Abstract
Behavioral adjustment plays an important role in the treatment and relapse of drug addiction. Nonetheless, few studies have examined behavioral adjustment and its plasticity following error commission in methamphetamine (METH) dependence, which is detrimental to human health. Thus, we investigated the behavioral adjustment performance following error commission in long-term METH addicts and how it varied with the application of repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLPFC). Twenty-nine male long-term METH addicts (for > 3 years) were randomly assigned to high-frequency (10 Hz, n = 15) or sham (n = 14) rTMS of the left DLPFC during a two-choice oddball task. Twenty-six age-matched, healthy male adults participated in the two-choice oddball task pretest to establish normal performance for comparison. The results showed that 10 Hz rTMS over the left DLPFC significantly decreased the post-error slowing effect in response times of METH addicts. In addition, the 10 Hz rTMS intervention remarkably reduced the reaction times during post-error trials but not post-correct trials. While the 10 Hz rTMS group showed a more pronounced post-error slowing effect than the healthy participants during the pretest, the post-error slowing effect in the posttest of this sample was similar to that in the healthy participants. These results suggest that high-frequency rTMS over the left DLPFC is a useful protocol for the improvement of behavioral adjustment after error commission in long-term METH addicts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methamphetamine (METH) dependence is a major problem and social service concern worldwide. A considerable body of evidence indicates that METH abuse can result in acute organ system dysfunction, serious and persistent cognitive impairment, and, more seriously, can lead to irreversible and permanent physiological damage [1, 2].
As an important aspect of cognitive function, the ability to adjust behavior is essential to success [3]. Especially, adjusting behavior after committing an error is crucial to decrease the possibility of erring again. In addition, deficits in behavioral adjustment are thought to be a factor contributing to falling into a cycle of drug addiction [4]. Thus, successfully adjusting inappropriate behavior may be pivotal for the rehabilitation and prevention of relapse in METH addicts. However, to the best of our knowledge, no empirical study to date has examined the ability to adjust behavioral performance following error commission in METH addicts.
People tend to slow their response once they have committed an error. This phenomenon is named post-error slowing (PES), which is operationally defined by a prolonged response latency in the subsequent trial after error commission in the preceding trial [5]. This is thought to reflect the activation of control processes that reset cognitive-motor operations to restore accurate responding [6]. PES may reflect a more cautious response mode providing the basis for more accurate behavior in the forthcoming trial [7]. If so, in accord with the speed-accuracy trade-off, the accuracy should be enhanced. However, the prediction that accuracy is enhanced following errors has been contradicted by many studies [8, 9]. Recent work suggests that PES can be explained by a combination of an increased decision threshold and a decrease of attentional sensitivity to perceptual information [10], both of which are closely linked with cognitive control [11].
Recently, it has been reported that borderline personality disorder is associated with prolonged PES, showing slowed recovery following errors [12]. Impaired inhibitory control is a cardinal feature of this disorder [13, 14], such as reduced task switching, distracter inhibition, and focused attention [15]. METH addiction is also characterized by deficits in inhibitory control, such as behavioral disinhibition [16, 17] and deficits in the neural circuits involved in emotional control [18, 19]. Paulus et al. [19] reported that response decision in METH addicts is more heavily influenced by the immediately preceding outcome than in healthy participants. Thus, we assumed that METH-dependent individuals would exhibit prolonged PES compared to a healthy group.
Transcranial magnetic stimulation (TMS) is a non-invasive technique that has been assessed in a great number of studies for its therapeutic potential in treating addiction; it involves projecting a fluctuating magnetic field (magnetic pulses) through the skull into the brain [20, 21]. Repetitive TMS (rTMS) refers to TMS pulses given consecutively. Generally, low-frequency (≤ 1 Hz) rTMS reduces neuronal activity and cortical excitability, whereas high-frequency (> 1 Hz) rTMS increases neuronal activity and cortical excitability [22]. High-frequency rTMS over specific regions increases the relative regional cerebral blood flow, and this is thought to enhance the cognitive functions mediated by the region [23, 24]. Study of rTMS as a treatment for addiction is at an early stage. Some laboratory studies have found that high-frequency rTMS targeted to the dorsolateral prefrontal cortex (DLPFC) significantly reduces spontaneous and cue-induced nicotine craving [25]. Furthermore, promising clinical evidence has been reported showing that 1 week of high-frequency rTMS over the left DLPFC reduces cocaine craving [26]. The left DLPFC plays a vital role in cognitive control [27], which is responsible for adjusting behaviors to satisfy contextual demands and goal-setting. Recent evidence suggests that high-frequency rTMS over the DLPFC is able to reduce cue-induced craving in smoking addicts by improving inhibitory control and response decision functions mediated by this region [28]. We therefore designed experiments to test the hypothesis that high-frequency rTMS targeted to the left DLPFC is an effective protocol to enhance behavioral adjustment following error commission in long-term METH abusers.
Materials and Methods
Participants
Twenty-nine male METH-addicted participants (21–55 years old; average 32 ± 7 years), with a history of regular (weekly) and exclusive (no other drugs) use of METH for > 3 years, were recruited. They were randomly assigned into the high-frequency 10 Hz rTMS (n = 15) or the sham rTMS group (n = 14). In addition, a sample of age-matched healthy male participants were recruited from the local community as the control group (n = 26, average age = 29 ± 7 years). The ages of the healthy participants were similar to those of the 10 Hz and sham rTMS groups (F (2, 52) = 1.794, P = 0.177).
All participants were healthy, free of mental disorders, a history of epilepsy, or cardiovascular complications, and all the METH addicts were in compulsory abstinence from drug use for more than one month and currently free of hallucinations and other acute withdrawal symptoms. They all participated in the study voluntarily and gave written informed consent. The study was approved by the Ethics Committee for Human Research at Southwest University, Chongqing, China. The experimental procedure was in accord with the ethical principles of the 1964 Declaration of Helsinki (World Medical Organization, 1996). The clinical trial registration is ChiCTR-ROC-16008541 (The brain’s cognitive function and its plasticity studies of drug addicts) at http://www.chictr.org.cn.
Data Collection and Measurement
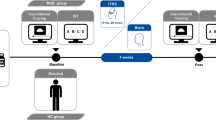
We used a two-choice oddball task to investigate how PES varied with rTMS. All the METH addicts performed the two-choice oddball task twice a day, that is, a pretest and a posttest, 5 min before and 5 min after the rTMS or sham stimulation (Fig. 1). Furthermore, the healthy controls performed the two-choice oddball task once, to set up a criterion for normal performance in a healthy group. This was designed to facilitate estimation of the PES performance of METH addicts and the extent to which rTMS may repair their performance relative to that of healthy individuals. The healthy group did not receive rTMS or sham stimulation.
The two-choice oddball task consisted of 300 trials, each of which started with a jittered fixation varying from 500 to 1500 ms. Then the task stimulus, either standard or deviant, was presented. In this task, participants were instructed to make a standard/deviant distinction by pressing different keys as accurately and quickly as possible [29]. Half of the participants were required to press “F” when the standard stimulus “W” (frequent stimulus, 80% of trials) was presented, and to press “J” when the deviant stimulus “M” (infrequent stimulus, 20% of trials; Fig. 1) appeared. For the remaining participants, the assignment of the response hand was reversed to control for the influence of response hand on reaction time (RT). Fifteen practice trials were used to familiarize participants with the stimuli and the procedure. To avoid practice effects, the formal experiment did not start, and the practice session was repeated, until participants achieved 100% accuracy for both the standard and the deviant stimulus. At the end of the experiment, the participants were informed of their accuracy for both stimuli as feedback on their performance.
In this study, we selected the traditional method to quantify post error slowing, that is, PES (traditional) = MRT (post error)—MRT (post correct), which has proven to have high validity [30]. As the standard stimulus in the two-choice oddball task triggers a habitual response pattern, error commission is most pronounced in deviant trials [31]. Because deviant trials, in most cases, were followed by a standard instead of deviant stimulus. We observed few post error trials for deviant stimuli. Thus, the final data analysis only included post correct and post error trials for the standard stimulus.
Data analysis was mainly based on the repeated measure analysis of variance model (ANOVA), as we were particularly interested in the error commission (post-error versus post-correct) by testing phase (pre and post) interaction, where significance denotes differences in the PES effect after versus before intervention.
rTMS Procedure
For rTMS or sham stimulation, the motor threshold was determined in all groups over the left motor cortex, by finding the lowest intensity that induced a motor response in the right abductor pollicis brevis muscle, which produced five motor-evoked responses of at least 50 mV in 10 trials. During treatment, the coil was placed over the left prefrontal area at a point 5 cm anterior to the scalp position at which the motor threshold was determined. High-frequency (10 Hz, strength at 100% of resting motor threshold; 5 s on, 10 s off for 10 min; 2000 pulses divided into 40 repeats at 15 s intervals) or sham TMS (1 Hz, coil turned away from the skull at 90°, with only one edge resting on the scalp) was applied over the left DLPFC. We used a figure-of-8 coil (radius, 45 mm for each lobe, center distance between the two lobes 76 mm) for accurately-targeted stimulation with a CCY-I TMS instrument (Yiruide Co., Wuhan, China). The left DLPFC stimulation site was defined as 5 cm anterior to the area of the optimal site for the primary motor cortex of the left hemisphere (method of Pascual-Leone). This method has been reported to be accurate in targeting the DLPFC area [32].
Results
Post-Error Slowing
To check the validity of our experimental manipulation, we determined whether the effect of PES existed in healthy participants and addicts. First, analysis of the data from the healthy group, with error commission (2 levels: Post-Error (PE) and Post-Correct (PC)) as a repeated factor, showed longer RTs during PE than during PC trials (RT (PE) = 504.90 ± 84.44 ms; RT (PC) = 464.60 ± 50.88 ms; P = 0.003). Second, using a similar method, we made this comparison of the PES effect in the addicts. Similarly, the results also showed a PES effect in the addicts (RT (PE) = 517.96 ± 116.87 ms; RT (PC) = 436.52 ± 57.68 ms; P < 0.001). These results verified the robustness of PES: that people tend to slow down once they have committed an error.
Analysis of the 10 Hz intervention effect (Fig. 2), with error commission (2 levels: PE and PC) and testing phase (2 levels: pretest and posttest) as repeated factors, showed significant main effects of error commission (P = 0.006) and testing phase (P = 0.01), as well as significant error commission by testing phase interaction (F (1, 14) = 6.270, P = 0.025; η 2p = 0.309). Furthermore, the 10 Hz rTMS intervention significantly decreased the RTs (P = 0.011) during PE trials but not PC trials (P > 0.06). However, this intervention effect was absent in the sham group, as the ANOVA with this sample showed neither a significant main effect of testing phase (P = 0.574) nor a significant interaction between error commission and testing phase (P = 0.502).
RTs in addicts and healthy participants for each trial type and testing phase. A RTs of healthy participants and METH addicts in the pretest stage. B 10 Hz rTMS intervention significantly decreased RTs during post-error trials (P = 0.011) but not post-correct trials (P > 0.06). C This intervention effect was absent in sham rTMS participants (n = 14). D Comparison of PES between healthy participants and 10 Hz addicts in the two testing stages (PES = PE − PC). * P < 0.05; ** P <0.01.
Subsequently, to compare the PES effect in addicts with that in healthy participants, we conducted two group comparisons. The first analysis aimed to compare the effect of PES in healthy and 10 Hz METH addicts during the pretest stage. To realize this, we first computed an index of the PES effect by subtracting the RTs in PC trials from those in PE trials (i.e., PES = PE – PC) separately for addicts and healthy participants. The results showed a significantly larger PES effect (F (1, 39) = 5.249, P = 0.027) in addicts (MeanPES = 103.96) than healthy participants (MeanPES = 40.30).
Using a similar method, we conducted a group comparison of the PES effect during the posttest stage. The results, different from those above, showed a similar PES effect across the healthy and the addicted (MeanPES = 28.71) participants (F (1, 39) = 0.269, P = 0.607).
Accuracy
Analysis of the response accuracy in the 10 Hz rTMS group, with testing phase (2 levels: pretest and posttest) and stimulus (standard and deviant) showed a significantly reduced accuracy for the deviant relative to the standard stimulus, irrespective of intervention (F (1, 14) = 38.33, P < 0.001). Neither the main effect of testing phase (F (1, 14) = 1.682, ns) nor the phase by stimulus interaction (F (1, 14) = 1.208, ns) was significant. Analysis of the response accuracy in the sham group showed no significant effects except for lower accuracy for deviant than for standard trials (F (1,13) = 32.34, P < 0.001) as well. This reduction of response accuracy for deviant relative to standard trials was also significant in the healthy participants (F (1,25) = 56.615, P < 0.001), most likely as a result of proponent response inhibition during deviant trials [33]. Thus, consistent with our hypothesis, this task induced sufficient erroneous responses which facilitated our assessment of PES effects (Fig. 3).
Manipulation check for the validity of the two-choice oddball task in inducing erroneous responses, which are the basis for assessing PES effects in healthy (n = 26) and METH-addicted (n = 29) participants. A Accuracy of healthy group and 10 Hz rTMS group during pretest and posttest. B Accuracy of addicts in sham rTMS group. * P < 0.05; ** P <0.01.
To assess the response accuracy across addicts and healthy participants, we compared the accuracy of addicts in the 10 Hz group with that of healthy participants during pretest and posttest. The results showed a similar pretest accuracy for the two groups [Mean (healthy) = 0.97 ± 0.01, Mean (addicts) = 0.97 ± 0.02, t (39) = 0.674, P = 0.504], while the posttest accuracy was significantly higher in the addicts than in the healthy participants [Mean (healthy) = 0.97 ± 0.01, Mean (addicts) = 0.98 ± 0.01, t (39) = 3.818, P < 0.001].
Discussion
METH use is highly correlated with various neuropsychiatric complications and has numerous adverse neurological and other health effects [34]. However, therapy for METH addiction suffers from high relapse rates [35]. A major reason for the failure of current METH-dependence therapies is the inability to prevent addicts from impulsive, episodic relapse into METH use, especially during the initiation of therapy [35, 36]. Deficits in behavioral adjustment may have reciprocal causation with drug addiction. Therefore, successfully adjusting post-error behaviors may be pivotal for the rehabilitation of METH addicts and the prevention of relapse. However, most prior studies focused on intervention for drug craving [37,38,39], the assessment of which is currently limited to subjective rating. Few studies have investigated the ability to adjust behavioral performance following error commission in METH addicts [40], leaving intervention in the behavioral adjustment of this group unconsidered.
TMS is a powerful tool in neuroscience, allowing transient interference with specific brain functions [25, 41]. Here, we report that high-frequency rTMS over the left DLPFC significantly reduced the PES in METH addicts. In addition, the 10 Hz rTMS intervention significantly decreased the RTs during PE trials but not PC trials. This intervention effect was absent with sham rTMS. When comparing the PES between the healthy and rTMS groups, we found a more pronounced PES in METH abusers during the pre-intervention stage, while the magnitude of the PES effects were similar during the post-rTMS stage. Comparison of the response accuracy in the rTMS group with that of healthy controls showed no significant effects except for a higher posttest accuracy in addicts than in healthy adults. From the results of response accuracy and the RTs of PES, we suggest that addicts in the rTMS group, after intervention, showed faster behavioral adjustment without the cost of a reduction in accuracy. The rTMS was well tolerated by all participants, and was free of reported or observed side-effects. Therefore, the 10 Hz rTMS protocol might be applicable to larger populations in future studies.
In general, when realizing an error has been committed, people tend to stop their current movement for a while or at least to slow down somewhat. This may help to improve accuracy [42, 43]. That is, commission of an error is often associated with a subsequent behavioral adjustment to increase accuracy at the cost of RT, consistent with the speed-accuracy trade-off. Currently, three accounts may contribute to understanding the mechanisms of PES. First, it has been argued that PES is related to cognitive control mechanisms serving to improve subsequent performance [7, 44]. However, certain data speak against the general assumption that PES serves to prevent future errors. In fact, the assumption that accuracy increases following errors has been contradicted by many studies [6, 9, 45, 46]. Second, it has been suggested that the commission of an error increases the response threshold for post-error trials, which entails more evidence before a choice is made [47, 48]. A third hypothesis is that PES arises from impairment of the participant’s sensitivity to incoming evidence following errors, perhaps because the negative feedback or unexpected outcome distracts and diverts attention [45, 49]. That is, the occurrence of response slowing is just because people are surprised at the unexpected event of committing an error [45, 49,50,51]. For instance, PES could be the outcome of distraction, or delayed startup of information accumulation due to time wasted on irrelevant processes such as overcoming disappointment [52]. By combining the second and third accounts, a recent study has indicated that PES is most likely the combined result of both an increased decision threshold and a decrease of the accumulator’s sensitivity to perceptual information [10]. Taking the above accounts together, it is clear that a common component underlies the different explanations. That is, PES entails the involvement of cognitive control mechanisms. In particular, according to the recent combination account [10], the increased PES would be a reflection of the impaired function of attentional control over prepotent, distracting information, and impaired focused attention on the evidence accumulation for a response decision.
High-frequency rTMS showed a significant intervention effect on behavioral adjustment in METH addicts, reflecting a faster behavioral adjustment without a loss of accuracy. Several potential mechanisms underlie the present findings. First, PES involves cognitive control, such as detaching from the consequences induced by error commission and focusing attention on the subsequent representation. In our experimental design, participants were required to achieve 100% accuracy before the formal test phase. When committing an error, they may have felt disappointment and anxiety, which was verified by a number of participants in the post-experiment debriefing. This might have contributed to the occurrence of response slowing after error commission. High-frequency rTMS of the DLPFC has been shown to induce dopamine release in the caudate nucleus and striatum, which play an important role in complex functions such as cognitive control [53, 54]. The nature of cognitive control lies in two opposing but complementary functions: (1) continuous focusing on task-relevant representations; and (2) inhibition of irrelevant or novel information [55, 56]. It is possible that the effect of high-frequency rTMS on PES is mediated by its effect on striatal dopamine release. Second, in our experiment, participants were required to complete a two-choice oddball task, which entails accumulating evidence before making a response decision. It has been suggested that rTMS of the DLPFC causes functional changes in subcortical regions including the reward system, which is associated with decision-making [57, 58]. Thus, the facilitation effect of high-frequency rTMS on post-error performance is potentially realized by its excitatory effect on brain regions related to decision-making (e.g. basal ganglia), consequently accelerating evidence accumulation for a response decision. Future studies are required to further elucidate the potential mechanisms underlying the post-error response changes following rTMS treatment.
Several limitations should be acknowledged. First, we did not subject healthy participants to rTMS, and they performed the task just once. This left us unable to investigate how TMS alters the PES effect in healthy people. Second, in the current study, driven by the purpose of investigating whether the PES phenomenon differs between healthy participants and METH addicts and how high-frequency rTMS modulates this difference, our data analysis focused on how the PES index differed between healthy participants and addicts before and after a real rTMS protocol. And analysis of the data in the sham group was to isolate a potential placebo or practice effect. However, we admit that if the intervention effect of 10 Hz rTMS was robust enough, we should have found a significant grouping (active versus sham) by testing phase interaction concerning the PES index. However, this interaction failed to reach statistical significance (P = 0.15). Thus, the results regarding the 10 Hz-rTMS effect on PES in METH addicts should be considered preliminary, and further studies are necessary to replicate the current findings with a larger sample size. Third, when locating the DLPFC, we did not take into consideration the shape and size of each individual’s head. MRI-guided neuro-navigation may achieve both better accuracy and superior efficacy for locating the DLPFC.
References
Gentry WB, Rüedi-Bettschen D, Owens SM. Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin 2009, 5: 206–213.
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine tranporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 2001, 21(23):9414–9418.
John Fantuzzo RB, Paul McDermottJohn Fantuzzo, Rebecca Bulotsky, Paul McDermott. A multivariate analysis of emotional and behavioral adjustment and preschool educational outcomes. School Psych Rev 2003, 32: 185–203.
Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry 2009, 65: 706–709.
Hajcak G, McDonald N, Simons RF. To err is autonomic: Error–related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiol 2003, 40: 895–903.
Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol 2011, 2: 233.
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 2004, 306: 443–447.
King JA, Korb FM, von Cramon DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. J Neurosci 2010, 30: 12759–12769.
Schroder HS, Moser JS. Improving the study of error monitoring with consideration of behavioral performance measures. Front Hum Neurosci 2014, 8: 178.
Purcell BA, Kiani R. Neural Mechanisms of Post-error Adjustments of Decision Policy in Parietal Cortex. Neuron 2016, 89: 658–671.
Posner MI, Rothbart MK, Vizueta N, Levy KN, Evans DE, Thomas KM, et al. Attentional mechanisms of borderline personality disorder. Proc Natl Acad Sci U S A 2002, 99: 16366–16370.
Saunders KE, Goodwin GM, Rogers RD. Borderline personality disorder, but not euthymic bipolar I disorder, is associated with prolonged post-error slowing in sensorimotor performance. J Affect Disord 2016, 198: 163–170.
Paris J. Borderline personality disorder. CMAJ 2005, 172: 1579–1583.
Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord 2009, 11: 280–288.
Minzenberg MJ, Poole JH, Vinogradov S. A neurocognitive model of borderline personality disorder: effects of childhood sexual abuse and relationship to adult social attachment disturbance. Dev Psychopathol 2008, 20: 341–368.
Meyer JS. 3,4-methylenedioxymethamphetamine (MDMA): current perspectives. Subst Abuse Rehabil 2013, 4: 83–99.
Greene SL, Kerr F, Braitberg G. Review article: amphetamines and related drugs of abuse. Emerg Med Australas 2008, 20: 391–402.
Abidin SZ, Leong JW, Mahmoudi M, Nordin N, Abdullah S, Cheah PS, et al. In Silico Prediction and Validation of Gfap as an miR–3099 Target in Mouse Brain. Neurosci Bull 2017, 33: 373–382.
Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and Functional Neuroimaging Evidence for Prefrontal Dysfunction in Methamphetamine-Dependent Subjects. Neuropsychopharmacology 2002, 26: 53–63.
Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol 2014, 2014: 815215.
Yang LZ, Yang Z, Zhang X. Non–invasive brain stimulation for the treatment of nicotine addiction: potential and challenges. Neurosci Bull 2016, 32: 550–556.
Amengual JL, Marco-Pallares J, Richter L, Oung S, Schweikard A, Mohammadi B, et al. Tracking post-error adaptation in the motor system by transcranial magnetic stimulation. Neuroscience 2013, 250: 342–351.
Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 2007, 68: 484–488.
Stern WM, Tormos JM, Press DZ, Pearlman C, Pascual-Leone A. Antidepressant effects of high and low frequency repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex: a double–blind, randomized, placebo–controlled trial. J Neuropsychiatry Clin Neurosci 2007, 19: 179–186.
Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, et al. 10-Hz Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biol Psychiatry 2016, 80: e13–14.
Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict 2008, 17: 345–346.
Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, et al. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex 2013, 23: 2677–2689.
Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013, 73: 714–720.
Yuan J, Xu S, Li C, Yang J, Li H, Yuan Y, et al. The enhanced processing of visual novel events in females: ERP correlates from two modified three-stimulus oddball tasks. Brain Res 2012, 1437: 77–88.
Dutilh G, van Ravenzwaaij D, Nieuwenhuis S, van der Maas HLJ, Forstmann BU, Wagenmakers E-J. How to measure post-error slowing: A confound and a simple solution. J Math Psychol 2012, 56: 208–216.
Yuan J, He Y, Qinglin Z, Chen A, Li H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiol 2008, 45: 986–993.
Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug–resistant depression. Lancet 1996, 348: 233–237.
Yuan J, Meng X, Yang J, Yao G, Hu L, Yuan H. The valence strength of unpleasant emotion modulates brain processing of behavioral inhibitory control: neural correlates. Biol Psychol 2012, 89: 240–251.
Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 2008, 27: 253–262.
Gentry WB, Ruedi-Bettschen D, Owens SM. Anti-(+)-methamphetamine monoclonal antibody antagonists designed to prevent the progression of human diseases of addiction. Clin Pharmacol Ther 2010, 88: 390–393.
Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Hood LE, Olive MF. Attenuation of reinstatement of methamphetamine-, sucrose-, and food-seeking behavior in rats by fenobam, a metabotropic glutamate receptor 5 negative allosteric modulator. Psychopharmacology (Berl) 2013, 225: 151–159.
Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol 2013, 23: 649–654.
Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 2012, 169: 406–414.
Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 2012, 336: 241–245.
Schütz CG. High dose stimulant substitution for the treatment of cocaine and crystal meth use disorders. J Addict Res Ther 2015, 6. e131. https://doi.org/10.4172/2155-6105.1000e131.
Wagner M, Rihs TA, Mosimann UP, Fisch HU, Schlaepfer TE. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex affects divided attention immediately after cessation of stimulation. J Psychiatr Res 2006, 40: 315–321.
Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post–error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci 2008, 20: 1021–1029.
Josep Marco-Pallares EC. Neural mechanisms underlying adaptive actions after slips. J Cogn Neurosci 2008, 20:9, 1595–1610.
Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci 2001, 21, 9430–9437.
Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition 2009, 111: 275–279.
Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci 1993, 4: 385–390.
Holroyd CB, Yeung N, Coles MG, Cohen JD. A mechanism for error detection in speeded response time tasks. J Exp Psychol Gen 2005, 134: 163–191.
Laming D. Choice reaction performance following an error. Acta Psychol 1979, 43: 199–224.
Nunez Castellar E, Kuhn S, Fias W, Notebaert W. Outcome expectancy and not accuracy determines posterror slowing: ERP support. Cogn Affect Behav Neurosci 2010, 10: 270–278.
Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev 2001, 25: 355–373.
Murphy PR vMM, Nieuwenhuis S. The pupillary orienting response predicts adaptive behavioral adjustment after errors. PLoS One 2016, 11: e0151763.
Carp J, Compton RJ. Alpha power is influenced by performance errors. Psychophysiol 2009, 46: 336–343.
Keck M WT, Muller M, Erhardt A, Ohl F, Toschi N. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neutopharmacol 2002, 43: 101.
Strafella AP PT, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001, 21:RC157, 1–4.
Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top–down cognitive control. Neuron 2011, 69: 680–694.
D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 2007, 362: 761–772.
Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci U S A 2006, 103: 7476–7481.
Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS, et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol 2011, 16: 654–666.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31371042, 31400906 and 31600886), and the Key Program of the Higher Education Institutions of Henan Province, China (17AJ90002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no conflicts of interest regarding this work.
Ethical Standards
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Rights and permissions
About this article
Cite this article
Liang, Q., Lin, J., Yang, J. et al. Intervention Effect of Repetitive TMS on Behavioral Adjustment After Error Commission in Long-Term Methamphetamine Addicts: Evidence From a Two-Choice Oddball Task. Neurosci. Bull. 34, 449–456 (2018). https://doi.org/10.1007/s12264-018-0205-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-018-0205-y