Abstract
The objective of our study was to evaluate the role of splenic artery embolization (SAE) in the management of traumatic splenic injuries. From September 2008 to September 2010, a total of 67 patients underwent nonoperative management (NOM) for blunt splenic injuries. Twenty-two patients were excluded from the study because of associated significant other organ injuries. Twenty-five patients underwent SAE followed by NOM (group A) and 20 patients underwent standard NOM (group B). Improvement in clinical and laboratory parameters during hospital stay were compared between two groups using Chi-square test and Mann–Whitney test. SAE was always technically feasible. The mean length of the total hospital stay was lower in the group A patients (5.4 vs. 6.6 day, [P = 0.050]). There was significant increase in hemoglobin and hematocrit levels and systolic blood pressure (SBP) in group A patients after SAE, whereas in group B patients there was decrease in hemoglobin and hematocrit levels and only slight increase in SBP (pre- and early posttreatment relative change in hemoglobin [P = 0.002], hematocrit [P = 0.001], and SBP [P = 0.017]). Secondary splenectomy rate was lower in group A (4 % [1/25] vs. 15 % [3/20] [P = 0.309]). No procedure-related complications were encountered during the hospital stay and follow-up. Minor complications of pleural effusion, fever, pain, and insignificant splenic infarct noted in 9 (36 %) patients. SAE is a technically feasible, safe, and effective method in the management of splenic injuries. Use of SAE as an adjunct to NOM of splenic injuries results improvement in hemoglobin, hematocrit levels, and SBP. SAE also reduces secondary splenectomy rate and hospital stay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past 3 decades, there have been major changes in the management of traumatic splenic injuries. Increased susceptibility of patients to infections after splenectomy—in particular, the risk of fatal postsplenectomy sepsis—has motivated trauma physicians towards splenic preservation procedures [1]. As a result, in most trauma care centres, non-operative management (NOM) is now believed to be the treatment of choice in hemodynamically stable patients regardless of the severity of the injury. However, this standard of practice is based only on prospective non-comparative studies, retrospective studies with control subjects, or retrospective analyses, for which there are wide variations in the reported rates (2–52 %) of NOM failure. Some investigators have shown increased risk of failed NOM for injuries of higher CT grade or if the CT reveals active contrast extravasation [2–8]. Judicious use of SAE as an adjunct for patients with blunt splenic injury avoids unnecessary surgery and expands the scope of NOM [9].
Purpose of Study
The purpose of our study was to prospectively evaluate the role of splenic artery embolization (SAE) in the non-surgical management of traumatic splenic injuries.
Patients and Methods
Patients
This prospective study was conducted after obtaining ethical clearance from our institutional ethical committee. From September 2008 to September 2010, a total of 67 patients underwent NOM for blunt splenic injuries. Twenty-two patients were excluded from the study because of associated significant other organ injuries. Twenty-five patients underwent SAE followed by NOM (group A) and 20 patients underwent standard NOM (group B).
Imaging
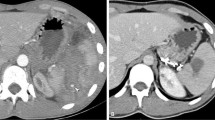
Contrast enhanced computed tomography (CECT) was performed on a 40 slice MDCT scanner (Somatom Sensation, Siemens, Erlangen, Germany). All splenic injuries were graded according to the AAST organ injury scale (Table 1) [10] (Fig. 1).
Management
After the CECT scan, a team was made (including both radiologists and surgeons) to decide the choice between SAE and standard NOM. CECT findings (grade of splenic injury, vascular injury, degree of hemoperitoneum), clinical and laboratory parameters (systolic blood pressure [SBP] at presentation, hemoglobin and hematocrit, RBC transfusion requirement) were taken into consideration for making a final decision. The patients who had relatively higher AAST grade of splenic injury (grades IV and V) and evidence of vascular injury on CECT scan were preferentially taken for SAE.
Splenic Artery Embolization
SAE was performed in the angiography suite. Vascular access was made through the right femoral artery. After securing vascular access, anteroposterior celiac and selective splenic arteriogram was obtained. Main splenic artery was selectively cannulated using 5-French catheter; tip of the catheter placed at least beyond the origin of the dorsal pancreatic artery. Whenever necessary, a coaxial 3-French microcatheter (SP, Terumo) was used for cannulation. Embolization was performed using 0.035-inch coils or 0.018-inch microcoils (Cook) in the splenic artery. In case of proximal SAE, the coils or microcoils were placed just distal to dorsal pancreatic artery (Fig. 2). Super selective distal embolization was performed with coils or microcoils as distally as possible, in a small arterial branch that supplies the segment in which extravasation/pseudoaneurysm was noted. Gel foam was also used in selected cases after placement of coils. The embolization end point was the complete absence of opacification of the splenic artery distal to the coils. Any complication occurring during the procedure was recorded. Patients were kept on conservative management after SAE.
Same patient as Fig. 1. Selective splenic arteriogram a showing multiple area of active contrast extravasation (arrows) in the lower pole. Proximal splenic artery embolization was done and post embolization DSA image b shows near complete absence of opacification of the splenic artery distal to the coil
Group B patients were kept on conservative management without surgical or angiographic intervention if they remained hemodynamically stable.
Hospital Stay
For both the groups of patients, notes were made for the amount of blood transfusion and total intravenous fluid required, hemoglobin and hematocrit levels, need for surgical exploration, duration of ICU and hospital stay, and mortality if any. In the SAE group patients, treatment-related complications were also noted.
Follow-up
The patients who underwent SAE were followed up after 1 month of discharge from hospital. During their visit, detailed clinical history, including pain, fever, and any infection, was taken. Repeat CECT scan was done in all the patients (Fig. 3). Notes were made for resolution of lesions, splenic infarction, collection, and abscess. A well-defined fluid density cystic lesion seen in the region of previous injury was considered resolving hematoma/collection. For resolution of lesions, imaging response was classified into three categories—complete, near complete, and partial response (Table 2). Percentage of imaging resolution was calculated by comparing the injuries seen on baseline images and follow-up CECT scan. Splenic infarct was also classified into two groups—significant infarct (involving >25 % of splenic volume) and insignificant infarct (involving <25 % of splenic volume).
Statistical Analysis
Statistical analysis was performed by our institution statistician using standard software. AAST CT grade, presence of active contrast extravasation, grade of hemoperitoneum, total hospital and ICU stay, total intravenous fluid, and RBC transfusion requirement were compared using the Student’s t test or Chi-square test in patients undergoing SAE followed by NOM and standard NOM. Pretreatment and posttreatment relative change in hemoglobin level, hematocrit level, and SBP were compared using Mann–Whitney test. A P value of 0.05 or less was considered significant. The end point of the statistical analysis was the comparison of the secondary splenectomy rate in the two groups of patients. Secondary splenectomy was defined as a splenectomy performed after an initial decision of NOM had been made.
Results
Group A consisted of 25 patients (21 male and 4 female, mean age 24.64 with range 5–60, mean ISS 19.2 with range 9–43, mean AAST grade 3.32 with range 2–5) with splenic injuries who were managed by SAE followed by NOM, and group B consisted of 20 patients (17 male and 3 female, mean age 24.95 with range 13–45, mean ISS 14.9 with range 10–27, mean AAST grade 3 with range 3) who were treated by standard NOM. There was a significant difference in group A when compared with group B with respect to the active contrast extravasation (32 % [8/32] vs. 0 % [0/20], respectively [P = 0.01]) as patients with evidence of vascular injury on CECT scan were preferentially taken for SAE. SAE was always feasible without immediate procedure-related complications.
On comparing the two groups (Table 3), the total intravascular fluid and RBC transfusion requirements (during hospital stay) were not statistically significantly different despite a trend toward lower requirements in group A. The mean length of ICU stay was lower in the group A patients (0.72 vs. 0.85 day, P = 0.797). The mean length of total hospital stay was also lower in the group A patients (5.4 vs. 6.6 days, P = 0.050). There was a significant increase in hemoglobin and hematocrit levels and SBP in group A patients, whereas in group B patients there was a decrease in hemoglobin, hematocrit levels and only a slight increase in SBP (pre- and posttreatment relative changes in hemoglobin [P = 0.002], hematocrit [P = 0.001], and SBP [P = 0.017]). Twenty-four patients (96 %) of group A and 17 patients (85 %) of group B were successfully managed and discharged from the hospital without requirement of exploratory laparotomy/surgery. The secondary splenectomy rate was lower in the group A (4 % [1/25] vs. 15 % [3/20], respectively [P = 0.309]).
Complications
During hospital stay—None of the 25 patients who underwent SAE had any major complications. Minor complications of pleural effusions, fever, pain, and insignificant infarct occurred in 9 (36 %) patients.
Follow-up—Of the 25 patients of group A, only 2 patients gave history of pain and fever. Abdominal CT examination showed large perisplenic fluid collection in both the patients. Aspiration was performed under USG guidance, the fluid was macroscopically typical of hematoma and the culture was negative. Rest of the 23 patients were asymptomatic. Insignificant infarct was seen in 7 patients. No patient had significant infarct. On follow-up CT scan, 3 (12 %) patients showed complete resolution of injury, whereas 17 (68 %) patients showed near complete resolution.
Discussion
The spleen is the most commonly injured organ in blunt abdominal trauma in both adults and children. NOM is the current standard of practice for hemodynamically stable patients. However, simple observation alone has been reported to have a failure rate as high as 34 %; the rate is even higher among patients with high-grade splenic injuries [1, 11]. With the help of SAE, success rates of more than 80 % have also been described for high-grade splenic injuries.
SAE was easily performed and always technically feasible without technique-related complications. In all the patients, the time gap between initial CT scan and SAE was less than 12 h. Of the total 25patients, 24 were hemodynamically stable. One patient who was hemodynamically unstable (SBP, 86 mm Hg; heart rate, 104 per minute) underwent SAE successfully, which allowed the hemodynamic status to normalize soon after embolization. This case, if confirmed in larger prospective series, can prove the efficacy of SAE even in hemodynamic unstable patients where spleen is the only source of bleed on imaging. Hagiwara et al. [12] have suggested that SAE can be used routinely in hypotensive patients who transiently respond to fluid administration.
Unlike Haan et al. [9], we chose to embolize patients even if the initial angiography examination was negative for active contrast extravasation, arteriovenous fistula, or pseudoaneurysm. Our choice may be supported by our splenectomy rate, which is lower than previous series that performed embolization only in cases of angiographic vascular abnormalities. Moreover, findings reported by Haan et al. [9] of 8 % continued bleeding requiring laparotomy and 3.5 % bleeding requiring secondary splenic embolization among their patients with negative angiography may support our choice.
As Sclafani et al. [13] have shown, we chose proximal SAE rather than selective distal embolization. In our study, proximal splenic artery embolization was done in 23 (92 %) patients. The end point of proximal embolization, as in surgical artery ligation, is to reduce the splenic bleeding by decreasing blood flow in the main splenic trunk [14] SAE allows reduction in the intrasplenic arterial blood pressure, a condition that may help clots to organize and the spleen to heal [15]. Moreover, it is theorized that proximal embolization allows the spleen to remain, at least partially, perfused by collaterals, thus, limiting the risk of splenic infarction [16]. A series of CT examinations of embolized spleens has shown proximal embolization to be associated with less frequent and smaller infarcts than distal embolization [17]. Consequently, splenic function impairment should be less marked in cases of proximal embolization, but this need to be proven in larger studies.
Efficacy of Splenic Artery Embolization
Despite significantly more severe splenic injuries in group A, that is, those who had a higher injury grade, higher rate of active contrast extravasation was found; during the hospital stay, these patients responded better than group B patients. The total RBC transfusion and total intravenous fluid requirements were not significantly different despite a trend toward lower requirements in the group A patients. This result is comparable to the study by Bessoud et al. [1] in which they reported a similar trend toward lower requirement of RBC transfusion in SAE group (1 vs. 1.7 units, respectively). The mean length of ICU stay was lower in the group A patients, though not statistically significant. The mean length of the total hospital stay was also lower in the group A patients (P = 0.050).
There was a significant increase in mean SBP, hemoglobin, hematocrit levels in group A patients, whereas in group B patients there was decrease in mean hemoglobin and hematocrit levels and only a slight increase in the mean SBP. This may be due to better hemostasis, facilitation of clot formation, and the early and rapid healing achieved by SAE. Whereas in group B patients, there may be continuous ongoing bleeding from the injured spleen leading to delayed and slow healing of injured spleen.
Outcome of Splenic Artery Embolization
In our study, trend towards lower failure rate was depicted in the SAE group (4 % [1/25] vs. 15 % [3/20], respectively). The only secondary splenectomy in group A was performed in a patient (ISS score - 35, grade V splenic injury with active contrast extravasation). On the third day after admission, the patient experienced sudden hemodynamic instability and decrease in hemoglobin and hematocrit levels. Exploratory laparotomy showed continued splenic hemorrhage, and splenectomy was done.
Compared with group A, secondary splenectomy rate was higher in group B ([3/20]15 %). All the three patients had grade III splenic injury without active contrast extravasation (ISS score, 10, 12, and 18), but developed sudden hemodynamic instability within 5 days of hospital admission (time span, 12–96 h). One patient died on the fourth day of admission due to septicemic shock. The overall success rate of NOM with the use of SAE ranges from 86 % to 100 % [1, 9, 12, 18–26], with most studies reporting success rates greater than 90 %. In King’s County Hospital study [13], 172 patients with blunt splenic injury were enrolled and 60 patients needed embolization because of evidence of angiographic arterial extravasation. The authors reported successful outcome in 93 % of the patients after embolization (proximal, distal, or both). A systematic review and meta-analysis by Schnüriger et al. [27] reported 10.2 % overall failure rate of angioembolization. Haan et al. [11] reported their experience with splenic embolization in 40 of 126 patients with angiographic evidence of vascular injury (e.g., arteriovenous fistula, pseudoaneurysm, or contrast extravasation). Successful outcome occurred in 92 % of patients, with 5 % of them requiring re-embolization. Recently, Mayglothling et al. [28] reviewed 97 adolescent patients of blunt splenic injury and they reported100 % splenic salvage rate in negative angiography group, with over all 87 % salvage rate in patients who underwent SAE. They concluded that SAE may be a valuable adjunct in adolescent blunt splenic injury, especially in higher-grade injuries or with evidence of splenic vascular injury on CECT. Bessoud et al. [1] reported 97.3 % successful outcome with SAE (36/37 patients). These series, and ours, showed that SAE is feasible, safe, and effective even in CT-grade injuries of III or higher or in cases of contrast extravasation (92 % in the study by Killeen et al. [17], 96 % in our study). Moreover, embolization has been shown to be useful in extending the type and number of splenic injuries that can be managed nonoperatively, as evidenced by the high success rates of NOM (>90 %) for AAST grade III–V injuries.
Safety of Splenic Artery Embolization
Procedural, early, and delayed complications of SAE were very few. Only minor complications including pleural effusion, pain, fever, and insignificant infarct were noted in 9 (36 %) patients. Minor complications occur more often after distal embolization. This is primarily explained by the higher rate of segmental infarctions by distal embolization [27]. Coil migration and splenic artery dissections have been described in the previous series [10, 11, 26], but we did not encounter any complication at the time of angiography. Our results are comparable with the study done by Bessoud et al. [1] where they reported no major complication. Haan et al. [19] in their series reported a 3 % splenic abscess rate (4/140) after embolization (proximal, distal, or combined). Duchesne et al. [29] reported higher incident of acute respiratory distress syndrome after SAE.
Follow-up
At 1-month follow-up we found 7 (25.9 %) patients with insignificant infarct. Haan et al. [19] reported significant infarction rate of 21 % that finally had limited short-term clinical implications because the infarcts were associated with minimal symptoms. Lower frequency of infarction in our study may be attributed to proximal SAE, which we performed in 92 % of our cases. Theoretically, proximal SAE may cause less impairment in splenic function than selective distal embolization because it allows the spleen to remain at least partially perfused by collaterals.
We also evaluated percentage resolution of lesion during the follow-up. Seventeen patients showed near complete resolution (50–90 %), 7 patients showed partial response (<50 %), and 3 patients (10.5 %) showed complete response (90–100 %). To the best of our knowledge, no previous study has evaluated resolution of splenic injury during follow-up.
Limitations of the Study
First, it is a nonrandomized study; thus, the choice between NOM with SAE and standard NOM was made by the team of several attending surgeons and radiologists at the time of each patient’s admission. Second, sample size was small; only 25 patients were in the SAE group. Lastly, follow-up period was short. Potential long-term complications resulting from SAE are unknown in the trauma population. No long-term follow-up has been described in the literature to date with regard to trauma patients. The status of splenic immunologic function after SAE is unknown. This area also requires further study.
Conclusion
SAE is a technically feasible, safe, and effective method in the management of splenic injuries. The use of SAE as an adjunct to NOM of splenic injuries results in improvement in the hemoglobin and hematocrit levels and SBP. SAE also reduces secondary splenectomy rate and hospital stay.
References
Bessoud B, Denys A, Calmes JM et al (2006) Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? AJR Am J Roentgenol 186:779–785
Pachter H, Guth A, Hofstetter S, Spencer F (1998) Changing patterns in the management of splenic trauma: the impact of nonoperative management. Ann Surg 227:708–717
Velmahos G, Chan L, Kamel E et al (2000) Nonoperative management of splenic injuries: have we gone too far? Arch Surg 135:674–679
Brasel K, DeLisle C, Olson C, Borgstrom D et al (1998) Splenic injury: trends in evaluation and management. J Trauma 44:283–286
Wei B, Hemmila MR, Arbabi S et al (2010) Angioembolization reduces operative intervention for blunt splenic injury. World J Surg 34(11):2745–2751
Federle M, Courcoulas A, Powell M, Ferris J, Peitzman A (1998) Blunt splenic injury in adults: clinical and CT criteria for management, with emphasis on active extravasation. Radiology 206:137–142
Velmahos G, Toutouzas K, Radin R, Cjan L, Demetriades D (2003) Nonoperative treatment of blunt injury to solid abdominal organs. J Trauma 138:844–851
Shapiro M, Krausz C, Durham R, Mazuski J (1999) Overuse of splenic scoring and computed tomographic scans. J Trauma 47:651–658
Haan J, Scott J, Boyd-Kranis R, Ho S, Kramer M, Scalea T (2001) Admission angiography for blunt splenic injury: advantages and pitfalls. J Trauma 51:1161–1165
Moore E, Cogbill T, Jurkovich G, Shackford S, Malangoni M, Champion H (1995) Organ injury scaling: spleen and liver (1994 revision). J Trauma 38:323–324
Sclafani S, Weisberg A, Scalea T, Phillips T, Duncan A (1991) Blunt splenic injuries: nonsurgical treatment with CT, arteriography, and transcatheter arterial embolization of the splenic artery. Radiology 181:189–196
Hagiwara A, Yukioka T, Ohta S, Nitatori T, Matsuda H, Shimazaki S (1996) Nonsurgical management of patients with blunt splenic injury: efficacy of transcatheter arterial embolization. AJR 167:159–166
Sclafani S, Shaftan G, Scalea T et al (1995) Nonoperative salvage of computed tomography–diagnosed splenic injuries: utilization of angiography for triage and embolization for hemostasis. J Trauma 39:818–825
Keramidas D, Buyukunal C, Senyuz O, Dolatzas T (1991) Splenic artery ligation: a ten-year experience in the treatment of selected cases of splenic injuries in children. Jpn J Surg 21:172–177
Bessoud B, Denys A (2004) Main splenic artery embolization using coils in blunt splenic injuries: effects on the intrasplenic blood pressure. Eur Radiol 14:1718–1719
Keramidas D, Kelekis D, Dolatzas T, Aivazoglou T, Voyatzis N (1984) The collateral arterial network of the spleen following ligation of the splenic artery in traumatic rupture of the spleen: an arteriographic study. Z Kinderchir 39:50–51
Killeen K, Shanmuganathan K, Boyd-Kranis R, Scalea T, Mirvis S (2001) CT findings after embolization for blunt splenic trauma. J Vasc Interv Radiol 12:209–214
Haan J, Ilahi O, Kramer M, Scalea T, Myers J (2003) Protocol-driven nonoperative management in patients with blunt splenic trauma and minimal associated injury decreases length of stay. J Trauma 55:317–321
Haan J, Biffl W, Knudson M et al (2004) Splenic embolization revisited: a multicenter review. J Trauma 56:542–547
Haan JM, Bochicchio GV, Kramer N et al (2005) Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma 58:492–498
Davis KA, Fabian TC, Croce MA et al (1998) Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma 44:1008–1013
Liu PP, Lee WC, Cheng YF et al (2004) Use of splenic artery embolization as an adjunct to nonsurgical management of blunt splenic injury. J Trauma 56:768–772, discussion 773
Dent D, Alsabrook G, Erickson BA et al (2004) Blunt splenic injuries: high nonoperative management rate can be achieved with selective embolization. J Trauma 56:1063
Smith HE, Biffl WL, Majercik SD et al (2006) Splenic artery embolization: have we gone too far? J Trauma 61:541–544, discussion 545–546
Peitzman A, Heil B, Rivera L et al (2000) Blunt splenic injury in adults: multi-institutional Study of the Eastern Association for the Surgery of Trauma. J Trauma 49:177–187
Raikhlin A, Baerlocher MO, Asch MR et al (2008) Imaging and transcatheter arterial embolization for traumatic splenic injuries: review of the literature. J Can Chir 51(6):464–472
Schnüriger B, Inaba K, Konstantinidis A et al (2011) Outcomes of proximal versus distal splenic artery embolization after trauma: a systematic review and meta-analysis. J Trauma 70(1):252–260
Mayglothling JA, Haan JM, Scalea TM et al (2011) Blunt splenic injuries in the adolescent trauma population: the role of angiography and embolization. J Emerg Med 41(1):21–28, Epub 2009 Jan 31
Duchesne JC, Simmons JD, Schmieg RE Jr, McSwain NE Jr et al (2008) Proximal splenic angioembolization does not improve outcomes in treating blunt splenic injuries compared with splenectomy: a cohort analysis. J Trauma 65(6):1346–1353
Funding
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parihar, M.L., Kumar, A., Gamanagatti, S. et al. Role of Splenic Artery Embolization in Management of Traumatic Splenic Injuries: A Prospective Study. Indian J Surg 75, 361–367 (2013). https://doi.org/10.1007/s12262-012-0505-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-012-0505-9