Abstract
The olfactory bulb (OB) is the first relay station in the olfactory system. In the OB, mitral/tufted cells (M/Ts), which are the main output neurons, play important roles in the processing and representation of odor information. Recent studies focusing on the function of M/Ts at the single-cell level in awake behaving mice have demonstrated that odor-evoked firing of single M/Ts displays transient/long-term plasticity during learning. Here, we tested whether the neural activity of M/Ts and sniffing patterns are dependent on anticipation and reward in awake behaving mice. We used an odor discrimination task combined with in vivo electrophysiological recordings in awake, head-fixed mice, and found that, while learning induced plasticity of spikes and beta oscillations during odor sampling, we also found plasticity of spikes, beta oscillation, sniffing pattern, and coherence between sniffing and theta oscillations during the periods of anticipation and/or reward. These results indicate that the activity of M/Ts plays important roles not only in odor representation but also in salience-related events such as anticipation and reward.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
As the sensory system that detects chemicals in the environment, olfaction is crucial for survival. Importantly, olfactory dysfunction is common in many neurodegenerative diseases including Parkinson’s disease [1,2,3]. The olfactory bulb (OB) is one of the most important odor information-processing centers in the olfactory system. It receives direct axonal input from the olfactory sensory neurons that express olfactory receptors and interact directly with odorants [4]. The OB is critically involved in the representation of many aspects of an odor, including its identity, intensity, duration, and other timing information [4,5,6,7]. While the OB sends this information to higher centers such as the anterior olfactory nucleus and piriform cortex via mitral/tufted cells (M/Ts), which are the main output neurons of the OB, it also receives extensive feedback and centrifugal inputs [4, 8]. For example, cholinergic, serotonergic, and noradrenergic innervation are extensive in the OB [9,10,11]. Since these modulatory systems play important roles in learning and memory and the plasticity of neurons in many brain areas, the odor representation by the OB is likely to be dramatically plastic and dependent on learning and task demands [12,13,14,15,16,17].
As an important olfactory center, the OB contains neurons that respond to odors by increasing or decreasing their firing rate in both anesthetized and awake states [18,19,20,21]. Interestingly, in awake behaving rodents, some neurons in the OB change their firing dramatically in response to other odor-related events such as light-on and a water reward but not the odor stimulus [22]. These findings indicate that the neural activity of OB neurons is rather complex, since it might carry key information not only on odor but also about internal brain states such as anticipation and reward. Unfortunately, the excellent pioneering work did not explore this important issue intensively and only very few responsive units were recorded [22]. Importantly, other than single units, local field potentials (LFPs), and sniffing signals that play key roles in olfactory perception [13, 23, 24], might also be plastic and dependent on behavioral context during an odor discrimination task within and/or outside the odor-sampling period. However, direct evidence is still lacking.
Here, we tested whether the sniffing pattern and neural activity in the OB show learning-dependent plasticity during odor sampling, anticipation, and water reward when the mice perform a go/no-go odor discrimination task in a head-fixed state. We found that while some of these critical signals showed learning-dependent plasticity during odor sampling, other signals showed such plasticity during anticipation and/or reward.
Materials and Methods
Animals
Eleven male C57BL/6J mice (8–16 weeks old) were housed in a vivarium under a 12/12 light/dark cycle with lights on at 08:00. Experiments were performed during the light cycle. Food and water were available ad libitum except during the behavioral procedure when water could be received in the experimental chamber. All experimental procedures were carried out in accordance with protocols approved by the Xuzhou Medical University Institutional Animal Care and Use Committee.
Implantation of Tetrodes
As in previous studies [25], mice were briefly anesthetized with pentobarbital (0.09 mg/g body weight, i.p.). Then, after the mouse was mounted in a stereotaxic frame, the fur on the scalp from the midline of the orbits to the midpoint between the ears was removed. A hole was drilled above the right OB (AP, 4.0 mm; ML, 1.0 mm) for the implantation of tetrodes.
For single-cell spiking and LFP recordings, tetrodes were implanted into the OB. Each tetrode consisted of four polyimide-coated nichrome wires (RO-800, Sandvik AB, Sandviken, Sweden), which were connected to a 16-channel electrode interface board (EIB-16, Neuralynx Inc.). The tetrodes were lowered to the mitral cell layer at an average depth between 1.8 mm and 2.0 mm [13, 20]. One stainless-steel screw, which was connected to ground as the reference electrode, was inserted into the bone (1 mm posterior to bregma and 1 mm from the midline). To ensure optimal placement of the tetrodes, spiking recordings were made during implantation. The signals recorded from the tetrodes were amplified (Plexon DigiAmp; bandpass, 1 Hz–5000 Hz, 2000 × gain), and sampled at 40 kHz by a Plexon Omniplex recording system. The tetrodes were finally sealed to the bone by dental acrylic. To fit the mice for the head-fixed recording system, an aluminum head plate was attached to the skull with two screws and dental cement.
Spike and LFP Recordings
After at least 7 days of recovery from the surgery, recordings were performed in the awake, head-fixed mice. The mice were head-fixed with two horizontal bars and were able to maneuver on a head-fixed go/no-go system (Thinkerbiotech, Nanjing, China). The procedure for spike recordings was similar to that for recordings made during the tetrode implantation described above. For LFP recordings, the signals were amplified (2000 × gain; Plexon DigiAmp), filtered at 0.1 Hz–300 Hz, and sampled at 1 kHz. Spikes or LFP signals along with other event markers, including odor stimulation and licking, were recorded via the same Plexon Omniplex recording system.
Sniffing Measurement
We implanted a cannula into one nasal cavity and connected it to a pressure sensor [Model No. 24PCEFA6G(EA), 0 psi–0.5 psi; Honeywell] to measure sniffing. Implantation of the cannula was performed as in previous studies [5, 13, 20]. The pressure transients were amplified (100 × gain, Plexon DigiAmp) and sampled at 1 kHz by the Plexon Omniplex recording system. Each sniff was detected at the point of transition from exhalation to inhalation.
Odor Presentation
The odors were presented by an odor delivery system (Thinkerbiotech). Four odor pairs, isoamyl acetate vs 2-heptanone, phenyl acetate vs benzaldehyde, acetophenone vs octyl aldehyde, and 1-nonanol vs 2-undecanone (Sinopharm Chemical Reagent Co., Shanghai, China) were used. All odorants were dissolved in mineral oil at 1% v/v dilution. During the odor delivery period, a stream of nitrogen at 100 mL/min flowed over the odor oil, and then was diluted to 1/20 by an olfactometer. Charcoal-filtered air at a constant rate of 1 L/min was delivered to the nose of the mouse to eliminate the effect of airflow.
The Go/No-go Behavioral Tasks
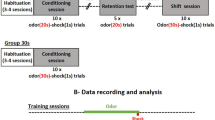
After the mice recovered from surgery for at least 1 week, they underwent a series of behavioral tasks. The water supply was removed from the mouse’s cage and the behavioral training began two days later if the weight of the mouse was 80%–85% of that before deprivation. Mice were trained to perform the go/go task before the go/no-go task. During the go/go task, mice learned to lick a tube to get a drop of water (20 μL) when either of the odor pair was present. Then the mice began to perform the go/no-go task in which they had to discriminate the two odors correctly to receive a water reward [13, 20]. The mice had to lick the tube when a rewarded odor (S+) was presented and not to lick when the unrewarded odor (S−) was presented. According to the responses of the mice to the odor presentation, if an S+ was presented and the mouse responded with licking (Hit), the water reward was delivered through the lickport; if they did not lick with the S+ (Miss), or the S− was delivered, no water reward was given regardless of the mouse’s actions (false alarm [FA] or correct rejection [CR], see Fig. 1C). Thus, Hit and CR were correct trials, and Miss and FA were incorrect trials. The electrophysiological signals and sniffing signals were simultaneously recorded during all tasks.
Paradigm for data recording and behavioral task. A Diagram of the recording setup for spikes, LFPs, and sniffs (OB, olfactory bulb; OE, olfactory epithelium; LFP, local field potential). B Schematic of the go/no-go task sequence. An odor is applied for 2 s followed by 2 s waiting time and then the water reward is given. C Trial structure of the go/no-go task (FA, false alarm; CR, correct rejection). D The odor discrimination performance during the go/no-go task across all sessions (n = 13 animal-odor pairs from 6 mice). The mean correct response across sessions is plotted for each block (20 trials). The chance level and learning threshold are indicated by dashed lines. Data are presented as the mean ± SEM. E Diagrams showing extraction of sniffing frequency, mean inhalation duration (MID), and inhalation (Inh.) volume from example nasal flow trace.
Data Analysis
For the go/no-go task, the performance of mice was calculated on each training day (one session). Further analysis was evaluated in blocks of 20 trials and the behavioral performance of each block was calculated separately. Usually mice performed 6–10 blocks in each session (Fig. 1D).
For offline spike sorting and statistics of single-cell spiking data, similar to previous studies, single units were sorted and identified by principal component analysis with Offline Sorter V4 software (Plexon) [20, 25, 26]. To generate peristimulus time histograms, the spikes 3 s before and 7 s after the odor stimulation in each trial were extracted and the spike firing rate was averaged with a bin-width of 100 ms. The spontaneous firing rate (2 s before odor stimulation) and the odor-evoked firing rate (during the 2 s odor stimulation) were calculated by averaging the spikes during the 2 s periods. We used multivariate permutation tests to determine whether an odor evoked a significant response. Excitatory, inhibitory, and non-responses were classified based on the P values and comparison of the firing rate between baseline and the odor-evoked response.
For analysis of LFP signals, a program written in MatLab (The MathWorks) was used. Similar to previous studies [1, 13, 25, 27], we divided LFP signals into 4 frequency bands: theta (2 Hz–12 Hz), beta (15 Hz–35 Hz), low gamma (36 Hz–65 Hz), and high gamma (66 Hz–95 Hz). However, we only focused on the beta and high gamma bands for further analysis since odors usually evoke strong and reliable responses within these two frequency bands [1, 25]. For odor-evoked responses, the data 4 s prior to and 8 s after the onset of odor stimulation were selected for further analysis. Spectral power was computed using the MatLab wavelet method, cwt, with Morlet wavelets. For each trial, the baseline was normalized to 1, and all the trials for each odor were averaged for further analysis.
To test the significance of differences between learning and learned (proficient) states, the Shapiro–Wilk test was first used to determine whether samples had a normal distribution. In the case of a normal distribution, the paired t-test was applied; in the case of a non-normal distribution, the Wilcoxon signed ranks test was used.
Results
Sniffing Pattern Shows Plasticity in the Water Reward Period During the Learning Process of the Go/No-go Task
The go/no-go task is extensively used as an olfactory discrimination model in rodents [13, 20, 28, 29]. During this task, animals usually sample the odors for 2 s and a water reward is obtained at the end of the odor delivery if the mice lick for the rewarded odor. This experimental design is sufficient for studying odor representation and reward; however, there is no clear time window in which to study anticipation. Thus, we inserted a 2 s waiting time between odor stimulation and water reward (Fig. 1A–C) and this waiting time represented anticipation by the mice if they expected a reward during this trial. During the task, the sniffing and neural activity from the OB, including spikes from single units and LFPs were recorded (Fig. 1A). In general, mice took ~ 5 blocks (100 trials) to learn to discriminate the odors (> 80% correct, Fig. 1D). To investigate how sniffing signals changed during the go/no-go task, we analyzed different aspects of the signals: sniffing frequency, mean inhalation duration (MID), and volume (Fig. 1E).
Representative sniffing signals during the go/no-go task are shown in Fig. 2A. For both learning (< 80% correct, left) and proficient (> 80% correct, right) states, the sniffing frequency began to increase at the late stage of odor stimulation, peaked during the waiting period, and then began to recover (Fig. 2B). Interestingly, in the learning state, S+ and S− trials showed similar sniffing frequencies during the odor stimulation and water reward periods but showed significantly different sniffing frequencies during the waiting period (Fig. 2B, left and Table S1). However, in the proficient state, S+ and S− trials only showed similar sniffing frequencies during the odor stimulation period and showed significantly different sniffing frequencies during both the waiting and reward periods (Fig. 2B, right and Table S1).
Sniffing pattern changes emerge across task learning. A, E Raw sniffing traces of responding to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during the go/no-go task (A) and the go/go task conditions (E) (S+ and Odor1, phenyl acetate; S− and odor2, 2-butanone). B, F Trial-averaged sniffing frequency to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during the go/no-go task (B) and the go/go task conditions (F). C, G As for B and F but for mean inhalation duration (MID) of sniffing. D, H As for B and F but for sniffing volume. Data are presented as the mean ± SEM.
Besides sniffing frequency, we also analyzed sniffing MID and volume, which are critical parameters of the pattern. Corresponding to sniffing frequency, the MID and volume began to decrease in the late stage of odor stimulation, descended to a trough during the waiting period, and then began to recover (Fig. 2C, D) in both the learning and proficient states. Similar to the results for sniffing frequency, S+ and S− trials showed significantly different MIDs during the waiting period (Fig. 2C, left, Table S2) in the learning stage and during the waiting and reward periods in the proficient stage (Fig. 2C, right, Table S2). However, for sniffing volume, S+ and S− trials only showed a significant difference during the reward period in the proficient state and not at any period in the learning state (Fig. 2D, Table S3). All together, these results indicate that the patterns of the sniffing signals are modulated in the reward period depending on the learning process of the go/no-go task.
During the go/no-go task, the learning state occurred in early trials while the proficient state occurred late in the session (Fig. 1D). This raises the question of whether the difference in the reward period between these behavioral states is due to general behavioral state differences such as thirst. To address this question, we analyzed the data focusing on S+ and S− during the early trials (first 30 trials) and the late trials (last 30 trials) of the go/go task where animals also received water and become satiated (see Materials and Methods). The sniffing signals in all the periods were similar for the odor 1 and odor 2 trials during both the early and late trials (Fig. 2E). Further analysis indicated that although the sniffing signals changed during different periods and these also occurred in the go/no-go task, no significant difference was found between odors 1 and 2 during any period, or in either early or late trials (Fig. 2F–H). Therefore, the change in sniffing pattern during the go/no-go task is established by learning-related plasticity as opposed to behavioral states such as thirst that differ between the beginning and end of the session.
Coherence Between Theta Oscillation and Sniffing in the Odor Sampling Period is Plastic During the Learning Process of the Go/No-go Task
The theta oscillation of the LFP recorded in the OB is strongly correlated with sniffing, and the correlation varies during different olfactory-related behaviors [13, 30, 31]. To test whether this correlation changed during the learning process of the go/no-go task, we recorded the sniffing signals and LFP signals simultaneously (Figs. 1A and 3A). In the learning state, the coherence between sniffing and theta oscillation showed no significant change during the odor stimulation, waiting, or reward period compared with baseline (− 4 s to 0 s, Fig. 3B, C, left; paired t-test, t = 0.77, 0.40, and − 0.16; P = 0.47, 0.70, and 0.87 for odor, waiting, and reward periods, respectively). However, in the proficient state, the coherence increased at the beginning of odor stimulation and then began to recover (Fig. 3B, C, right). Further comparison between S+ and S− trials showed no significant difference during any period in the learning stage (Fig. 3C, left, Table S4), but a significant difference was found during the odor period in the proficient state (Fig. 3C, right, Table S4). Moreover, data from the go/go experiment showed that the coherence was neither significant between baseline and any event period nor between S+ and S− trials at an early or late state (Fig. 3D, E, Table S4). Thus, these results indicate that the coherence between theta oscillation and sniffing in the odor sampling period is plastic during the learning process of the go/no-go task.
Coherence between sniffing and theta oscillations under go/no-go and go/go task conditions. A Raw traces (upper) and filtered theta (middle) oscillations of the LFP signals and sniff (lower) responses to one odor (phenyl acetate) from a representative mouse during the go/no-go task. B, D Coherence spectra between sniffing and theta oscillations responding to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during go/no-go (B) and go/go (D) task conditions (S+ and odor 1, phenyl acetate; S− and odor 2, 2-butanone). C, E Trial-averaged coherence between sniff and theta oscillations to one odor pair for example in B and D. Data are presented as the mean ± SEM.
Power of LFP Beta Oscillation Shows Plasticity for Anticipation and Reward During the Learning Process of the Go/No-go Task
In both anesthetized and awake rodents, odor evokes strong increases in beta oscillations in the OB [19, 25, 32, 33], and these oscillations are critically involved in olfactory learning and perception [34, 35]. Next, we explored whether the beta oscillations changed during learning of the go/no-go task. Similar to previous studies, we found that odor evoked increased responses during the odor stimulation (Fig. 4A, B). Generally, there was no significant difference between S+ and S− trials during any event period in the learning state, but significant differences were found during the waiting and reward periods in the proficient state (Fig. 4C, Table S5). Data from the go/go experiment showed that the power of beta oscillations did not significantly differ between S+ and S− trials during any event period, either early or late (Fig. 4D–F, Table S5). Thus, these results indicate that the beta oscillation is plastic for the anticipation and reward during the learning process of the go/no-go task.
Odor-evoked beta responses under go/no-go and go/go task conditions. A, D Raw traces (upper) and filtered beta (lower) oscillations of LFP signals responding to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during the go/no-go (A) and go/go (D) task conditions (S+ and odor 1, phenyl acetate; S− and odor 2, 2-butanone). B, E Power spectra of beta responding to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during the go/no-go (B) and go/go (E) task conditions (S+ and odor 1, phenyl acetate; S− and odor 2, 2-butanone). C, F Trial-averaged normalized power of beta responses to the odor pair in B and E. Data are presented as the mean ± SEM.
During the go/no-go task, for S+, the behavioral outputs were mainly Hits and there were few Miss trials (73/617, 11.8%), since we trained the mice to learn the go/go task before the go/no-go task. For S−, the behavioral outputs were FA and CR. However, FA and CR reflect anticipation differently, and the difference would be evident from the power of the LFP beta oscillation. Thus, we performed further analysis to compare the beta power for Hit, CR, and FA. We found that the beta power during the waiting period for Hit and FA trials, which represent anticipation, were similar, and were significantly stronger than CR trials, which represent no anticipation (Fig. S1). This result provides further evidence that the power of beta oscillation reflects anticipation.
The Power of LFP Gamma Oscillation is Stable During the Learning Process of the Go/No-go Task
Besides beta oscillations, gamma oscillations also play important roles in olfactory learning [34, 35]. We then investigated whether the gamma oscillations changed during learning of the go/no-go task. Similar to previous studies in awake animals [32], we found that odor evoked dramatically decreased responses during odor stimulation (Fig. 5A, B). However, no significant difference between S+ and S− trials was found during any event period in either the learning or the proficient stage (Fig. 5B, C, Table S6). Data from the go/go experiment showed similar results (Fig. 5D–F, Table S6). Therefore, these data indicate that the gamma oscillation is stable and shows no plasticity for odor stimulation, anticipation, or reward during the learning process of the go/no-go task.
Odor-evoked high gamma responses under go/no-go and go/go task conditions. A, D Raw traces (upper) and filtered high gamma (lower) oscillations of the LFP signals responding to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during go/no-go (A) and go/go (D) task conditions (S+ and odor 1, phenyl acetate; S− and odor 2, 2-butanone). B, E Power spectra of high gamma responding to one odor pair (phenyl acetate vs 2-butanone) from a representative mouse during go/no-go (B) and go/go (E) task conditions (S+ and odor 1, phenyl acetate; S− and odor 2, 2-butanone). C, F Trial-averaged normalized power of high gamma responses to the odor pair in B and F. Data are presented as the mean ± SEM.
The Firing of Single M/Ts Shows Plasticity in the Odor Sampling and Water Reward Periods During Learning of the Go/No-go Task
Finally, we recorded spikes from M/Ts, which are the major output neurons of the OB and play important roles in odor representation. Single M/T units were identified by tetrode recording (Fig. 6A). As in previous studies [13, 20], we recorded strong spontaneous firing of M/Ts, and odors evoked both excitatory and inhibitory responses (Fig. 6B). The proportions of each type of response during the learning and proficient states are shown in Fig. 6C. We then investigated whether the single cell firing changed during learning of the go/no-go task (Fig. 7A, B). Both S+ and S− evoked strong inhibitory responses during odor stimulation and the firing peaked in the waiting period in both the learning and proficient states (Fig. 7C, D). Interestingly, in the proficient state, there was a significant difference between S+ and S− during the odor sampling period across all the cell-odor pairs (Fig. 7E). No significant difference was found between S+ and S− during the waiting period in either the learning or proficient state (Fig. 7F). Similar to the odor sampling period, during the reward period there was a significant difference between S+ and S− across all the cell-odor pairs in the proficient state (Fig. 7G). These results indicate that the firing of single M/Ts is plastic for the odor response and the reward during the learning process of the go/no-go task. Data from the go/go experiment showed that the firing of single M/Ts did not significantly differ between S+ and S− trials during any event period either early or late (Fig. 7H–J).
M/T spikes recorded from the OB of awake mice. A Example of spike sorting using principal component analysis scan clustering of extracellular voltage recordings by tetrodes, resulting in the separation of three units (a, b, and c). B Three examples of firing induced by one odor (phenyl acetate) during the go/go task. From left to right: excitatory response, inhibitory response, and no response. Upper, raster plots; lower, peristimulus time histograms of the firing rate; green lines, odor stimulation (2 s). C Pie charts showing the percentages of excitatory (red), inhibitory (blue), or no response (gray) under the go/no-go task conditions.
Learning-induced plasticity of spikes during the odor sampling and reward periods. A, B Raster plots of firing induced by S+ and S− in the learning state (A) and the proficient state (B) during the go/no-go task from a representative unit (S+ , phenyl acetate; S−, 2-butanone). C, D Trial-averaged peristimulus time histograms for the example in A and B, smoothed by a Gaussian filter with a standard deviation of 1500 ms. E Comparison of the changes in mean firing rate (ΔMFR) between S+ and S− in the learning state [left: paired t-test, P = 0.073 (df = 118, t = 1.810)] and the proficient state [right: paired t-test, ***P < 0.001 (df = 118, t = 3.454)] during the odor sampling period of the go/no-go task for all units and odors (n = 119, 9 mice and 8 odors). The dashed line shows the diagonal, where the ΔMFR in S+ equals that in S−. F As for E but for the waiting period [left: paired t-test, P = 0.913 (df = 118, t = 0.109); right: paired t-test, P = 0.826 (df = 118, t = − 0.220)]. G As for E but for the reward period [left: paired t-test, P = 0.284 (df = 118, t = 1.077); right: paired t-test, **P = 0.004 (df = 118, t = 2.977)]. H Comparison of the ΔMFR between odor 1 and odor 2 early [left: paired t-test, P = 0.241 (df = 133, t = 1.178)] and late [right: paired t-test, P = 0.189 (df = 133, t = − 1.321)] in the odor sampling period of the go/go task for all units and odors (n = 134, 10 mice with 8 odors). The dashed line shows the diagonal, where ΔMFR for odor 1 equals that for odor 2. I As for H but for the waiting period [left: paired t-test, P = 0.462 (df = 133, t = 0.738); right: paired t-test, P = 0.094 (df = 133, t = − 1.686)]. J As for H but for the reward period [left: paired t-test, P = 0.113 (df = 133, t = 1.595); right: paired t-test, P = 0.810 (df = 133, t = 0.241)].
Discussion
The OB is the first relay station in the olfactory system; it receives direct input from the olfactory sensory neurons in which the olfactory receptors are located. However, recent studies in awake behaving rodents have demonstrated that the odor responses of the neurons in the OB are largely dependent on behavioral and brain states [4, 17, 36,37,38,39]. In the present study, we found that both the sniffing pattern and neural activity in the OB were plastic during the learning process. The plasticity not only occurred during odor sampling, which is correlated with olfactory information, but also occurred during the anticipation and reward periods. Specifically, learning-induced plasticity of single unit spikes and sniffing/theta oscillation coherence occurred during odor sampling; plasticity of beta oscillation, sniffing frequency, and MID occurred during anticipation of the reward; and plasticity of single unit spikes and sniffing frequency, MID, and volume occurred during the water reward (Fig. 8). These findings provide direct evidence that signals such as single units, LFPs, and sniffing, which play key roles in olfactory perception, show plasticity and are behavioral context-dependent during an odor discrimination task within and/or outside the odor sampling period.
Diagram showing the results indicating that learning-induced plasticity of spikes and beta oscillations occurred during odor sampling, and plasticity of spikes, beta oscillations, sniffing patterns, and coherence between sniffing and theta oscillations occurred during the period of anticipation and/or reward (OB, olfactory bulb; OE, olfactory epithelium).
The go/no-go task was initially designed for free-moving rodents [28], and recently it has been extensively used in head-fixed mice [14, 17, 23, 40]. Odor-evoked LFP responses during the learning process of the go/no-go task have been investigated in many studies of free-moving but not head-fixed rodents [13, 32, 33, 41, 42]. Behavioral studies have reported similar odor-discrimination performance under these two conditions [43], indicating that the odor-processing strategy is similar in the two conditions. However, the neural activity may differ because of differences in behavioral status and the level of stress. Moreover, odor sampling is vastly different under the two conditions since the sampling in the head-fixed state is relatively stable while in the free-moving state it is variable due to the movements of the mice [4]. Thus, it is not unexpected that the results from our study in the head-fixed state differ somewhat from previous studies in the free-moving state.
As regards the LFP, odor-evoked increases in beta response and decreases in gamma response were recorded both here and in previous studies [32, 34]. However, while the odor-evoked plasticity of both beta and gamma oscillations during the learning process has been found in previous studies [32], we only found that the beta but not the gamma oscillations were plastic during learning. This difference is likely due to the different behavioral conditions. Future studies are needed to test this by recording LFPs in mice that perform the go/no-go task in both free-moving and head-fixed states. Similar studies have compared the odor-evoked responses in different olfactory tasks (go/no-go vs 2-alternative choice) [42].
Interestingly, consistent with previous studies that have revealed that the spikes of single M/Ts show plasticity to the odor during learning, and the odor-evoked spikes are more divergent after the mice learn to discriminate the odor pair in the free-moving go/no-go task [13, 21, 29], our study demonstrated that learning-induced plasticity of single unit spikes occurred during odor sampling. These results indicate that the plasticity of M/T spiking during the learning process is reliable and independent of behavioral conditions such as free-moving or head-fixed. In addition, besides spikes, the coherence between sniffing and LFP theta oscillation also showed plasticity during odor sampling in the learning process, but this has not been reported in previous studies. The sniffing pattern and LFP theta oscillation are strongly but not completely correlated in awake rodents [13, 31, 44], and their coherence varies from moment to moment [30, 31]. However, the functional significance of the coherence remains elusive. Our data suggest that this coherence at least plays important roles in the olfactory learning process during the go/no-go task in head-fixed mice.
Besides odor sampling, a previous study has reported that other odor-related events, such as light signals and door openings during an olfactory discrimination task, also evoke significant changes of M/T firing [22]. We further confirmed and extended this finding, showing that both the sniffing pattern and neural activity in the OB responded to anticipation and/or reward that were odor-related events in the go/no-go task. Although the neural plasticity of M/Ts responding to odors during the learning process has been extensively investigated by both electrophysiological recording in free-moving rodents and single-cell imaging in head-fixed mice [13, 14, 21, 29, 36, 40], no study has tested whether the neural response to odor-related events shows similar plasticity during learning. Thus, our study provides the first evidence that, while odor-related events evoke neural responses in the OB and sniffing pattern changes, these signals also show plasticity during the learning process, at least in the head-fixed go/no-go paradigm.
The response and plasticity of neurons to odor-related events are linked to higher cognitive functions since they are associated with leaning and reward [45, 46]. It is surprising that neural activity in the OB shows this property since the OB is the first relay station in the olfactory system that receives direct input from the olfactory periphery [4, 47]. Therefore, higher brain centers must be involved in the mechanisms by which neural activity in the OB and the sniffing pattern show plasticity during the learning process. While the OB receives direct input from olfactory sensory neurons, it also receives intense cortical feedback and modulatory innervation from a wide range of higher brain centers [9,10,11]. Some of these centers are critically involved in learning, motivation, anticipation, and reward. For example, cholinergic and noradrenergic inputs to the OB are important for learning [29, 48, 49], and serotonergic inputs to the OB likely play crucial roles in reward and anticipation [50, 51]. In addition, the sniffing pattern usually changes during exploratory behavior and other non-olfactory events [24]. A recent study has reported that the prefrontal cortex and sniffing pattern are strongly correlated during freezing behavior [52], suggesting that the sniffing pattern is modulated by higher brain centers that play important roles in learning, motivation, and reward. Therefore, the learning-induced plasticity of the sniffing pattern and neural activity in the OB responding to odor stimulation and odor-related events is the result of complex interactions between the olfactory system and other centers involved in higher brain functions.
In addition, although both the sniffing pattern and neural activity in the OB showed plasticity during the learning process, it is likely that the change of sniffing signals makes a major contribution since the neural activity in the OB is largely modulated by sniffing patterns in both anesthetized and awake rodents [24]. For example, theta and gamma oscillations in the OB LFP are tightly coupled with the sniffing pattern [35], and the firing of single units recorded from M/T cells is precisely locked to a specific phase of the sniffing cycle [53]. Importantly, a recent study using patch clamping in awake behaving mice has revealed that the activity of tufted cells is modulated by active sniffing [23]. Therefore, the changes of neural activity in the OB during the learning process might also be due to the different sniffing strategies adopted during the different learning states.
In summary, we investigated the sniffing pattern and neural activity in the OB responding to odor and odor-related events in the learning process of the go/no-go task in head-fixed mice. Our results revealed the occurrence of plasticity of the sniffing pattern and neural activity in the OB of behaving mice during odor sampling, anticipation, and reward. These findings are important for understanding the function of the OB beyond representing olfactory information in awake behaving rodents.
References
Zhang W, Sun C, Shao Y, Zhou Z, Hou Y, Li A. Partial depletion of dopaminergic neurons in the substantia nigra impairs olfaction and alters neural activity in the olfactory bulb. Sci Rep 2019, 9: 254.
Jin H, Zhang JR, Shen Y, Liu CF. Clinical significance of REM sleep behavior disorders and other non-motor symptoms of Parkinsonism. Neurosci Bull 2017, 33: 576–584.
Fullard ME, Morley JF, Duda JE. Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull 2017, 33: 515–525.
Li A, Rao X, Zhou Y, Restrepo D. Complex neural representation of odour information in the olfactory bulb. Acta Physiol (Oxf) 2020, 228: e13333.
Li A, Gire DH, Bozza T, Restrepo D. Precise detection of direct glomerular input duration by the olfactory bulb. J Neurosci 2014, 34: 16058–16064.
Roland B, Deneux T, Franks KM, Bathellier B, Fleischmann A. Odor identity coding by distributed ensembles of neurons in the mouse olfactory cortex. Elife 2017, 6.
Chong E, Rinberg D. Behavioral readout of spatio-temporal codes in olfaction. Curr Opin Neurobiol 2018, 52: 18–24.
Wen P, Rao X, Xu L, Zhang Z, Jia F, He X, et al. Cortical organization of centrifugal afferents to the olfactory bulb: mono- and trans-synaptic tracing with recombinant neurotropic viral tracers. Neurosci Bull 2019, 35: 709–723.
Fletcher ML, Chen WR. Neural correlates of olfactory learning: Critical role of centrifugal neuromodulation. Learn Mem 2010, 17: 561–570.
Linster C, Cleland TA. Neuromodulation of olfactory transformations. Curr Opin Neurobiol 2016, 40: 170–177.
Lizbinski KM, Dacks AM. Intrinsic and extrinsic neuromodulation of olfactory processing. Front Cell Neurosci 2017, 11: 424.
Restrepo D, Doucette W, Whitesell JD, McTavish TS, Salcedo E. From the top down: flexible reading of a fragmented odor map. Trends Neurosci 2009, 32: 525–531.
Li A, Gire DH, Restrepo D. Upsilon spike-field coherence in a population of olfactory bulb neurons differentiates between odors irrespective of associated outcome. J Neurosci 2015, 35: 5808–5822.
Chu MW, Li WL, Komiyama T. Balancing the robustness and efficiency of odor representations during learning. Neuron 2016, 92: 174–186.
Gire DH, Restrepo D, Sejnowski TJ, Greer C, De Carlos JA, Lopez-Mascaraque L. Temporal processing in the olfactory system: can we see a smell? Neuron 2013, 78: 416–432.
Abraham NM, Vincis R, Lagier S, Rodriguez I, Carleton A. Long term functional plasticity of sensory inputs mediated by olfactory learning. Elife 2014, 3: e02109.
Koldaeva A, Schaefer AT, Fukunaga I. Rapid task-dependent tuning of the mouse olfactory bulb. Elife 2019, 8: e43558.
Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci 2006, 26: 8857–8865.
Li A, Gong L, Xu F. Brain-state-independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci U S A 2011, 108: 5087–5092.
Li A, Guthman EM, Doucette WT, Restrepo D. Behavioral status influences the dependence of odorant-induced change in firing on prestimulus firing rate. J Neurosci 2017, 37: 1835–1852.
Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol 2008, 6: e258.
Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci 1999, 2: 1003–1009.
Jordan R, Fukunaga I, Kollo M, Schaefer AT. Active sampling state dynamically enhances olfactory bulb odor representation. Neuron 2018, 98: 1214–1228.
Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron 2011, 71: 962–973.
Sun C, Tang K, Wu J, Xu H, Zhang W, Cao T, et al. Leptin modulates olfactory discrimination and neural activity in the olfactory bulb. Acta Physiol (Oxf) 2019: e13319.
Jeanne JM, Sharpee TO, Gentner TQ. Associative learning enhances population coding by inverting interneuronal correlation patterns. Neuron 2013, 78: 352–363.
Zhou Y, Wang X, Cao T, Xu J, Wang D, Restrepo D, et al. Insulin modulates neural activity of pyramidal neurons in the anterior piriform cortex. Front Cell Neurosci 2017, 11: 378.
Slotnick B, Restrepo D. Olfactometry with mice. Curr Protoc Neurosci 2005, Chapter 8: Unit 8 20.
Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron 2011, 69: 1176–1187.
Khan AG, Sarangi M, Bhalla US. Rats track odour trails accurately using a multi-layered strategy with near-optimal sampling. Nat Commun 2012, 3: 703.
Nguyen Chi V, Muller C, Wolfenstetter T, Yanovsky Y, Draguhn A, Tort AB, et al. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J Neurosci 2016, 36: 162–177.
Ravel N, Chabaud P, Martin C, Gaveau V, Hugues E, Tallon-Baudry C, et al. Olfactory learning modifies the expression of odour-induced oscillatory responses in the gamma (60–90 Hz) and beta (15–40 Hz) bands in the rat olfactory bulb. Eur J Neurosci 2003, 17: 350–358.
Martin C, Gervais R, Hugues E, Messaoudi B, Ravel N. Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: a correlate of odor recognition? J Neurosci 2004, 24: 389–397.
Martin C, Ravel N. Beta and gamma oscillatory activities associated with olfactory memory tasks: different rhythms for different functional networks? Front Behav Neurosci 2014, 8: 218.
Kay LM. Olfactory system oscillations across phyla. Curr Opin Neurobiol 2015, 31: 141–147.
Kato HK, Chu MW, Isaacson JS, Komiyama T. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron 2012, 76: 962–975.
Ross JM, Fletcher ML. Learning-dependent and -independent enhancement of mitral/tufted cell glomerular odor responses following olfactory fear conditioning in awake mice. J Neurosci 2018, 38: 4623–4640.
Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol 2008, 100: 1160–1168.
Carlson KS, Gadziola MA, Dauster ES, Wesson DW. Selective attention controls olfactory decisions and the neural encoding of odors. Curr Biol 2018, 28: 2195–2205.
Yamada Y, Bhaukaurally K, Madarasz TJ, Pouget A, Rodriguez I, Carleton A. Context- and output layer-dependent long-term ensemble plasticity in a sensory circuit. Neuron 2017, 93: 1198–1212.
Gourevitch B, Kay LM, Martin C. Directional coupling from the olfactory bulb to the hippocampus during a go/no-go odor discrimination task. J Neurophysiol 2010, 103: 2633–2641.
Frederick DE, Brown A, Tacopina S, Mehta N, Vujovic M, Brim E, et al. Task-dependent behavioral dynamics make the case for temporal integration in multiple strategies during odor processing. J Neurosci 2017, 37: 4416–4426.
Abraham NM, Guerin D, Bhaukaurally K, Carleton A. Similar odor discrimination behavior in head-restrained and freely moving mice. PLoS One 2012, 7: e51789.
Rojas-Libano D, Frederick DE, Egana JI, Kay LM. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci 2014, 8: 214.
Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in posterior piriform cortex during odor discrimination and reversal learning. Cereb Cortex 2007, 17: 1342–1349.
Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex 2007, 17: 643–652.
Gelperin A, Ghatpande A. Neural basis of olfactory perception. Ann N Y Acad Sci 2009, 1170: 277–285.
Ogg MC, Ross JM, Bendahmane M, Fletcher ML. Olfactory bulb acetylcholine release dishabituates odor responses and reinstates odor investigation. Nat Commun 2018, 9: 1868.
Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci 2012, 32: 10105–10116.
Liu ZX, Zhou JF, Li Y, Hu F, Lu Y, Ma M, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 2014, 81: 1360–1374.
Luo M, Li Y, Zhong W. Do dorsal raphe 5-HT neurons encode “beneficialness”? Neurobiol Learn Mem 2016, 135: 40–49.
Moberly AH, Schreck M, Bhattarai JP, Zweifel LS, Luo W, Ma M. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat Commun 2018, 9: 1528.
Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AT. Two distinct channels of olfactory bulb output. Neuron 2012, 75: 320–329.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31571082, 31872771 and 31700895), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (16KJA180007), and the Natural Science Foundation of Jiangsu Province (BK20170260). We thank Siqi Jing and Lingzhi Zhang for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, P., Cao, T., Xu, J. et al. Plasticity of Sniffing Pattern and Neural Activity in the Olfactory Bulb of Behaving Mice During Odor Sampling, Anticipation, and Reward. Neurosci. Bull. 36, 598–610 (2020). https://doi.org/10.1007/s12264-019-00463-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00463-9