Abstract
Hyperphosphorylated tau is the major protein component of neurofibrillary tangles in the brains of patients with Alzheimer’s disease (AD). However, the mechanism underlying tau hyperphosphorylation is not fully understood. Here, we demonstrated that exogenously expressed wild-type human tau40 was detectable in the phosphorylated form at multiple AD-associated sites in cytoplasmic and nuclear fractions from HEK293 cells. Among these sites, tau phosphorylated at Thr205 and Ser214 was almost exclusively found in the nuclear fraction at the conditions used in the present study. With the intracellular tau accumulation, the Ca2+ concentration was significantly increased in both cytoplasmic and nuclear fractions. Further studies using site-specific mutagenesis and pharmacological treatment demonstrated that phosphorylation of tau at Thr205 increased nuclear Ca2+ concentration with a simultaneous increase in the phosphorylation of Ca2+/calmodulin-dependent protein kinase IV (CaMKIV) at Ser196. On the other hand, phosphorylation of tau at Ser214 did not significantly change the nuclear Ca2+/CaMKIV signaling. Finally, expressing calmodulin-binding protein-4 that disrupts formation of the Ca2+/calmodulin complex abolished the okadaic acid-induced tau hyperphosphorylation in the nuclear fraction. We conclude that the intracellular accumulation of phosphorylated tau, as detected in the brains of AD patients, can trigger nuclear Ca2+/CaMKIV signaling, which in turn aggravates tau hyperphosphorylation. Our findings provide new insights for tauopathies: hyperphosphorylation of intracellular tau and an increased Ca2+ concentration may induce a self-perpetuating harmful loop to promote neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common senile dementia. It is characterized by the accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles, the latter mainly composed of hyperphosphorylated tau protein [1,2,3]. Upregulation of several protein kinases and/or inhibition of protein phosphatases have been proposed to cause tau hyperphosphorylation [4].
Deregulation of intracellular Ca2+ signaling has been implicated in the pathogenesis of AD [5]. Ca2+ dysfunction elicits the accumulation of β-amyloid and tau hyperphosphorylation [6,7,8]. Increased [Ca2+]i induces degeneration or antigenic changes in the microtubule-associated protein tau in neuronal cultures of rat hippocampus and the human cerebral cortex [9]. Several studies have also shown that β-amyloid enhances Ca2+ entry [10,11,12,13], and the expression of mutant presenelin 1 (PS1) increases Ca2+ release from the endoplasmic reticulum in neurons [14, 15].
Tau hyperphosphorylation promotes its intracellular accumulation and the formation of neurofibrillary tangles in the AD brain. The normal function of tau is to promote microtubule assembly and to maintain the stability of microtubules. Recently, tau phosphorylation has been found to be involved in regulating cell viability, i.e., phosphorylation of tau renders cells more resistant to apoptosis [16], while dephosphorylation of tau by protein phosphatase-2A facilitates apoptosis [17]. The extracellular tau released from dead neurons increases the [Ca2+]i and alkaline phosphatase promotes the neurotoxic effects of tau [18]. In 3xTg-AD mice expressing mutant PS1, amyloid protein precursor (APP), and tau, an exaggerated endoplasmic reticulum Ca2+ signal was also detected when compared with non-Tg mice [15]. These data suggest an intrinsic link of abnormal tau with intracellular Ca2+ homeostasis. However, it has not been reported whether and how the intracellular expression of phosphorylated tau may affect nuclear Ca2+ signaling, and in turn whether nuclear Ca2+ signaling affects tau phosphorylation.
In the present study, by transfecting human wild-type tau40 into HEK293 cells, we found that the expression of tau increased the [Ca2+]i both in the cytoplasm and nucleus with simultaneous hyperphosphorylation and nuclear localization of tau protein. Further studies demonstrated that phosphorylation of tau at Thr205 activated nuclear Ca2+/CaMKIV, which in turn aggravated tau hyperphosphorylation.
Materials and Methods
Antibodies and Reagents
Rabbit polyclonal antibodies (pAb) pS396 (1:1000), pS404 (1:1000), pT231 (1:1000), pS262 (1:1000), pS214 (1:400), and pT205 (1:400) against tau phosphorylated at the corresponding sites were from Signalway Antibody (Pearland, TX). Mouse monoclonal antibody (mAb) tau-5 (1:1000) against total tau was from Lab Vision Co. (Westinghouse, CA). PAb against histone-3 (1:1000) was from Millipore (Billerica, MA). PAb against CaMKIV (1:400) and pAb against phosphorylated CaMKIV (1:400) at Ser196 were from Santa Cruz Biotechnology (Santa Cruz, CA). Okadaic acid (OA) was from Calbiochem (Darmstadt, Germany). Fluo 3-AM was from Dojindo Laboratories (Kumamoto, Japan). The nuclear and cytoplasmic protein extraction kit was from KeyGen Biotech (Nanjing, China).
Construction of Plasmids
DsRed2-N1-human tau40 was constructed using the polymerase chain reaction (PCR). The mutants T205A, T205E, S214A, and S214E were prepared by site-directed mutagenesis, which was directly performed on DsRed2-N1-human tau40 by PCR amplification with self-complementary primers using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, Shanghai, China). The primers were CCCGGCTCCCCAGGCGCTCCCGGCAGCCGC (PF, T205A), GCGGCTGCCGGGAGCGCCTGGGGAGCCGGG (PR, T205A) CAGCCCCGGCTCCCCAGGCGAACCCGGCAGCCGCTCCC (PF, T205E), GGGAGCGGCTGCCGGGTTCGCCTGGGGAGCCGGGGCTG (PR, T205E), TCCCGCACCCCGGCTCTTCCAACCCCA (PF, S214A), TGGGGTTGGAAGAGCCGGGGTGCGGGA (PR, S214A), CGCACCCCGGAACTTCCAACCCCACCCACCCG (PF, S214E), and CGGGTGGGTGGGGTTGGAAGTTCCGGGGTGCG (PR, S214E). A recombinant adeno-associated virus (rAAV) containing an expression cassette for calmodulin-binding protein-4 (rAAV-CMV/CBA-CaMBP4) plasmid and its control (rAAV-CMV/CBA-LacZ) were kind gifts from Dr. Hilmar Bading (University of Heidelberg, Heidelberg, Germany).
Cell Culture
Human embryonic kidney 293 (HEK293) cells and HEK293/tau cells (stably tau-transfected HEK293 cells) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco BRL, Gaithersburg, MD) and grown at 37 °C in a humidified atmosphere containing 5% CO2. Transfection was carried out with Lipofectamine2000 according to the manufacturer’s instructions.
Fluo 3-AM Ca2+ Imaging and Calibration Procedure for Intracellular Ca2+ Concentration
The [Ca2+]i was measured using the following previous procedures [19,20,21]. The cells were loaded with Fluo 3-AM as described [19, 20]. Briefly, Fura 3-AM was solubilized in anhydrous dimethyl sulfoxide (DMSO) and then diluted with HEPES buffered saline (HBS). The final concentration of Fura 3-AM was 6 μmol/L. The cells were incubated with Fluo 3-AM (pH 7.4) for 20 min at 37 °C and washed three times with HBS. An additional 20 min was allowed for de-esterification of Fluo 3-AM. Images were recorded with a laser-scanning confocal microscope (LSM 510, Zeiss, Oberkochen, Germany) for at least 40 min.
The fluorescence intensity was converted into [Ca2+] using the following equation: [Ca2+] = [(F − F min)/(F max − F)] × K D. The dissociation constant (K D) value was 400 nmol/L. F represents the fluorescence intensity measured in a region of interest (ROI). F min was the minimum fluorescence intensity of the ROI when the cells were treated with the Ca2+ ionophore ionomycin (10 μmol/L; Sigma-Aldrich) in Ca2+-free solution. F max was the maximum fluorescence intensity of the ROI when the cells were treated with ionomycin (10 μmol/L; Sigma-Aldrich) in high-Ca2+ solution (10 mmol/L Ca2+; Sigma-Aldrich). The values of F min and F max were averaged [20, 21].
Western Blotting
Western blotting was performed according to a well-established method in our laboratory. Briefly, cells were collected and the nuclear and cytoplasmic proteins were prepared using an extraction kit according to the manufacturer’s instructions (KeyGen Biotech, Nanjing, China). The protein concentrations in the extracts were measured using a BCA kit (Pierce, Rockford, IL). The protein was separated by 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The membranes were blocked in 5% non-fat milk for 1 h at 15 °C–25 °C, incubated with primary antibodies at 4 °C overnight, and then incubated with anti-rabbit or anti-mouse IgG conjugated to IRDye™ (800CW; Licor Biosciences, Lincoln, NE) for 1 h at room temperature. The blots were detected by the Odyssey Infrared Imaging System (Licor Biosciences) and quantitatively analyzed using Kodak Digital Science 1D software (Eastman Kodak Co., New Haven, CT).
Statistical Analysis
Data are presented as mean ± SEM and were analyzed with SPSS 11.0 statistical software. Statistical significance was determined by one-way ANOVA followed by the Student–Newman–Keuls post hoc test with 95% confidence and Student’s two-tailed t test.
Results
Expression of Phosphorylated Tau Increases [Ca2+] in Cytoplasmic and Nuclear Fractions
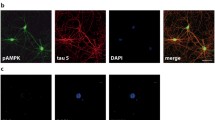
The human wild-type tau40 plasmid was transfected into HEK293 cells to explore the effect of AD-like tau accumulation on intracellular Ca2+ homeostasis, and the empty vector was transfected as control. At 24 h after transfection, the cells were carefully fractionated for the measurement of tau expression/phosphorylation and cellular localization. HEK293 cells expressed almost no endogenous tau protein (Fig. 1A). Exogenously-expressed human tau in HEK293 cells was phosphorylated at multiple AD-associated sites in the cytoplasmic and nuclear fractions. Interestingly, phosphorylation at Thr205 and Ser214 was almost exclusively in the nucleus (Fig. 1B, C). Co-immunofluorescence staining also showed that pT205-tau and pS214-tau were nicely co-localized with a nuclear marker (Hoechst33342, Invitrogen) (Fig. 1D, E). With the expression of phosphorylated tau, the [Ca2+] was significantly elevated in both cytoplasmic and nuclear fractions (Fig. 1F). These data indicate that the accumulation of human phosphorylated tau disrupts intracellular Ca2+ homeostasis.
Expression of phosphorylated tau increases Ca2+ in cytoplasm and nucleus. A Western blots of extracts from HEK293 cells transiently transfected with DsRed2-N1-human tau40 plasmid for 24 h, showing phosphorylation at Thr205, Ser214, Thr231, Ser396, and Ser404 and total tau (probed by Tau-5). B, C Western blots and levels of tau proteins in the cytoplasmic (Cyto) and nuclear (Nuc) fractions. D, E Representative images showing co-localization of Thr205- (pT205) and Ser214- (pS214) phosphorylated tau with a nuclear marker (Hoechst) after transfection in HEK293 cells. F Basal [Ca2+] increased in the nucleus and cytoplasm of HEK293 cells transfected with human wild-type tau40 compared with vehicle (Vec) for 24 h (>100 cells). Data are expressed as mean ± SEM; *P < 0.05, ***P < 0.001 vs Vec or Cyto.
Tau Phosphorylation at Thr205 But Not Ser214 Activates Nuclear Ca2+-CaMKIV Signaling
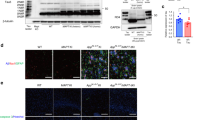
As the Thr205- and Ser214-phosphorylated tau were mainly in the nuclear fraction (Fig. 1B), we further studied their role in modulating nuclear Ca2+ homeostasis. Phospho-mimic (T205E and S214E) and non-phospho-mimic (T205A and S214A) tau plasmids were constructed and each transfected into HEK293 cells. After 24 h, the expression of tau was confirmed by using antibody Tau-5 in the nuclear fraction of the T205E-transfected cells and the T205A-transfected cells (Fig. 2A, B). Furthermore, an increased phosphorylation level of CaMKIV at Ser196 was detected in the nuclear fraction of T205E-transfected cells (Fig. 2A, B). Then the cells were loaded with Fluo-3AM and the nuclear Ca2+ was measured [20]. The results showed that expression of T205E increased the nuclear [Ca2+] compared with T205A expression (Fig. 2C).
Phosphorylation of tau at Thr205 activates the nuclear Ca2+-CaMKIV pathway. A, B HEK293 cells were transfected with DsRed2-N1-phospho-mimic (T205E) or non-phospho-mimic (T205A) mutant tau plasmids for 24 h and then the nuclear fraction was prepared for Western blotting. An increased phosphorylation level of CaMKIV was detected in the nuclear fraction of T205E-transfected cells compared with T205A. C An increased basal [Ca2+] was detected in the nuclear fraction of T205E-transfected cells compared with T205A (>100 cells). Data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs T205A.
On the other hand, expression of S214E did not significantly change CaMKIV phosphorylation at Thr196 (Fig. 3A, B), with no significant change on the nuclear Ca2+ level compared with S214A expression (Fig. 3C). These data indicate that phosphorylation of tau at Thr205 but not at Ser214 activates nuclear Ca2+/CaMKIV signaling.
Phosphorylation of tau at Ser214 does not affect nuclear Ca2+ and CaMKIV activity. A, B HEK293 cells were transfected with DsRed2-N1-phospho-mimic (S214E) or non-phospho-mimic (S214A) mutant tau plasmids for 24 h and then the nuclear fraction was prepared for Western blotting. Compared with S214A, expression of S214E did not significantly change the levels of total CaMKIV and pCaMKIV in the nuclear fraction. C Compared with S214A, expression of S214E did not change the level of nuclear Ca2+. Data are expressed as mean ± SEM.
Okadaic Acid Induces Tau Hyperphosphorylation with Nuclear Ca2+/CaMKIV Upregulation
To further verify the relationship of tau hyperphosphorylation with activation of Ca2+ signaling, we transiently transfected HEK293 cells with human wild-type tau40 for 24 h and then treated them with OA (10 nmol/L) for 12 h to induce tau hyperphosphorylation [22]. The results showed that the phosphorylation level of tau further increased in the nuclear fraction after OA treatment (Fig. 4A, B). Simultaneously, the phosphorylation level of CaMKIV at Ser196 (Fig. 4C, D) and the [Ca2+] in the nuclear fraction (Fig. 4E) also significantly increased after OA treatment. These data further verify the correlation of tau hyperphosphorylation with nuclear Ca2+/CaMKIV upregulation.
OA induces tau hyperphosphorylation with nuclear Ca2+/CaMKIV upregulation. A, B HEK293 cells with stable expression of human wild-type tau (293Tau) were treated with OA (10 nmol/L) for 12 h, and the increased phosphorylation level of tau at Thr204, Ser214, Thr231, Ser396, and Ser404 was detected in the nuclear fraction by Western blotting (Tau5 probes total tau proteins). C, D OA treatment further increased pCaMKIV in the nuclear fraction. E OA treatment further increased nuclear [Ca2+]. Data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs OA.
Inhibition of Ca2+/CaMKIV Attenuates OA-Induced Nuclear Tau Hyperphosphorylation
To explore whether activation of nuclear Ca2+/CaMKIV signaling in turn affects tau phosphorylation, we transfected CAMBP4, a calmodulin-binding protein that disrupts formation of the Ca2+/calmodulin complex [23,24,25], into HEK293 cells that stably express human tau40. Inactivation of CaMKIV shown by the decreased pCaMKIV level in the nuclear fraction was confirmed at 24 h after transfection (Fig. 5A, B). Then, we treated the cells with OA or DMSO (as control) for 12 h, and measured the phosphorylation level of tau in the nuclear fraction. We found that expression of CaMBP4 attenuated the OA-induced nuclear tau hyperphosphorylation at Thr205, Ser214, Thr231, Ser262, Ser396, and Ser404, but inactivating CaMKIV by expressing CaMBP4 did not affect tau phosphorylation at the basal level (Fig. 5C, D). These data suggest that activation of CaMKIV contributes to the OA-induced tau hyperphosphorylation.
Inhibition of CaMKIV abolishes OA-induced tau hyperphosphorylation in the nuclear fraction. A, B HEK293 cells with stable expression of human wild-type tau (293Tau) were transfected with CaMBP4 plasmid or vector for 24 h, and a decreased level of pCaMKIV in the nuclear fraction of the CaMBP4-trasfected cells was detected by Western blotting (**P < 0.01 vs Vec). C, D HEK293 cells with stable expression of human wild-type tau were transfected with CaMBP4 or the vector for 24 h, and then treated with OA or DMSO for 12 h. CaMBP4 expression reduced the OA-induced tau hyperphosphorylation at Thr205, Ser214, Thr213, Ser396, and Ser404 in the nuclear fraction. Tau-5 probes total tau proteins. Data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs Vec + DMSO; # P < 0.05, ## P < 0.01 vs Vec + OA.
Discussion
The molecular mechanisms underlying Alzheimer’s neurodegeneration are not fully understood. In the past two decades, a large body of evidence has suggested that an important contributing factor is an imbalance in the homeostasis of Ca2+ signaling [21, 26], which promotes β-amyloid production [6, 7], tau hyperphosphorylation [8, 9], synaptic impairments [27], and neuronal death [8, 9]. It has also been reported that overproduction of β-amyloid triggers Ca2+ dyshomeostasis [11,12,13], and 3XTg-AD mice that express mutant PS1, APP, and tau proteins show an exaggerated Ca2+ signal in the endoplasmic reticulum [15]. However, it is not known whether intracellular tau hyperphosphorylation/accumulation also modulates Ca2+ homeostasis in AD. Using pharmacological and genetic approaches, we investigated the effects of tau phosphorylation on intracellular Ca2+ signaling, and found that the expression of phosphorylated tau caused Ca2+ elevation in both the cytoplasmic and nuclear fractions with the activation of nuclear CaMKIV, and the tau-triggered activation of CaMKIV in turn stimulated tau phosphorylation. Previous studies have been mainly focused either on the influence of Ca2+ on β-amyloid production/tau hyperphosphorylation [6,7,8] or on the impact of β-amyloid on Ca2+ [21]. Although recent studies have also suggested that the extracellular [18] or intracellular tau [28] can increase intracellular Ca2+, the finding presented in the current paper is the first direct evidence to identify the “calciumopathy” induced by site-specific tau phosphorylation.
Tau protein harbors >80 putative Ser/Thr-phosphorylation sites, among which at least 30 have been identified in AD brain samples [29,30,31]. Normally, tau is mainly located in neuronal axons. Upon abnormal hyperphosphorylation, tau proteins accumulate in the cell bodies where they form tangles. We found that a great proportion of tau was located in the nuclear fraction after transfection into HEK293 cells, in which the nuclear localization of the Thr205 and Ser214 phosphorylated tau was most significant. Therefore, we further studied the effects of Thr205 and Ser214 on the nuclear Ca2+ signaling by site-specific mutagenesis. The results showed that Thr205-phosphorylation of tau was critical in increasing the nuclear Ca2+ signaling, whereas phosphorylation at Ser214 did not significantly change the nuclear [Ca2+]. By expressing DsRed-tau441, we consistently found that the apparent molecular weight of the fusion protein was >100 kDa on 10% SDS-PAGE analysis, exceeding the expected molecular mass of the DsRed-tau fusion protein. We speculate that this upshift of fusion tau protein bands may be related to a hyperphosphorylation-induced conformational change.
The mechanisms underlying the pathological alterations elicited by Ca2+ overload are sophisticated. For instance, Ca2+ can enhance the polymerization rate and fibrillar stability of β-amyloid in a dynamic manner [32]. Ca2+-calcineurin signaling also affects the interaction of β-amyloid with receptor of advanced glycation end products (RAGEs), to disrupt tight junctions of the blood–brain barrier [33]. A Ca2+-regulated protease, calpain, stimulates the conversion of the cdk5 activator protein p35 into a C-terminal fragment p25 that causes deregulation of cdk5 activity [34]. Tau is a cytoskeletal protein with the conventionally recognized biological functions of promoting microtubule assembly and maintaining the stability of microtubules [35]. Recent studies have also shown that tau phosphorylation plays a crucial role in regulating cell viability [36]. It is currently not understood how the abnormal tau expression/phosphorylation affects intracellular Ca2+ homeostasis. Since a Ca2+ elevation was detected in both the cytoplasmic and nuclear fractions upon expression of tau, we speculate that the endoplasmic reticulum and mitochondria, organelles actively participating in modulating store-operated Ca2+ entry under physiological conditions and in AD model cells, must be involved [37]. Future studies may investigate whether the storage or release of Ca2+ from these organelles contributes to the tau-induced elevation of cytoplasmic and nuclear Ca2+.
A previous study showed that the CaMKK2-AMPK kinase pathway mediates the synaptotoxic effects of β-amyloid through tau phosphorylation [38]. Here, we demonstrated that tau phosphorylation per se also activated nuclear Ca2+/CaMKIV. Nuclear Ca2+ signaling regulates many important functions, including dendritic elaboration [39, 40], spine remodeling [41, 42], and activity-dependent regulation of synapse number, size, and function [43,44,45,46]. We found that activation of nuclear CaMKIV in turn aggravated OA-induced tau hyperphosphorylation at Thr205, Ser214, Thr231, Ser262, Ser396, and Ser404. We also found that blockade of CaMKIV did not change tau phosphorylation at the basal level, suggesting that upregulation of CaMKIV may only aggravate phosphorylation of tau molecules that were already in a hyperphosphorylated state. These data suggest that intracellular tau hyperphosphorylation and Ca2+ rise may elicit a self-perpetuating harmful loop to promote neurodegeneration, providing new insights into the abnormal tau-induced neurodegeneration in AD.
Taken together, in the present study we found that the expression of hyperphosphorylated tau elevates cytoplasmic and nuclear [Ca2+] and activates nuclear Ca2+/CaMKIV signaling, which in turn aggravates tau hyperphosphorylation.
References
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 1986, 261: 6084–6089.
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 1986, 83: 4913–4917.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 1985, 82: 4245–4249.
Wang JZ, Wang ZH, Tian Q. Tau hyperphosphorylation induces apoptotic escape and triggers neurodegeneration in Alzheimer’s disease. Neurosci Bull 2014, 30: 359–366.
Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J Biol Chem 2006, 281: 39907–39914.
Buxbaum JD, Ruefli AA, Parker CA, Cypess AM, Greengard P. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc Natl Acad Sci U S A 1994, 91: 4489–4493.
Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry 1994, 33: 4550–4561.
Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron 1990, 4: 105–117.
Mattson MP, Lovell MA, Ehmann WD, Markesbery WR. Comparison of the effects of elevated intracellular aluminum and calcium levels on neuronal survival and tau immunoreactivity. Brain Res 1993, 602: 21–31.
Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci 2008, 31: 454–463.
Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59: 214–225.
Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L. Mitochondrial Ca2 + overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One 2008, 3: e2718.
Shirwany NA, Payette D, Xie J, Guo Q. The amyloid beta ion channel hypothesis of Alzheimer’s disease. Neuropsychiatr Dis Treat 2007, 3: 597–612.
Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, et al. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 2008, 58: 871–883.
Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci 2006, 26: 5180–5189.
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A 2007, 104: 3591–3596.
Liu XA, Liao K, Liu R, Wang HH, Zhang Y, Zhang Q, et al. Tau dephosphorylation potentiates apoptosis by mechanisms involving a failed dephosphorylation/activation of Bcl-2. J Alzheimers Dis 2010, 19: 953–962.
Diaz-Hernandez M, Gomez-Ramos A, Rubio A, Gomez-Villafuertes R, Naranjo JR, Miras-Portugal MT, et al. Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J Biol Chem 2010, 285: 32539–32548.
Mazzucato M, Cozzi MR, Battiston M, Jandrot-Perrus M, Mongiat M, Marchese P, et al. Distinct spatio-temporal Ca2+ signaling elicited by integrin alpha2beta1 and glycoprotein VI under flow. Blood 2009, 114: 2793–2801.
Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 1999, 79: 1089–1125.
Garwood C, Faizullabhoy A, Wharton SB, Ince PG, Heath PR, Shaw PJ, et al. Calcium dysregulation in relation to Alzheimer-type pathology in the ageing brain. Neuropathol Appl Neurobiol 2013, 39: 788–799.
Zhu LQ, Zheng HY, Peng CX, Liu D, Li HL, Wang Q, et al. Protein phosphatase 2A facilitates axonogenesis by dephosphorylating CRMP2. J Neurosci 2010, 30: 3839–3848.
Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci 2005, 25: 4279–4287.
Wang J, Campos B, Jamieson GA, Jr., Kaetzel MA, Dedman JR. Functional elimination of calmodulin within the nucleus by targeted expression of an inhibitor peptide. J Biol Chem 1995, 270: 30245–30248.
Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, et al. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet 2009, 5: e1000604.
Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, Stutzmann GE. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J Neurosci 2012, 32: 8341–8353.
Nanou E, Scheuer T, Catterall WA. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to long-term potentiation and spatial learning. Proc Natl Acad Sci U S A 2016, 113: 13209–13214.
Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye J, et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci U S A 2016, 113: E3773–E3781.
d’Abramo C, Ricciarelli R, Pronzato MA, Davies P. Troglitazone, a peroxisome proliferator-activated receptor-gamma agonist, decreases tau phosphorylation in CHOtau4R cells. J Neurochem 2006, 98: 1068–1077.
Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem 1998, 71: 2465–2476.
Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience 1997, 78: 309–324.
Brannstrom K, Ohman A, Lindhagen-Persson M, Olofsson A. Ca(2+) enhances Abeta polymerization rate and fibrillar stability in a dynamic manner. Biochem J 2013, 450: 189–197.
Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci 2012, 32: 8845–8854.
Nath R, Davis M, Probert AW, Kupina NC, Ren X, Schielke GP, et al. Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem Biophys Res Commun 2000, 274: 16–21.
Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol 2008, 85: 148–175.
Sun XY, Tuo QZ, Liuyang ZY, Xie AJ, Feng XL, Yan X, et al. Extrasynaptic NMDA receptor-induced tau overexpression mediates neuronal death through suppressing survival signaling ERK phosphorylation. Cell Death Dis 2016, 7: e2449.
Ma T, Gong K, Yan Y, Song B, Zhang X, Gong Y. Mitochondrial modulation of store-operated Ca(2+) entry in model cells of Alzheimer’s disease. Biochem Biophys Res Commun 2012, 426: 196–202.
Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron 2013, 78: 94–108.
Mauceri D, Freitag HE, Oliveira AM, Bengtson CP, Bading H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron 2011, 71: 117–130.
Redmond L. Translating neuronal activity into dendrite elaboration: signaling to the nucleus. Neurosignals 2008, 16: 194–208.
Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron 2005, 45: 741–752.
Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 2002, 34: 999–1010.
Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol 2012, 970: 377–405.
Buchthal B, Lau D, Weiss U, Weislogel JM, Bading H. Nuclear calcium signaling controls methyl-CpG-binding protein 2 (MeCP2) phosphorylation on serine 421 following synaptic activity. J Biol Chem 2012, 287: 30967–30974.
Hagenston AM, Bading H. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harb Perspect Biol 2011, 3: a004564.
Schlumm F, Mauceri D, Freitag HE, Bading H. Nuclear calcium signaling regulates nuclear export of a subset of class IIa histone deacetylases following synaptic activity. J Biol Chem 2013, 288: 8074–8084.
Acknowledgements
We thank Dr. Hilmar Bading of the University of Heidelberg, Germany, for the kind gift of CaMBP4 plasmid. This work was supported by the National Natural Science Foundation of China (91632305) and the National Key Research and Development Program of China (2016YFC13058001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, YP., Ye, JW., Wang, X. et al. Tau-Induced Ca2+/Calmodulin-Dependent Protein Kinase-IV Activation Aggravates Nuclear Tau Hyperphosphorylation. Neurosci. Bull. 34, 261–269 (2018). https://doi.org/10.1007/s12264-017-0148-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0148-8