Abstract
Skin grafts can be used effectively to cover burn injuries. A critical element of this treatment is the adherence of the graft to the wound bed. Honey has been shown to increase the adherence of skin grafts to wound beds and have antibacterial and anti-inflammatory effects and increase healing rate of wounds. We therefore devised a clinical trial to determine the effect of honey on skin graft fixation in burn injuries. Sixty patients were included in this study (in 30 patients, graft was fixed with medical honey, and in 30 patients, it was fixed with dressing or suturing). All patients in two groups were evaluated for infection, graft loss, graft contraction, severity of pain, and need for re-operation. The most common cause of burn was kerosene. Honey significantly decreased infection rate on fifth day and reduced the patient pain. The mean hospital stay was shorter in honey group. Contraction of graft was significantly less in honey group. Honey has strong adhesive properties for skin graft fixation. Medical honey is a natural material, not synthetic. For this reason, we can advise the application of medical honey for the fixation of split thickness skin graft.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey has been used for medicinal purposes since ancient times [1]. It was used topically in the Ayurvedic medicine of 2,500 B.C., and the Egyptians, Greeks, and Romans used it as well [2]. Hippocrates prescribed honey for various indications, including the management of wounds and gastritis, and the wound-healing properties of honey were mentioned in the Qur’an and the Bible. After having played an important part in the traditional medicine for centuries, honey was subjected to laboratory and clinical investigations during the past few decades [3–5]. The most remarkable discovery was the antibacterial activity of honey that has been confirmed in numerous studies [4, 6]. This antibacterial activity is related to four properties of honey. First, honey is a supersaturated sugar solution, and this results in a strong interaction between the sugar molecules and water molecules. This “osmotic effect” leaves very few water molecules for growth support of microorganisms. The rate of inhibition of growth depends on the species of bacteria and the concentration of honey [11]. Second, the pH of honey is between 3.2 and 4.5, and this acidity is low enough to inhibit the growth of most microorganisms [7–10]. Hydrogen peroxide produced by the glucose oxidase is the third and probably the most important antibacterial component, although some authors believe the nonperoxide activity to be more important. Lastly, several phytochemical factors for antibacterial activity have been identified in honey. When applied on the tissue defect areas, the osmotic effect and acidity of honey decrease on dilution in recipient fluids—more so because these fluids are well buffered. Contrary to this decrease, hydrogen peroxide activity increases 2.5–50.0 times on dilution. In this dosage, hydrogen peroxide is still antiseptic without damage to the tissues [11, 12]. Likewise, most phytochemical factors withstand dilution in wound fluids. Overall, honey has a restraining influence on the growth of most bacteria, including some methicillin-resistant Staphylococcus aureus strains. This makes honey attractive for the prevention and treatment of infections in chronic wounds [11, 13], as well as for the treatment of acute wounds. Unlike most conventional local chemotherapeutics, honey does not lead to the development of antibiotic-resistant bacteria, and it may be used continuously. Although there is obvious interest in the application of honey in wound treatment, medical practitioners hesitate to use it because the mode of application often is messy [14–19]. Hence, honey that is to be used for medicinal purposes has to meet certain criteria. As such, it has to be free of residual herbicides, pesticides, heavy metals, and radioactivity. It has to be sterilized to prevent secondary wound infection. Furthermore, glucose oxidase in honey has to be controlled during processing to maintain the potency for infection prevention without doing harm to the wound tissues. Besides these primary conditions, the application of honey should be easy [20–23]. Based on these requirements, fixation by using honey was developed by impregnation of a modern synthetic material with decontaminated and regulated honey (HoneySoft; MediProf, Moerkapelle, The Netherlands). We performed a split thickness skin graft fixation to assess the clinical applicability of this dressing and present the outcome here.
Materials and Methods
Sixty patients were included in this study (in 30 patients, graft was fixed with medical honey, and in 30 patients, it was fixed with dressing or suturing). The Human Subjects Committee of the Tabriz University of Medical Sciences approved the study. Eligible participants (1) were III degree burns, (2) have no previous or comorbid diseases, (3) provided informed consent, and (4) have no sensitivity to honey and honey products.
All patients were treated as inpatients and went on to continue therapy after discharge. Follow-up period was average 17 months (10–24 months). All wounds were opened on the fifth day of grafting. First observation was done on the fifth day. This was followed by alternate day dressings with wet gauze. At the next dressing in honey group, topical application of neither antimicrobial pomad nor systemic antibiotics was used. In all patients of honey group, the decontaminated and regulated honey (HoneySoft; MediProf, Moerkapelle, The Netherlands) was used for split skin grafts fixations. All the honey products were sterilized with cobalt 60 gamma radiation [16]. Honey was applied on the defect areas drop by drop with a medicine dropper. The severity of pain was evaluated by visional analog system (VAS) on 3rd hour, 6th hour, 12th hour, 24th hour, and third day. Data was collected and analyzed statistically using SPSS 16 (SPSS Inc., Chicago, Illinois). Tests for normality were performed for all quantitative outcome variables. Independent and paired t-tests were used for comparison between pretreatment and post-treatment test results between groups and within groups, respectively. Chi square test was performed for qualitative variables. The level of significance was set at 0.05.
Results
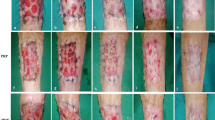
There were 39 (65 %, 21 in honey group, 18 in dressing or suturing group) women and 21 men (35 %, 9 in honey group, 12 in dressing or suturing group). The mean age of patients was 36.72 ± 13.44 in honey group and 39.43 ± 13.03 in dressing or suturing group. The mean percent of burn surface area was 23.66 ± 16.62 in honey group and 22.7 ± 17.66 in dressing or suturing group (Table 1). No significant differences were observed according to age, percentage of burns, etiology of burns, and sex. Areas burnt are shown in Fig 1. The most common cause of burn in two groups was flame burn due to kerosene.
During honey application for the fixation of split thickness skin graft, we observed the decrease of edema and wound exudates from the recipient area. No allergic reactions occurred during this study. Infection rate was shown in Fig 2. Infection rate was greater in dressing or suturing group at all times in the study, but only significant difference was on the fifth day. Variation of severity of pain was shown in Fig 3. Decrease of pain was faster and greater in honey group (P < 0.001). According to VAS, the amount of pain was significantly less on 3, 6, 12, and 24 h (P < 0.001). But difference in pain score at 72 h in two fixation group was not significant (P = 0.06). Contraction rate of graft in 1 month in two fixation groups was shown in Fig 4. Contraction of graft was significantly less in honey group (P < 0.001). There was no graft loss in two groups. The mean hospital stay was 7.53 ± 3.48 in honey group and 10.76 ± 4.05 in dressing or suturing group (P = 0.002).
Discussion
The success of skin grafting, or “take,” depends on the ability of the graft to receive nutrients and, subsequently, the vascular in-growth from the recipient bed. The grafts will survive transfer based on a defined sequence of events that culminates in vascular independence. These events are (1) serum imbibition—direct absorption of nutrients from recipient capillary beds that generally takes place in the first 24 h; (2) inosculation—the connecting of donor and recipient vessels that typically begins in the 24- to 72-h period (“kissing” capillaries); and (3) angiogenesis—vascular ingrowth of vessels from the recipient bed into the graft that starts after 72 h. Factors that interrupt this process—such as fluid collection under the graft, hematoma, seroma, pus, non-viable tissue from inadequate excision, or mechanical shear forces—will compromise the graft take. Since the graft is only held in place by the natural fibrin, there is little resistance to shear, so staples, sutures, fibrin glue, or complete immobilization are required. New blood vessels invade the graft by angiogenesis (as described above) and by a process of “inosculation,” where old capillaries in the wound bed are said to “hook up” with those in the graft. Any shear at this time affects collagen bridges form between the wound bed and the graft in the final phase of maturation. This process takes months, with the graft tending to get thicker and more vascular for 3–4 months before finally fading out over the ensuing months. This maturation process parallels the maturation phase described earlier and can take 1–2 years for completion.
The most common cause of graft failure is blood or serous fluid collection beneath the graft, raising the graft from the bed and preventing revascularization. Movement of the graft on the bed interrupts revascularization, and immobilization techniques include the use of bolster dressings on the face or trunk or splinting of the extremities. In our trial, honey fixed the graft to the burn wound bed and prevents from hematoma and seroma formation. This is due to adhesive properties of honey [6, 12, 20–23].
The second most common cause of graft failure is infection. The risk of infection can be minimized by careful preparation of the recipient site and early inspection of grafts applied to contaminated beds. Wounds that contain more than 105 organisms per gram of tissues will not support a skin graft. In addition, an infection at the graft donor site can convert a partial-thickness dermal loss into a full-thickness skin loss [6, 12, 20–23]. In our study, honey significantly decreased infection rate on the fifth day. This is due to antibacterial effects of honey.
In the current study, honey significantly reduced the patient pain. This is may be due to suppression of inflammation by honey [9, 23]. According to our search of literature, there is no study in reduction of pain by honey.
In our study, contraction of graft was significantly less in honey group. This is may be due to fixation properties, minimization of scarring, and stimulation of angiogenesis as well as tissue granulation and epithelium growth by honey [9, 23].
The revascularization of a skin graft depends on immobilization of the graft on the wound bed. In cases in which circular dressings are needed, the graft may be displaced under the dressing, leading to partial graft loss [24]. For this reason, several methods of graft fixation have been described. A common technique is to use stitches [25], but this is time consuming, and the stitches need to be removed after graft takes. Placing surgical drapes over the graft is another method [26], but these must be removed on the second or third day when the grafts are still not well vascularized, and this can lead to failure of the graft. For these reasons, fixation with skin staples was introduced as a fast and reliable method for graft fixation [27]; however, it is expensive and also stressful for the patient as the staples must be removed, a procedure which is very painful and also frightening, particularly for children. Another technique uses Steri-Strips W microporous tapes for fixation [24], but the surrounding skin must be completely healthy to retain the tapes. Other methods applying different materials such as cyanoacrylate [28] or amniotic membrane [29] have been designed. Particularly, most of the cyanoacrylate and other fibrin or tissue glues are synthetic materials, and they can cause inflammatory responses in an open wound, and we do not know about the long-term side effects of these synthetic elements in future. We would like to present a method of graft fixation pioneered at our burn center and found convenient for both patient and surgeon, using honey as fixator. This is particularly appropriate in cases involving children or burns of the extremities. Medical honey has been used in wound management, treatment of pressure ulcers, and infected leg ulcers, and it has been shown to be a very effective agent. We used medical honey first on skin graft fixation because of its advantages such as: its antibacterial activity (due to contained inhibin factor), its adhesive properties, and its anti-inflammatory effect (it can reduce the severity of pain) [30]. Especially, we observed that it has strong adhesive properties for skin graft fixation. Most important factor in this study is that our fixation material called medical honey is a natural material, not synthetic. For this reason, we can advise the application of medical honey for the fixation of split thickness skin graft.
Conclusion
Medical honey has been found to be a very effective agent in skin graft fixation because of its properties. This agent has prevented negative factors of skin graft loss such as infection and graft mobility. There is no need of skin graft suturing. Major advantages of this procedure are that it is time saving, easy in application, and cheap. But, it should be used sterile.
Therefore, we can recommend honey as a skin graft fixator, particularly for children and for burns involving the extremities.
References
Majno G (1975) The healing hand. Man and wound in the ancient world. Harvard University Press, Cambridge, MA, p 571
Ransome HM (1937) The sacred bee in ancient times and folklore. Allen & Unwin, London, p 308
Gunther RT (1959) The Greek herbal dioscorides. Hafner, New York [Goodyear J, trans.]
Zumla A, Lulat A (1989) Honey—a remedy rediscovered. J R Soc Med 82:384–5
Allen KL, Molan PC, Reid GM (1991) A survey of the antibacterial activity of some New Zealand honeys. J Pharm Pharmacol 43:817–22
Bergam A, Yanai J, Weis J et al (1983) Acceleration of wound healing by topical application of honey: an animal model. Am J Surg 145:374–6
Maghsoudi H, Moradi S. Comparison between topical honey and mafenide acetate in treatment of burn wounds. Annals of burns and fire disasters - vol. XXIV - n. 3 -September 2011
Obaseiki-Ebor EE, Afonya TCA (1983) Preliminary report on the antimicrobial activity of honey distillate. J Pharm Pharmacol 35:748–9
Efem SEE (1988) Clinical observations on the wound healing properties of honey. Br J Surg 75:679–81
Cooper R, Molan PC (1999) The use of honey as a antiseptic in managing Pseudomonas infection. J Wound Care 8:161–4
Molan PC (1999) The role of honey in the management of wounds. J Wound Care 8:415–8
Postmes T, Bosch MMC, Dutrieux R et al (1997) Speeding up the healing of burns with honey. An experimental study with histological assessment of wound biopsies. In: Mizrahi A, Lensky Y (eds) Bee products: properties, applications and apiotherapy. Plenum Press, New York, pp 27–37
Postmes T, Boogaard AE, van den Hazen M (1993) Honey for wounds, ulcers, and skin graft preservation. Lancet 341:756–7
Subrahmanyam M, Archan M, Pawar SG (2001) Antibacterial activity of honey on bacteria isolated from wounds. Ann Burns Fire Disasters 14:22–4
Postmes T, van den Boogaard A, Hazen M (1995) The sterilization of honey with cobalt 60 gamma radiation: a study of honey spiked with spores of Clostridium botulinum and Bacillus subtilis. Experientia 51:986–9
Molan PC, Allen KL (1996) The effect of gamma-irradiation on the antibacterial activity of honey. J Pharm Pharmacol 48:1206–9
Molan PC (1996) Honey as an antimicrobial agent. In: Mizrahi A, Lensky Y (eds) Bee products. Plenum Press, New York, pp 27–37
Bogdanov S (1996) Non-peroxide antimicrobial activity of honey. In: Mizrahi A, Lensky Y (eds) Bee products. Plenum Press, New York, pp 39–47
Tovey FI (2000) Honey and sugar as a dressing for wounds and ulcers. Trop Doct 30:1, Editorial
Snowdon JA, Cliver DO (1996) Micro-organisms in honey. Int J Food Microbiol 31:1–26
Birch J, Branemark PI (1969) The vascularization of a free full thickness skin graft: a vital microscopic study. Scand J Plast Surg 3:1
Converse JM, Rapaport FT (1956) The vascularization of skin autografts and homografts: an experimental study in man. Ann Surg 143:306
Haller JA, Billingham RE (1967) Studies of the origin of vasculature in free skin grafts. Ann Surg 166:896
Rudge R (2007) An easy and safe method of split-thickness skin graft fixation. Burns 33:1074–5
Mah E, Morrison WA (2006) A stitch in time (save mines). Plast Reconstr Surg 117:2535–6
Yenidünya MO, Özdengill E, Emsen I (2000) Split-thickness skin graft fixation with surgical drape. Plast Reconstr Surg 106:1429–30
Alexander G, Al-Rasheed AA (2005) Skin stapler removal by artery forceps: a hazardous practice? Burns 31:116
Adler N, Nachumovsky S, Meshulam-Derazon S, Ad-El D (2007) Skin graft fixation with cyanoacrylate tissue adhesive in burn patients. Burns 33:803
Mohammadi A, Johari HG (2008) Amniotic membrane: a skin graft fixator convenient for both patient and surgeon. Burns 34:1051–2
Dunford C (2005) The use of honey-derived dressing to promote effective wound management. Prof Nurse 20(8):35–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maghsoudi, H., Moradi, S. Honey: A Skin Graft Fixator Convenient for Both Patient and Surgeon. Indian J Surg 77 (Suppl 3), 863–867 (2015). https://doi.org/10.1007/s12262-014-1039-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-014-1039-0