients, especially those who are older, obese, or nonwhite, or who have low UV-B exposure. Another approach is to counsel patients that sun exposure for 10 to 15 min twice per week generally provides a sufficient vitamin D dose, except in northern states during winter. For patients who prefer to obtain vitamin D through food or supplements, many experts recommend a total daily dose of 800 to 1,000 IU. Vitamin D is found in fatty fish (one serving, 250–360 IU), cod liver oil (one tablespoon, 1,360 IU), eggs (one yolk, 20 IU), fortified milk (one cup, 100 IU), and fortified cereals [50] and is also available in multivitamins, some calcium tablets and osteoporosis medications, and as an individual supplement. Most experts prefer vitamin D3 (cholecalciferol) to vitamin D2 (ergocalciferol) supplementation because of its greater efficacy at raising 25(OH)D levels, longer shelf life, and other physiologic considerations [27]. (However, in contrast to earlier trials [2, 66], a recent trial [26] found that vitamin D2 was as effective as vitamin D3 at maintaining 25(OH)D levels.) The current “safety limit” for vitamin D intake is 2,000 IU/day [30], but some experts have proposed that daily doses of up to 10,000 IU carry little toxicity risk [24]. Indeed, for patients with clear deficiency (25(OH)D < 25 nmol/L), many clinicians administer 50,000 IU of vitamin D once weekly for 8 weeks and every 2 weeks thereafter until 25(OH)D levels are no longer inadequate or recommend maintenance doses of 800–1,000 IU/day to achieve a healthful vitamin D status. Additional research is required to clarify the relative advantages and disadvantages of high-dose vitamin D supplementation.
References
Aihara, K., Azuma, H., Akaike, M., Ikeda, Y., Yamashita, M., Sudo, T., et al. (2004). Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. Journal of Biological Chemistry, 279(34), 35798–35802.
Armas, L. A., Hollis, B. W., & Heaney, R. P. (2004). Vitamin D2 is much less effective than vitamin D3 in humans. Journal of Clinical Endocrinology and Metabolism, 89(11), 5387–5391.
Ascherio, A., & Munger, K. (2008). Epidemiology of multiple sclerosis: from risk factors to prevention. Seminars in Neurology, 28(1), 17–28.
Autier, P., & Gandini, S. (2007). Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Archives of Internal Medicine, 167(16), 1730–1737.
Bischoff-Ferrari, H. A., Giovannucci, E., Willett, W. C., Dietrich, T., & Dawson-Hughes, B. (2006). Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. American Journal of Clinical Nutrition, 84(1), 18–28.
Buell, J. S., & Dawson-Hughes, B. (2008). Vitamin D and neurocognitive dysfunction: preventing "D"ecline? Molecular Aspects of Medicine, 29(6), 415–422.
Cade, C., & Norman, A. W. (1986). Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology, 119(1), 84–90.
Canning, M. O., Grotenhuis, K., de Wit, H., Ruwhof, C., & Drexhage, H. A. (2001). 1-α,25-dihydroxyvitamin D3 (1,25(OH)2D3) hampers the maturation of fully active immature dendritic cells from monocytes. European Journal of Endocrinology, 145(3), 351–357.
Choi, S. H., An, J. H., Lim, S., Koo, B. K., Park, S. E., Chang, H. J., et al. (2009). Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and post-menopausal women. Clinical Endocrinology (Oxford). doi:10.1111/j.1365-2265.2009.03535.x.
Chonchol, M., Cigolini, M., & Targher, G. (2008). Association between 25-hydroxyvitamin D deficiency and cardiovascular disease in type 2 diabetic patients with mild kidney dysfunction. Nephrology Dialysis Transplantation, 23(1), 269–274.
Cui, Y., & Rohan, T. E. (2006). Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiology, Biomarkers & Prevention, 15(8), 1427–1437.
Cutolo, M., Otsa, K., Uprus, M., Paolino, S., & Seriolo, B. (2007). Vitamin D in rheumatoid arthritis. Autoimmunity Reviews, 7(1), 59–64.
Davis, C. D., & Dwyer, J. T. (2007). The “sunshine vitamin”: benefits beyond bone? Journal of the National Cancer Institute, 99(21), 1563–1565.
Dawson-Hughes, B. (2008). Serum 25-hydroxyvitamin D and functional outcomes in the elderly. American Journal of Clinical Nutrition, 88(2), 537S–540S.
Dobnig, H., Pilz, S., Scharnagl, H., Renner, W., Seelhorst, U., Wellnitz, B., et al. (2008). Independent association of low serum 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Archives of Internal Medicine, 168(12), 1340–1349.
Forman, J. P., Giovannucci, E., Holmes, M. D., Bischoff-Ferrari, H. A., Tworoger, S. S., Willett, W. C., et al. (2007). Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension, 49(5), 1063–1069.
Giovannucci, E., Liu, Y., Rimm, E. B., Hollis, B. W., Fuchs, C. S., Stampfer, M. J., et al. (2006). Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Journal of the National Cancer Institute, 98(7), 451–459.
Giovannucci, E., Liu, Y., Hollis, B. W., & Rimm, E. B. (2008). A prospective study of 25-hydroxyvitamin D and risk of myocardial infarction in men. Archives Internal Medicine, 168(11), 1174–1180.
Gorham, E. D., Garland, C. F., Garland, F. C., Grant, W. B., Mohr, S. B., Lipkin, M., et al. (2007). Optimal vitamin D status for colorectal cancer prevention: a quantitative meta-analysis. American Journal of Preventive Medicine, 32(3), 210–216.
Gregori, S., Giarratana, N., Smiroldo, S., Uskokovic, M., & Adorini, L. (2002). A 1α, 25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes, 51(5), 1367–1374.
Gysemans, C. A., Cardozo, A. K., Callewaert, H., Giulietti, A., Hulshagen, L., Bouillon, R., et al. (2005). 1, 25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology, 146(4), 1956–1964.
Harris, S. S. (2006). Vitamin D and African Americans. Journal of Nutrition, 136(4), 1126–1129.
Harris, S. S., & Dawson-Hughes, B. (2007). Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. Journal of Clinical Endocrinology and Metabolism, 92(8), 3155–3157.
Hathcock, J. N., Shao, A., Vieth, R., & Heaney, R. (2007). Risk assessment for vitamin D. American Journal of Clinical Nutrition, 85(1), 6–18.
Hofbauer, L. C., Brueck, C. C., Shanahan, C. M., Schoppet, M., & Dobnig, H. (2007). Vascular calcification and osteoporosis–from clinical observation towards molecular understanding. Osteoporosis International, 18(3), 251–259.
Holick, M. F., Biancuzzo, R. M., Chen, T. C., Klein, E. K., Young, A., Bibuld, D., et al. (2008). Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. Journal of Clinical Endocrinology and Metabolism, 93(3), 677–681.
Houghton, L. A., & Vieth, R. (2006). The case against ergocalciferol (vitamin D2) as a vitamin supplement. American Journal of Clinical Nutrition, 84(4), 694–697.
Hsia, J., Heiss, G., Ren, H., Allison, M., Dolan, N. C., Greenland, P., et al. (2007). Calcium/vitamin D supplementation and cardiovascular events. Circulation, 115(7), 846–854.
Hyder, J. A., Allison, M. A., Wong, N., Papa, A., Lang, T. F., Sirlin, C., et al. (2009). Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. American Journal of Epidemiology, 169(2), 186–194.
Institute of Medicine Food and Nutrition Board. (1999). Dietary reference intakes: Calcium, phosphorous, magnesium, vitamin D and fluoride. Washington, D.C.: National Academies.
Kim, D. H., Sabour, S., Sagar, U. N., Adams, S., & Whellan, D. J. (2008). Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). American Journal of Cardiology, 102(11), 1540–1544.
Kovesdy, C. P., Ahmadzadeh, S., Anderson, J. E., & Kalantar-Zadeh, K. (2008). Association of activated vitamin D treatment and mortality in chronic kidney disease. Archives of Internal Medicine, 168(4), 397–403.
Krause, R., Buhring, M., Hopfenmuller, W., Holick, M. F., & Sharma, A. M. (1998). Ultraviolet B and blood pressure. Lancet, 352(9129), 709–710.
Lappe, J. M., Travers-Gustafson, D., Davies, K. M., Recker, R. R., & Heaney, R. P. (2007). Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. American Journal of Clinical Nutrition, 85(6), 1586–1591.
Li, Y. C., Kong, J., Wei, M., Chen, Z. F., Liu, S. Q., & Cao, L. P. (2002). 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. Journal of Clinical Investigation, 110(2), 229–238.
Li, Y. C., Qiao, G., Uskokovic, M., Xiang, W., Zheng, W., & Kong, J. (2004). Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. Journal of Steroid Biochemistry and Molecular Biology, 89–90(1–5), 387–392.
Li, H., Stampfer, M. J., Hollis, J. B., Mucci, L. A., Gaziano, J. M., Hunter, D., et al. (2007). A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Medicine, 4(3), e103.
Liu, S., Song, Y., Ford, E. S., Manson, J. E., Buring, J. E., & Ridker, P. M. (2005). Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care, 28(12), 2926–2932.
Looker, A. C., Pfeiffer, C. M., Lacher, D. A., Schleicher, R. L., Picciano, M. F., & Yetley, E. A. (2008). Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. American Journal of Clinical Nutrition, 88(6), 1519–1527.
Luo, G., Ducy, P., McKee, M. D., Pinero, G. J., Loyer, E., Behringer, R. R., et al. (1997). Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature, 386(6620), 78–81.
Manson, J. E., & Bassuk, S. S. (2009). Vitamin D and cardiovascular disease. Menopause Management, 18(1), 28–31.
Mathew, S., Lund, R. J., Chaudhary, L. R., Geurs, T., & Hruska, K. A. (2008). Vitamin D receptor activators can protect against vascular calcification. Journal of the American Society of Nephrology, 19(8), 1509–1519.
Mathieu, C., Waer, M., Laureys, J., Rutgeerts, O., & Bouillon, R. (1994). Prevention of autoimmune diabetes in NOD mice by 1, 25 dihydroxyvitamin D3. Diabetologia, 37(6), 552–558.
Mattila, C., Knekt, P., Mannisto, S., Rissanen, H., Laaksonen, M. A., Montonen, J., et al. (2007). Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care, 30(10), 2569–2570.
Melamed, M. L., Michos, E. D., Post, W., & Astor, B. (2008). 25-hydroxyvitamin D levels and the risk of mortality in the general population. Archives of Internal Medicine, 168(15), 1629–1637.
Melamed, M. L., Muntner, P., Michos, E. D., Uribarri, J., Weber, C., Sharma, J., et al. (2008). Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(6), 1179–1185.
Mikhak, B., Hunter, D. J., Spiegelman, D., Platz, E. A., Hollis, B. W., & Giovannucci, E. (2007). Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D, and prostate cancer risk. Prostate, 67(9), 911–923.
National Osteoporosis Foundation. (2009). http://bones.nof.org/site/PageServer?pagename=NOF_25th_Anniversary_Bone_Facts Accessed April 16, 2009.
Norman, A. W., Frankel, J. B., Heldt, A. M., & Grodsky, G. M. (1980). Vitamin D deficiency inhibits pancreatic secretion of insulin. Science, 209(4458), 823–825.
Office of Dietary Supplements. (2008). Dietary Supplement Fact Sheet: Vitamin D. Available at: http://ods.od.nih.gov/factsheets/vitamind.asp. Accessed May 12, 2009.
Pfeifer, M., Begerow, B., Minne, H. W., Nachtigall, D., & Hansen, C. (2001). Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. Journal of Clinical Endocrinology and Metabolism, 86(4), 1633–1637.
Pilz, S., Marz, W., Wellnitz, B., Seelhorst, U., Fahrleitner-Pammer, A., Dimai, H. P., et al. (2008). Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. Journal of Clinical Endocrinology and Metabolism, 93(10), 3927–3935.
Pittas, A. G., Dawson-Hughes, B., Li, T., Van Dam, R. M., Willett, W. C., Manson, J. E., et al. (2006). Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care, 29(3), 650–656.
Pittas, A. G., Lau, J., Hu, F. B., & Dawson-Hughes, B. (2007). The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism, 92(6), 2017–2029.
Poole, K. E., Loveridge, N., Barker, P. J., Halsall, D. J., Rose, C., Reeve, J., et al. (2006). Reduced vitamin D in acute stroke. Stroke, 37(1), 243–245.
Price, P. A., Faus, S. A., & Williamson, M. K. (2001). Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arteriosclerosis, Thrombosis, and Vascular Biology, 21(5), 817–824.
Radimer, K., Bindewald, B., Hughes, J., Ervin, B., Swanson, C., & Picciano, M. F. (2004). Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. American Journal of Epidemiology, 160(4), 339–349.
Schleithoff, S. S., Zittermann, A., Tenderich, G., Berthold, H. K., Stehle, P., & Koerfer, R. (2006). Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. American Journal of Clinical Nutrition, 83(4), 754–759.
Scragg, R., Jackson, R., Holdaway, I. M., Lim, T., & Beaglehole, R. (1990). Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. International Journal of Epidemiology, 19(3), 559–563.
Seibert, E., Levin, N. W., & Kuhlmann, M. K. (2005). Immunomodulating effects of vitamin D analogs in hemodialysis patients. Hemodialysis International, 9(Suppl 1), S25–29.
Shea, M. K., Booth, S. L., Massaro, J. M., Jacques, P. F., D’Agostino, R. B., Sr., Dawson-Hughes, B., et al. (2008). Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. International Journal of Epidemiology, 167(3), 313–320.
Shoji, T., Shinohara, K., Kimoto, E., Emoto, M., Tahara, H., Koyama, H., et al. (2004). Lower risk for cardiovascular mortality in oral 1α-hydroxy vitamin D3 users in a haemodialysis population. Nephrology Dialysis Transplantation, 19(1), 179–184.
Targher, G., Bertolini, L., Padovani, R., Zenari, L., Scala, L., Cigolini, M., et al. (2006). Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clinical Endocrinology (Oxford), 65(5), 593–597.
Teng, M., Wolf, M., Ofsthun, M. N., Lazarus, J. M., Hernan, M. A., Camargo, C. A., Jr., et al. (2005). Activated injectable vitamin D and hemodialysis survival: a historical cohort study. Journal of the American Society of Nephrology, 16(4), 1115–1125.
Timms, P. M., Mannan, N., Hitman, G. A., Noonan, K., Mills, P. G., Syndercombe-Court, D., et al. (2002). Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM, 95(12), 787–796.
Trang, H. M., Cole, D. E., Rubin, L. A., Pierratos, A., Siu, S., & Vieth, R. (1998). Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. American Journal of Clinical Nutrition, 68(4), 854–858.
Trivedi, D. P., Doll, R., & Khaw, K. T. (2003). Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ, 326(7387), 469.
Van den Berghe, G., Van Roosbroeck, D., Vanhove, P., Wouters, P. J., De Pourcq, L., & Bouillon, R. (2003). Bone turnover in prolonged critical illness: effect of vitamin D. Journal of Clinical Endocrinology and Metabolism, 88(10), 4623–4632.
van Etten, E., & Mathieu, C. (2005). Immunoregulation by 1, 25-dihydroxyvitamin D3: basic concepts. Journal of Steroid Biochemistry and Molecular Biology, 97(1–2), 93–101.
Vieth, R., Bischoff-Ferrari, H., Boucher, B. J., Dawson-Hughes, B., Garland, C. F., Heaney, R. P., et al. (2007). The urgent need to recommend an intake of vitamin D that is effective. American Journal of Clinical Nutrition, 85(3), 649–650.
Wactawski-Wende, J., Kotchen, J. M., Anderson, G. L., Assaf, A. R., Brunner, R. L., O’Sullivan, M. J., et al. (2006). Calcium plus vitamin D supplementation and the risk of colorectal cancer. New England Journal of Medicine, 354(7), 684–696.
Wang, L., Manson, J. E., Buring, J. E., Lee, I. M., & Sesso, H. D. (2008). Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension, 51(4), 1073–1079.
Wang, T. J., Pencina, M. J., Booth, S. L., Jacques, P. F., Ingelsson, E., Lanier, K., et al. (2008). Vitamin D deficiency and risk of cardiovascular disease. Circulation, 117(4), 503–511.
Watson, K. E., Abrolat, M. L., Malone, L. L., Hoeg, J. M., Doherty, T., Detrano, R., et al. (1997). Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation, 96(6), 1755–1760.
Wong, M. S., Delansorne, R., Man, R. Y., & Vanhoutte, P. M. (2008). Vitamin D derivatives acutely reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. American Journal of Physiology. Heart and Circulatory Physiology, 295(1), H289–296.
Zittermann, A. (2006). Vitamin D and disease prevention with special reference to cardiovascular disease. Progress in Biophysics and Molecular Biology, 92(1), 39–48.
Zittermann, A., Schleithoff, S. S., Tenderich, G., Berthold, H. K., Korfer, R., & Stehle, P. (2003). Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? Journal of the American College of Cardiology, 41(1), 105–112.
Zittermann, A., Schleithoff, S. S., & Koerfer, R. (2005). Putting cardiovascular disease and vitamin D insufficiency into perspective. British Journal of Nutrition, 94(4), 483–492.
Zittermann, A., Schleithoff, S. S., & Koerfer, R. (2007). Vitamin D and vascular calcification. Current Opinion in Lipidology, 18(1), 41–46.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bassuk, S.S., Manson, J.E. Does Vitamin D Protect Against Cardiovascular Disease?. J. of Cardiovasc. Trans. Res. 2, 245–250 (2009). https://doi.org/10.1007/s12265-009-9111-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-009-9111-z