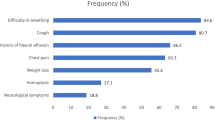
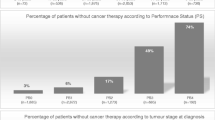
Abstract
Background
In Bulgaria, lung cancer incidence and mortality rates are rising in both men and women. The study aims to present a picture of lung cancer diagnosis and treatment process and to identify factors affecting survival in advanced lung cancer patients (LCP) treated with systemic therapy.
Patients and methods
Data from LCP admitted at the Medical Oncology Department were retrospectively collected from electronic and hard-paper database for a 10-year period (January 2005–2015). The test for frequencies was used to describe parameters. Kaplan–Mayer estimates with two-sided 95 % confidence interval (CI) were calculated for clinical and laboratory prognostic factors in advanced LCP who received medical therapy. Cox-regression model was used for the evaluation of significant prognostic factors’ impact on survival. Statistical analyses were performed using SPSS 9.0 software.
Results
Data from 204 LCP were retrospectively analyzed for a period between January 2005 and January 2015. LCP characteristics were as follows: median age 60.2 years (range 28–78), male/female (M/F) 159/55, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1/> 2 136/63, no comorbidity/with comorbidity 36/168, smoking history never/ever 37/149; 86.3 % LCP had stage IIIB and IV disease. In 43.2 % of LCP with negative or not performed fibrobronchoscopy (FBS), pathological diagnosis was obtained by other methods including surgical. Before treatment, patients obtained morphological verification of lung cancer—98.5 % histologically versus 1.5 % cytologically. The prevalent histo-type found was adenocarcionoma. In all, 88.7 % of LCP received systemic medical treatment while 11.3 %—palliative care. Only 2.5 % received adjuvant and 2.0 % neoadjuvant chemotherapy, while 84.2 % received medical therapy for advanced disease. In the last group, prognostic value for survival according to Cox-regression model reached ECOG performance status (PS) (HR 0.4; CI 0.23–0.63; p < 0.0001); weight loss (WL) prior to diagnosis (HR 2.03; CI 1.22–3.37; p < 0.01); number of treatment lines (HR 1.65; CI 1.2–2.67; p < 0.05); and platelet to lymphocyte ratio (PLR) (HR 0.48;CI 0.24–0.95; p < 0.001).
Conclusions
Lung cancer diagnosis and treatment in Bulgaria are managed according to the European guidelines. ECOG PS and WL are known prognostic factors in advanced LCP. Our results support prognostic impact of PLR on survival. However, the confirmation of this finding needs further prospective validation. The fact that the number of treatment lines impacts survival point out the importance of “continuum of care” concept in advanced LCP, treated with medical therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths in both sexes, with increasing incidence and mortality worldwide. In Europe, crude incidence rates are between 2/100,000–80/100,000 and 1/100,000–39/100,000 for men and women, respectively [1,2]. In Bulgaria, crude incidence/mortality rate for men is 86.2/74.8 per 100,000 and for women is 16.1/14.6 per 100,000 [3]. In 2010, Bulgaria prevalence crude data for lung cancer is 138/100,000 with distribution between man/women 207.2/73.4 [3].
The prognosis for patients with lung cancer has improved recently, mainly because of development of histology-directed, platinum-based systemic chemotherapy. In the past years, major molecular biology discoveries defined the role of targeted therapy in lung cancer patients (LCP). But the prognosis of patients remain still poor, with a median overall survival (OS) approaching 15 months [4, 5].
Multidisciplinary approach has also a significant impact on lung cancer patients’ survival [4]. Scientific approaches have been focused on an identification of prognostic factors enabling tailoring the treatment of advanced LCP for decades [6, 7]. In routine clinical practice, some prognostic models of baseline clinical and biological factors had proved to be efficient [8–10].
In Bulgaria, there are 21 state and 6 private facilities where patients with lung cancer receive medical therapy and in some of which patients can also undergo radiotherapy. The diagnostic work-up of lung cancer patients are performed in the pulmonology or thoracic surgery departments which are located usually in different hospitals from those where medical or/and radiotherapy is performed. There is National Hospital for Pulmonary Disease, where diagnosis and surgical treatment of large amount of lung cancer patients is made. There is no Lung Cancer Research Group in the country.
Our department of medical oncology takes a part of Military Medical Academy (MMA). There are also Pulmonology and Thoracic Surgery Departments. It is a small department with 10 beds and day hospital. Till 2012, the unit was permitted to admit only military cancer patients, but since 2012 the treatment of civil cancer patients has started. Here, we report our experience with lung cancer treatment management. Some data regarding prognostic for survival factors are also reported.
Material and methods
Data collection
Data from lung cancer patients admitted at the Medical Oncology Department were retrospectively collected from electronic and hard-paper database for a 10-year period (January 2005–2015). The following demographic and clinical parameters were collected: age; gender; Eastern Cooperative Oncology Group (ECOG) performance status (PS); smoking history; weight loss in previous 6 months (> or < 5 %); stage; comorbidity; diagnostic work-up procedure (fibrobronchoscopy (FBS), surgical diagnosis); number of metastatic sites in advanced LCP; histological subtypes; immunohistochemistry (IHC); malignancy grade and Ki67 %; thyroid transcription factor-1(TTF1) expression and epidermal growth factor receptor (EGFR) mutation status; the type of surgical treatment; radiotherapy performed; medical therapy, supportive and palliative care performed; the time from the first symptoms to diagnosis (symptom lead time (SLT)); the time from the first symptoms to the medical treatment; additionally in advanced LCP started primary medical treatment at our department with at least once cycle performed were collected: baseline values of hemoglobin (Hb) (g/L), lactate dehydrogenase (LDH) (U/L), albumin (g/L) mean corpuscular value (MCV) (f)L, white blood cells (WBC) count (109/L), platelets count (109/L), neutrophil to lymphocyte ration (NLR), platelet to lymphocyte ratio (PLR), type of medical therapy, and number of treatment lines.
Patients treated before 2010 were restaged according to the actual American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM (Tumor Node Metastases classification) staging system [12, 13].
Survival analyses
Overall survival was defined as the time from the pathological diagnosis to patients’ death or last follow-up. Survival data was updated in January 2015. For patients who were loss of follow-up, survival data was measured by the last time they have been seen. Patients diagnosed previously with cancer different from lung cancer were excluded from survival analysis with exception of patients with noninvasive bladder cancer. In prognostic to survival evaluations and in regression model were enrolled for analyses only advanced LCP who received at least one cycle first line therapy at our department.
Statistical analysis
The test for frequencies was used to describe demographic, clinical, morphological treatment and laboratory parameters. Kaplan–Meier event rates at various time points CIs were summarized and Kaplan–Meier estimates with two-sided 95 % confidence interval (CI) were calculated for clinical and laboratory prognostic factors in advanced LCP who received medical therapy. Cox-regression model was used for the evaluation of significant prognostic factors’ impact on survival.
Statistical analysis were performed using SPSS 9.0 software (SPSS Inc Chicago, IL). All statistical measurements were two-sided and p-value of < 0.05 was considered statistically significant.
Results
Patients’ demographic and clinical characteristics
Between January 2005 and January 2015, data from 204 lung cancer patients admitted at our department were retrospectively analyzed. Their characteristics are summarized in Table 1. In all, 159 patients (78 %) were men and 55 women (22 %). Median age of patients was 60.2 years (range 28–78).
A total of 136 patients (66.7 %) had ECOG PS 0–1. Ninety (44 %) patients had weight loss of more than 5 % in the 6 months prior to diagnosis. Regarding comorbidity, only 36 (17.6 %) patients had no comorbidity at lung cancer diagnosis. For the rest 168 patients with comorbidity, the most frequent concomitant diseases were arterial hypertension (75.6 %), followed by ischemic heart disease (23.2 %) and chronic pulmonary disease (16.7 %). Prior to the lung cancer diagnosis 14 patients had been diagnosed previously with another type of cancer.
Data from smoking history revealed only 37 (18.1 %) LCP who had never smoked in their life, in contrast to the 149 patients (73.1 %) who had smoked in their lifetime, current and exsmokers.
At diagnosis, 158 (77.5 %) patients had stage IV cancer. Prevalence of patients with one metastatic site (38.7 %) among all metastatic patients (at the time of diagnosis plus recurrent metastatic disease) was noticed (Table 1).
Patients’ characteristics related to lung cancer diagnosis
According to our data available only for 183 patients mean SLT was 2.77 months (range 1–13) and mean time from the symptoms’ appearance to the start of cancer treatment was 3.14 months (range 1–14). As it is shown in Table 2, it took less than 2 months to obtain lung cancer diagnosis and consecutive treatment only for 52 patients (28.4 %) and for 38 (20.9 %) patients, respectively.
Regarding the diagnostic work-up, once imaging had been done, FBS was performed in 138 (67.7 %) patients. A total of 86 patients (43.2 %) (without or with negative FBS) received pathological diagnosis by additional invasive methods (Table 2).
All patients had morphological verification of lung cancer before treatment—201 (98.5 %) had histological, while only 3 patients (1.47 %) had cytological confirmation of the disease. (Table 3). The prevalent histological type found was adenocarcinoma (45.1 %), followed by squamous cellular (26 %) and small-cell lung cancer (15.7 %). From patients with large cell cancer, slightly more were those with neuroendocrine differentiation (4.9 %) compared with those without such differentiation (2.9 %).
In 107 patients (52.5 %) IHC was done. In 14.7 % of those patients, the lack of correspondence between primary histological lung diagnosis and IHC results was found. The prevalent part of these discrepancies (78 %) regarded cases with low differentiated squamous-cell carcinoma, which on IHC revealed immunophenotype of adenocarcinoma (data not shown). Regarding TTF1 expression and EGFR mutation status, the positive TTF1 expression was found in 37.3 % of patient in whom IHC was performed. EGFR mutations were detected in 14 patients (6.9 %) of all analyzed patients. Malignancy grade of tumor differentiation was described in histological reports of 180 patients with prevalence of low differentiated tumors which count for 69.6 % of all tumors (data not shown). In 37 patients Ki-67 evaluation was done with mean expression of 67.3 % (range 15–100) (data not shown).
Patients’ characteristics related to lung cancer treatment
As shown in Fig. 1, the number of treated patients at our department has been gradually increasing. As before January 2010, there were 46 treated patients, while after January 2010 their number increased to 155. In all, 167 patients (81.9 %) were diagnosed at the MMA and started their primary medical treatment at our department, while 18.1 % of analyzed patients (37) were referred from other part of the country because of the difficult treatment decisions (8 patients), treatment complications (16 patients), or after consultations abroad (13 patients) (data not shown).
Only 11 patients (5.4 %) had undergone radical pulmonary resection. Radiotherapy as a part of complex lung cancer treatment was conducted in 75 (36.8 %) patients. In majority of patients, radiotherapy was palliative mainly for pain relief, while only in 2.6 % definitive radiotherapy to the thorax was performed with maximal dose of 50 Gy.
In total, 181 (88.7 %) patients received medical treatment (chemotherapy and/or targeted therapy), while 23 advanced LCP (11.3 %) received only palliative care (Table 4). Only small proportion of patients received adjuvant (2.5 %) and neoadjuvant (2.0 %) chemotherapy, while majority of them (84.3 %) received medical therapy for advanced disease.
Prognostic factors for survival in advanced LCP, treated with medical treatment
From the survival analysis were excluded 7 patients who had undergone previously surgery for other cancer types (1-laryngeal, 1-renal cell, 2-prostate, 1-rectum, 1-germ cell, 1-pancreatic). The estimated mean overall survival (OS) was 13 months (range 1–88). All clinical and laboratory parameters were collected from baseline assessments.
A total of 155 patients with locally advanced and metastatic lung cancer, primarily treated at our department with first and further lines of therapy were included in this analysis. Majority of analyzed advanced LCP were with non-small-cell lung carcinoma (NSCLC) (79.4 %). More than a half of the patients (56 %) received only one line of therapy. The distribution according to the type of first line therapy is shown in Table 5. The rest of advanced LCP performed second (28 %), third (14 %) and more than third (2 %) treatment lines. From 44 patients who had undergone second line therapy 63 % were treated with docetaxel. Maintenance therapy was received by 25 patients (16.1 %).
The statistically significant difference between survival of advanced LCP treated in two different time spans (from January 2005 to January 2010 and January 2010 to January 2015) was not found. Regarding histology, only survival of advanced LCP with squamous cell carcinoma treated before 2010 was shorter in comparison to OS of patients with the same histology but treated after 2010. The difference was with borderline significance (Log rank = 8.85; p = 0.055) (data not shown).
As it is shown in Table 6, mean OS differs statistically significant between patients with ECOG PS 0–1 and those with ECOG PS 2 (p < 0.001). Smokers live less than non-smoker patients (p = 0.04). Patients with weight loss more than 5 % in the past 6 months before diagnosis live less than lung cancer patient without significant weight loss (p = 0.02). Significant difference between mean OS of patients treated with one and two or more than two treatment lines was also found (p = 0.02).
Among baseline laboratory factors, significant impact on OS had white blood cell (WBC) (p = 0.02), platelets (p = 0.01), Hb (p = 0.02) as shown in Table 6. NLR impacts patients’ OS (p = 0.01) with patients with lower than median values having longer survival times than those with baseline values above the median 3.57. Statistically significant difference in OS was noticed in patients with PLR bellow the median value of 188 compared to those and above, respectively to the median value of 188 (p = 0.004).
Survival analyses by Kaplan–Meier method of 155 chemo-naïve advanced LCP treated with medical therapy revealed statistically significant impact on prognosis for 9 clinical and laboratory factors among all studied 16 factors (Table 6).
In Cox-regression model among nine statistically significant clinical and laboratory baseline factors in Kaplan–Meier analyses only four factors retained their significant influence on survival—ECOG PS (HR 0.4; p < 0.0001); weight loss (WL) prior to diagnosis (HR 2.03; p < 0.01), the number of treatment lines (HR 1.65; p < 0.043), and PLR (HR 0.48; p < 0.001) (Table 7).
The impact of ECOG PS, WL prior to diagnosis, the number of treatment lines, and PRL on survival of advanced LCP treated with medical therapy estimated by Kaplan–Meier survival curves is shown in Fig. 2, 3, 4, and 5.
Discussion
Lung cancer is a life-threatening disease and the only effective method of prevention is the smoking cessation. Smoking is not only the major risk factor for lung cancer, but it also has negative impact on lung cancer treatment efficacy. Our study results proved a significant impact of smoking history on survival (p = 0.04) of advanced LCP under systemic medical treatment. For advanced NSCLC patients nonsmoking is associated with longer PFS (Progression Free Survival) than ever smoking after EGFR-tyrosine-kinase inhibitor (TKIs) treatment [14]. O’Malley M et al. [15] after the review analysis of several prospective studies, pointed out that small changes in plasma concentrations in smokers may result in suboptimal therapy and poor outcomes because of systemic therapy narrow therapeutic index. According to National Statistical Institute [16] data, 40 % of men and 20 % of women in Bulgaria are heavy smokers and 10 % are time-to-time smokers for both sexes.
Our results revealed that almost 80% of all 204 studied LCP needed more than 2 months to obtain diagnosis. At the beginning of this year, researchers from UK published data regarding SLT defined as the time between symptoms caused by cancer and eventual diagnosis. Their results found in patients with lung and colorectal cancer mean SLT were between 4.1 and 6.0 months, with medians between 2.0 and 3.2 months, respectively [17]. Our results about SLT are in agreement with UK results and our mean SLT is 2.38 months with median 2 months. These results could not be probably generalized for all Bulgarian lung cancer patients. There are many reasons for this situation like slow going health system reform, little contribution of primary care physicians in lung cancer diagnosis, and so on. Our results are mainly connected with diagnostic work-up of military lung cancer patients for whom all diagnostic procedure which are unpaid from Health Basket are free of charge in MMA.
Multidisciplinary approach has a major role in lung cancer diagnosis and treatment [4]. The insufficient activity of multidisciplinary groups has additional negative impact on lung cancer diagnosis. The high percentage of negative (11 %) and not performed FBS (33 %) with concomitant high proportion of explorative thoracothomy supports the abovementioned conclusion.
The study results showed the prevalence of adenocarcinoma histotype (45 %) in comparison to squamous cell cancer (26 %), which is not in agreement with National data. According to the National Cancer Registry [3], the prevalence of adenocarcinoma from morphologically confirmed lung cancer is 14.4 %, while squamocellular carcinoma accounts 48.3 %. This disconcordance between our data and National Registry could be of not routinely applied IHC method in lung cancer diagnosis across the country. IHC was performed in 53 % of our patients, which means that in the last 5 years almost all treated with systemic therapy LCP at our department have IHC confirmation of diagnosis.
In Bulgaria, neither IHC analysis nor EGFR mutation status testing is covered by Health Basket. As a result of the pharma-industry support, lung cancer patients are tested genetically in several genetic labs across the country. Thus, the existing problem of small biopsy in advanced lung cancer [4] is more apparent in Bulgaria. At current time point there is no possibility to test ALK-rearrangements in Bulgaria.
Kaplan–Meier survival analyses of 155 chemo-naïve patients with advanced LCP treated with medical therapy revealed significant impact on prognosis of nine clinical and laboratory factors among which four factors retained their significance in Cox regression model: ECOG PS (p < 0.0001), WL prior to diagnosis (p < 0.01), the number of treatment lines (p < 0.001) and PLR (p < 0.043). The importance of ECOG PS and WL as significant prognostic factors in advanced LCP is proved by many publications and is stated in European and American guidelines [4–6, 8, 10, 18]. The number of treatment lines as survival determinant in advanced LCP needs special attention. For patients who have passed through all indicated treatment lines, and have good performance status it remains unclear whether to continue treatment after new progression. It could be reasonable to introduce the continuum of care paradigm in advanced LCP as it is so widely accepted in the treatment of metastatic colorectal cancer [19]. The “liquid biopsy” in the future would guide more precisely such kind of therapeutic strategy.
PLR together with NLR represent an important part of systemic inflammatory response in chronic disease and their strong impact on cancer survival including advanced LCP’ survival was confirmed in several studies [20–23]. Kaplan–Meier analyses revealed significant impact on prognosis for PLR (p = 0.004) and for NLR (p = 0.02). As it is known the host’s immune response to tumors is lymphocyte dependent [24]. The inflammatory-based prognostic factors representing the host inflammatory response to cancer might help in identification of patients with poor outcomes. Among these markers, PLR is a representative index of systemic inflammation and significant prognostic factor in many cancer types including NSCLC [25]. The platelets’ role in cancer is related to vascular endothelial growth factor release and promotion of angiogenesis. More over patients with neutrophilia and with lymphocytopenia or in other words patients with high NLR might have a poorer lymphocyte-mediated immune response to cancer, which might increase the metastatic potential for cancer progression [24].
Lung cancer diagnosis and treatment in Bulgaria are managed according to the European guidelines, but several issues still exist. They have economic dimensions and are mostly National Health System and hospital-organization related.
Smoking cessation is a national problem with obvious consequences.
ECOG PS and WL are known prognostic factors in advanced LCP. Our results support prognostic impact of PLR on survival. However, the confirmation of this finding needs further prospective validation. The fact that the number of treatment lines impacts survival point out the importance of “continuum of care” concept in advanced LCP, treated with medical therapy.
Conflict of interest
Zh. Mihaylova, V. Megdanova, V. Petrova, D. Petkova, A. Fakirova, M. Petrova, R. Asenov, I. Kisjova, M. Encheva, H. Dinev declare that they have no conflict of interest.
Ethical standards
Every patient signed informed consent before admission in the hospital about usage of data regarding her/his disease for educational and/or research purposes.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2012. Ann Oncol. 2012;23:1044–52.
Cancer Incidence in Bulgaria 2010- National Cancer Registry, 2012: Vol.XX1;pp 18-20: Publisher Avia-24,Ltd.
M. Reck, S. Popat, N. Reinmuth, et al., on behalf of the ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;00:1–13.
Hotta K, Fujiwara Y, Kiura K, et al. Relationship between response and survival in more than 50,000 patients with advanced non-small cell lung cancer treated with systemic chemotherapy in 143 phase III trials. J Thoracic Oncol. 2007;2:402–7.
Brundage MD, Davies D, Mackillop WJ Prognostic factors in non-small cell lung cancer: adecade of progress. Chest 2002;122:1037–57.
Kulesza P, Ramchandran K, Patel JD Emerging concepts in the pathology and molecular biology of advanced non-small cell lung cancer. Am J Clin Pathol. 2011;136: 228–38.
Ulas A, Turkoz FP, Silay K, et al. A laboratory prognostic index for patients with advanced non-small cell lung cancer. Plos One. 2014;9(12):e114471. doi:10.1371/journal.pone.0114471.
Kasymjanova G, MacDonald N, Agulnik JS, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010;17(4):52–58.
Trape J, Montesinos J, Catot S, Buxo J, Franquesa J, et al. A prognostic score based on clinical factors and biomarkers for advanced non-small cell lung cancer. Int J Biol Markers. 2012;27(3):257–62.
Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158–67.
Edge SB, Byrd DR, Compton CC, editor. AJCC Cancer Staging Handbook. 7th ed. New York: Springer, 2010
Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14.
Zhang Y, Kang S, Fang W, et al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer. 2014. doi:10.1016/j.cllc.2014.09.008. (pii: S1525–7304(14)00196–X)
O’Malley M, King AN, Conte M, et al. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J Thorac Oncol. 2014;9(7):917–26. doi:10.1097/JTO.0000000000000191.
Bulgarian National Statistical Institute. http://www.nsi.bg. Accessed Jan 2015.
Biswas M, Ades AE, Hamilton W. Symptom lead times in lung and colorectal cancers: what are the benefits of symptom-based approaches to early diagnosis? Br J Cancer. 2015;112(2):271–7. doi:10.1038/bjc.2014.597.
National Comprehensive Cancer Network Guidelines V 2. 2013. http://www.nccn.org. Accessed May 11, 2013.
Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12(1):38–50.
McMilan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009:12(3):223–6
Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00–03. Eur J Cancer. 2009;5:1950–8.
Qiu M, Zhou Y, Jin Y, et al. Prognostic effect of high pretreatment neutrophil to lymphocyte ratio on survival of patients with gastric adenocarcinoma in China. Int J Bio Markers. 2014;30:e96–103. doi:10.5301/jbm.5000123.
Supoken A, Kleebkaow P, Chumworathayi B, et al. Elevated preoperative platelet to lymphocyte ratio associated with decreased survival of women with ovarian clear cell carcinoma. Asian Pac J Cancer Prev. 2014;15(24):10831–6.
Kusumanto Y, Dam W, Hospers G, et al. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003;6:283–7
Zhou X, Du Y, Huang Z, et al. Prognostic value of PRL in various cancers: a meta-analysis. PLoS One. 2014;9(6):e101119.
Author information
Authors and Affiliations
Corresponding author
Additional information
To my mentor Prof. Maurizio Tonato
Rights and permissions
About this article
Cite this article
Mihaylova, Z., Megdanova, V., Petrova, V. et al. Lung cancer in Bulgaria ‒ diagnosis, treatment, and factors affecting survival. memo 8, 136–143 (2015). https://doi.org/10.1007/s12254-015-0214-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-015-0214-8