Abstract
Several monoclonal antibodies and inhibitors targeting signalling pathways are being used in personalised medicine. Anti-EGFR antibodies seem to be effective, however, therapy resistance often occurs in colon carcinoma cases. mTOR inhibitors (mTORIs) could have a potential role in the breakthrough of therapy resistance. The mTOR activity related protein expression patterns and the in vitro effects of EGFR inhibitors (EGFRIs), mTORIs and their combinations were studied in different colon carcinoma cell lines (with different genetic backgrounds). Alamar Blue test and flow cytometry were used to analyse the in vitro proliferation and apoptotic effects of cetuximab, gefitinib, cisplatin, rapamycin, PP242 and NVP-BEZ235. The expressions of mTOR activity related proteins (p-70S6K, p-S6, Rictor, p-mTOR, Raptor) were studied by Western blot, immunocytochemistry and Duolink staining. The EGFRI resistance of the studied colon carcinoma cell lines related to their known mutations were confirmed, neither gefitinib nor cetuximab inhibited the proliferation or induced apoptosis in vitro. Individual differences in Rictor and Raptor expressions were detected by Western blot and immunocytochemistry beside elevated mTOR activity of these different colon carcinoma cell lines. These expression patterns correlated to the mTORIs sensitivity differences, moreover, mTORIs could enhance the effects of EGFRIs and other in vitro treatments. Our results suggest that mTORI combinations could be helpful in both EGFRI and platinum-based therapy of colon carcinomas. Moreover, we suggest determining both mTOR complex activity and mutations in Akt/mTOR signalling pathways for selecting the appropriate mTORIs and patients in potential future combination treatments.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is one of the most frequent malignancies worldwide and the prognosis of these patients is largely dependent on the stadium of the cancer at the time of the diagnosis. The identified new molecular targets contributed to therapy development for personalised medicine of these type of cancers in the last decade [1,2,3,4]. In case of metastatic tumours, the systemic therapy is based on chemotherapy, either traditional or targeted (e.g. fluoropirimidin-based drugs or monoclonal antibodies against molecular targets, such as anti-EGFR drugs –cetuximab, panitumumab). Using monoclonal antibodies in the therapy of colon carcinomas, even single agent anti-EGFR antibodies, seems to be effective in particular setting of the related patients. However, the in vivo drug resistance development is one of the unsolved problems in cancer therapy [5]. The reasons are manifold, including the hyperactivated regulatory pathways related to several oncogenic mutations in different genes such as growth factor receptors like EGFR or in the molecules of signalling network (e.g. Ras, Raf, PI3KCA, PTEN, TSC1/2, mTOR) or cell cycle regulatory proteins (e.g. p53, CDKIs), as well [2, 6, 7]. In many times can be detected an elevated activity of PI3K/Akt/mTOR pathway and all participants of these pathway could serve as drivers.
The activated mTOR signalling was described in many tumours [8, 9]. mTOR (mammalian target of rapamycin) kinase forms two different protein complexes: mTORC1 and mTORC2. These complexes countain characteristic elements such as Raptor (mTORC1) and Rictor (mTORC2), different targets in the signalling network (e.g. p70S6K, 4EBP1 – mTORC1 and Akt, SGK – mTORC2 besides many others) and different sensitivity to inhibitors (rapamycin, their analogs (rapalogs) and other new mTOR inhibitors, which are being developed) [10]. High mTOR activity is characteristic for most of colon carcinomas as we and others described, where this activity could be mTORC2 complex related in about 60% of the cases [11]. Rapamycin, the first described mTORC1 inhibitor originally was described as an immunosuppressant agent, its analogs (e.g. everolimus, temsirolimus) are used to treat such malignancies as renal cell carcinoma, mantle cell lymphoma, breast cancer etc. thanks to their anti-proliferative, anti-tumour growth effects. However, many other clinical trials related to these inhibitors are ongoing with different success [10, 12, 13]. Both EGFR inhibitors (EGFRIs) and mTOR inhibitors are in the highlight of the recently developing treatment combinations.
In our work we studied the mTOR activity related protein expression patterns and the in vitro effects of EGFRIs, mTORIs and their combinations in different colon carcinoma cell lines were described by some known signalling pathway mutation.
Methods
In Vitro Cell Cultures and Different Treatments
Colon carcinoma cell lines with different genetic backgrounds [14] (mKRAS – SW620, SW480, HCT116, mBRAF – RKO, Colo205, HT29; mPI3KCA – HCT116, RKO, HT29, mP53 – CaCo2, HT29, SW480, SW620, GC3; these cell lines were purchased from Institutional Cell Line Collection see acknowledgements) were cultured in RPMI 1640/DMEM/MEM (depending on cell line request) with 10% FCS (Gibco) medium supplemented with 100 U/ml penicillin and 100 ng/ml streptomycin (Sigma). Cells were treated with cetuximab (10 μg/ml, Erbitux Bristol-Myers,), gefitinib (1–20 μM, Cayman Chemical), cisplatin (0.1–10 μg/ml, EBEWE Pharma), rapamycin (50 ng/ml, Sigma), PP242 (1 μM - ATP-competitive inhibitor inhibits mTOR kinase activity in both mTORC1 and mTORC2, Tocris Bioscience), NVP-BEZ235 (1 μM - dual ATP-competitive PI3K and mTOR inhibitor, Cayman Chemical) for 24–72 h. For Alamar Blue proliferation tests 1500–5000 cells/100 μl were applied depending on the individual doubling time of the cell cultures which were previously tested.
Detection of Cell Proliferation and Apoptosis
Alamar Blue test (Thermo Fisher Scientific) was used with 4 h incubation time to analyse the proliferation capacity. Fluorescence was measured by Fluoroscan Ascent FL fluorimeter software (Labsystems International) and the percentage of proliferation was given relative to control samples. Apoptosis detection was performed by flow cytometry after fixation in 70% ethanol (−20 °C) and was followed alkalic extraction (200 mM Na2HPO4, pH 7.4 and 100 μg/ml RNase, Sigma, St. Louis, MO, USA) and ethidium bromide staining (10 μg/ml, Sigma, St. Louis, MO, USA). 10,000–20,000 events/samples were measured using a FACScan flow cytometer (Becton-Dickinson, BD Biosciences, San Diego, CA, USA). Apoptotic subG1 cells were evaluated by WinList software (Verity Software House, Topsman, ME, USA).
Protein Expression Studies – Western Blot, Immunocytochemistry and Duolink Staining
Whole cellular extracts were prepared and quantitated with Quant-iT protein assay (Invitrogen). SDS-PAGE protein extracts (20 μg) were transferred to PVDF membranes. Membranes were incubated with antibodies (anti-phosphorylated-p70S6K, −phosphorylated-S6, −Rictor, phosphorylated-mTOR from Cell Signaling Technologies #9234, #2211, #2140, #2972; anti-Raptor from Novus Biotechnologies #89603), followed by biotinylated secondary antibodies and avidin-HRP complex (Vectastain Elite ABC Kit, Vector), and detected by enhanced chemiluminescence (Pierce ECL Western Blotting Substrate). Membranes were stripped (Re-Blot Plus, Millipore) and the equal protein loading was confirmed by anti-β-actin (A2228; Sigma).
Cytospin slides were prepared and fixed by methanol (−20 °C, 80%) and treated by endogenous peroxidase blocking solutions. These were followed by blocking and primary antibody (anti-Rictor from Bethyl, anti-phosphorylated-mTOR #2976 from Cell Signaling and Technologies and anti-Raptor from Biolegends), incubation overnight at 4 °C followed by Novolink Polymer Detection System (Novocastra, Wetzlar, Germany) and DAB staining and haematoxylin counterstaining. Immunostainings were evaluated by 2 independent pathologists (both the intensity of the staining and the percentage of the positive cells were evaluated - +: 90% of the cells show low intensity of staining, ++: 10% < higher intensity stained cells, +++: 30% < higher intensity stained cells).
Duolink staining was used to detect and quantify the amount of phosphorylated S6 protein or mTOR-Rictor protein complexes. For these stainings cell cytospins were fixed by paraformaldehyde (10 min, 4%) and permeabilised by TritonX-100 (0.4%). p-S6 – S6 (#2211 and #2317 from Cell Signaling and Technologies) and mTOR – Rictor (Bethyl A300-503A and A500-002A, Cell Signaling and Technologies) antibody pairs were used with the following Duolink staining development kit (Olink/Sigma) applying manual instruction and were detected the bounded antibodies by FITC labelled probes and fluorescent microscope.
Statistical Analysis
Mean values and SD were calculated from three independent experiments with three or more parallels, depending on the used assays. Student’s t-test and one-way analysis of variance (ANOVA) were used for statistical analysis; p ≤ 0.05 was considered to be statistically significant.
Results
The EGFRI resistance related to known mutations in the studied colon carcinoma cell lines was confirmed in our experiments. Neither gefitinib nor cetuximab inhibited the proliferation or induced apoptosis after 72 h treatment in vitro. High dose gefitinib treatment – with less specific kinase inhibitor effect – has significantly inhibited the proliferation in almost every cell line and induced apoptosis in certain cells such as HT29 and GC3 (Fig. 1).
The EGFR inhibitor resistance of RKO and the other studied colon carcinoma cells. a The in vitro effect of different doses of gefitinib and 10 μg/ml cetuximab treatment on the proliferation of RKO colon carcinoma cells detected by Alamar Blue test (result of the representative experiment - #: 1 μM gefitinib treatment was repeated every day). b Different assays – cell numbers, Alamar Blue test and apoptosis measurements by flow cytometry – confirmed that gefitinib has no effect on RKO colon carcinoma cells after 72 h treatment (result of representative experiment). c The higher dose gefitinib treatment could inhibit the proliferation of different colon carcinoma cells, however, induced apoptosis could not be detected after 72 h treatment neither in the lower dose nor cetuximab treated cell cultures (apoptosis data were not shown). * labelled the significant changes (p < 0.05)
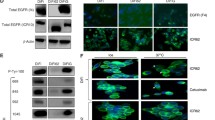
High mTOR activity was detected in the studied colon carcinoma cell lines. Different techniques such as Western blot, ICC and Duolink staining showed that elevated mTOR activity could be present in both mTORC1 and C2 complexes. Cell lines showed individual differences in the amount of the Rictor and Raptor (Fig. 2.) similarly to our previously described data related to human colon carcinoma cases [11]. These expression patterns - expression of the active mTOR kinase (phosphorylated mTOR – p-mTOR), mTORC1 and C2 complex characteristic Raptor or Rictor, mTORC1 activity related expression of phosphorylated p70S6K (p-p70S6K) and its target protein ribosomal S6 (p-S6) - correlated to mTOR inhibitor sensitivity differences.
Characteristic high mTOR activity in different colon carcinoma cell lines. a mTOR activities of SW620, HT29 and RKO colon carcinoma cell lines (Western blot analysis). The amount of different proteins, which are in different complexes and related to mTOR activity were shown by representative Western blot (phospho-mTOR – p-mTOR, active mTOR kinase in both mTORC1 and C2; Raptor and Rictor – the characteristic proteins of mTORC1 and C2 respectively and the phosphorylated direct and indirect targets of mTORC1 – p-p70S6K and p-S6). b Summarised results of the evaluation of immunocytochemical (ICC) stainings with different antibodies characterising the activity of mTOR complexes (+ low; ++ intermediate; +++ high expression based on evaluation of 2 pathologists). c Representative photos about Duolink stainings in HCT116 and in CaCo2 cell lines. Higher mTORC2 activity related high amount of the detected mTOR-Rictor antibodies in HCT116 cells and higher mTORC1 activity related high amount of p-S6-S6 antibodies in CaCo2 cells. The green signals represent the amplified target sequences at the sites where the used two different antibodies detect interacting proteins in complex (mTOR and Rictor – mTORC2 complex) or the specific modification of the proteins (phosphorylated S6 (Ser 235/236) and S6 as the sign of mTORC1 activity) (400X). d Representative photos about different scores after immunocytochemistry using HRP conjugated secondary antibodies, DAB substrate (brown colour) and haematoxylin counterstaining (200X)
It was also found that the cells which express higher amount of Rictor (+++, GC3, HCT116 and HT29) were less sensitive to mTOR, especially to mTORC1 inhibitors than other cells with characteristic lower Rictor (related mTORC2 complex) expression. Where rapamycin and the other dual or mTORC1 and C2 inhibitors showed significant inhibition in proliferation in vitro. Moreover, in the most mTORI sensitive cell line - RKO - both dual and mTORC1 and C2 inhibitors inhibited the proliferation significantly higher than rapamycin (Fig. 3.). In these cells the level of the mTORC1 activity related protein, the p-S6 was reduced rapidly after 24 h rapamycin and NVP-BEZ235 treatment, as well. In an other - less sensitive - HT29 cells the p-S6 protein level could be reduced significantly higher by NVP-BEZ235 (Dual inhibitor) treatment than rapamycin at 24 h. This difference was detected at 72 h treatments, as well. p-S6 level was reduced by both rapamycin and NVP-BEZ235, however, Rictor expression was decreased under detectable level after 72 h NVP-BEZ235 treatment (Fig. 3c).
mTOR inhibitor sensitivity of different colon carcinoma cell lines. a Time dependent significant anti-proliferative effect of 50 ng/ml rapamycin treatment was detected by Alamar Blue test in RKO cells (the result of representative experiment). b The effects of rapamycin (50 ng/ml), NVP-BEZ235 (1 μM - mTOR and PI3K. c dual inhibitor) and PP242 (1 μM - mTORC1 and C2 inhibitor) on the proliferation of different colon carcinoma cells. The untreated cell cultures were considered 100%, the significantly reduced proliferation (p < 0.05) were labelled (*) (Alamar Blue test results of representative experiments after 72 h treatment). d The mTOR inhibitors - rapamycin (50 ng/ml - Rapa) and NVP-BEZ235 (1 μM – NVP) treatments reduce the amount of mTORC1 activity related phosphorylated-S6 (p-S6) and Rictor (characteristic protein of mTORC2 complex) in RKO and HT29 cells (Western blot results of representative experiments)
It was also detected that rapamycin and especially other more effective mTOR inhibitors such as NVP-BEZ235 (mTOR and PI3K dual inhibitor) and PP242 (mTORC1-C2 inhibitor) in combination with EGFRI could be more effective in the less mTORI sensitive and EGFRI resistant colon carcinoma cells (GC3, HCT116 and HT29). However, mTORI and EGFRI combination could have no more additional effects compared with the mTORIs in other mTORI sensitive cell lines (Fig. 4a and b). mTOR inhibitors, especially the NVP-BEZ235, dual inhibitor induced the effect of cisplatin and could be effective in cisplatin and EGFRI resistant RKO colon carcinoma cells, as well. Moreover, the dual mTOR and PI3K inhibitor could significantly induce the effect of cisplatin in both HT29 and SW620, less mTOR sensitive and EGFRI resistant cell lines (Fig. 4c).
Rapamycin induces the effects of gefitinib and cisplatin in certain colon carcinoma cells. a The combined rapamycin (50 ng/ml) and EGFR inhibitor (gefitinib 1 μM, cetuximab 10 μM) treatments could be significantly effective in less mTORI sensitive and EGFR inhibitor resistant colon carcinoma cells (Alamar Blue test results of representative experiments after 72 h in vitro treatments). b Dual mTOR inhibitor NVP-BEZ235 and mTORC1-C2 inhibitor PP242 with gefitinib could be effective in anti-tumour growth of GC3, EGFR inhibitor and rapamycin resistant colon carcinoma cells and in other less mTORI sensitive cells (representative experiments after 72 h treatments with 50 ng/ml rapamycin, 1 μM NVP-BEZ235, 1 μM PP242, 1 μM gefitinib; Alamar Blue test). c mTOR inhibitors - especially NVP-BEZ235 (mTOR and PI3K dual inhibitor) -induce the effect of cisplatin and could be effective in cisplatin and EGFRI resistant RKO colon carcinoma cells (representative experiments after 72 h treatments with cisplatin 0.1 μM - 10 μM, rapamycin 50 ng/ml, NVP-BEZ235 1 μM and Alamar Blue test)
Discussion
The therapy resistance of both EGFR inhibitor and traditional chemotherapy is well described in many different cancers and in colon carcinomas, as well. To breakthrough this resistance or prevent the fatal development of carcinomas further new approaches are needed. mTOR inhibitors could have potential role in these, based on their in vitro and in vivo effects. In spite of the previously expected therapeutic results, the clinical results give only few benefits because of many background reasons [15,16,17]. In our work we could detect the effect of different mTORIs in many EGFRI resistant colon carcinoma cell lines which showed different mTOR complex activity patterns. These inhibitors enhanced the effects of cisplatin or EGFRI especially in less mTORI sensitive cells. This anti-proliferative effect could be interesting because it is known that long-term mTORI treatment could significantly inhibit the tumour growth in vivo in many xenograft models. Several mechanisms such as induced apoptotic effect in vivo could support this effectivity comparing with short in vitro treatment [18,19,20,21]. Dual or mTORC1 and C2 inhibitor monotreatments were very effective in many studied cell lines except for GC3 (known p53 mutant, but wildtype K-Ras, B-Raf, PI3KCa) and HCT116 (Ras and PI3KCa mutant) cells. It was previously described that PI3KCa mutation determines the sensitivity to rapamycin in different tumours such as breast cancers [22]. HCT116 cells could be referred as mTOR resistant cell line, however, they carry PI3KCa mutation. We could detect high level Rictor expression and potential mTORC2 complex activity in these cells. It is well-known that HCT116 cells also contain Ras mutation in cellular signalling network, this could explain the resistance of rapamycin and other mTORI. It was suggested in lot of previous publications that EGFRI resistance could be override in Ras mutant cells by mTORIs [23, 24]. We could found this effect in HCT116 cells in the EGFRI and rapamycin combination treatment, where the proliferation was significantly inhibited in spite of its single rapamycin treatment resistance. It was also shown that cisplatin resistance can be overloaded. The reduced dose of cisplatin (lower dose 1 μg/ml) can be more effective in combination with mTORI treatment in the studied cells. Similar effects were previously described in other carcinomas. According to these data, low dose cisplatin administration could induce autophagy and its combination with rapamycin treatment could enhance the anti-tumour effect in other cancers [25,26,27,28].
Our work suggests that mTORI combination could be helpful in both EGFRI and platinum-based traditional therapy in colon carcinoma cases. However, in these cases the determination of the mTOR complex activity [11], and the mutation in Akt/mTOR signalling pathways [16, 29] will help to select the optimal mTORI for combination [30]. These could have an important role in therapeutic decisions. In these kind of combination therapies of lower dose treatments with less side effects could have similar or more effective results. However, combination therapy could have several side effects with individual differences. To evaluate the optimal combinations, tolerable doses and its effectiveness clinical trials are needed and these could help to find appropriate biomarkers, as well [31,32,33]. Our recently published results [11] underline the correlation between the mTORC2 complex related mTOR activity and the worse prognosis of traditionally treated colon carcinoma cases. These and the presented in vitro data suggest to determine the patient selection criteria, which could help to choose the right target and the optimal therapeutic combination for the right patients. Based on our results the mTORC1 and C2 activity pattern besides EGFR and Akt/mTOR pathway related mutations are good candidates for such selections as in gastric cancers where the amplification of Rictor and rapamycin resistance was found [34].
The determination of the above mentioned parameters could contribute to patient selection criteria. Moreover, mTORIs could also have an inhibitory effect on tumour progression without performing therapy resistant clone selection as applying low dose mTORIs either at an early tumour stage or after an aggressive therapy promoting the extended tumour and disease free survival [35, 36]. Our previous and the presented results and the already available other data highlight the potential application of different mTORIs in EGFRI and/or conventional therapy resistant colon carcinomas. However, our data underline the importance of appropriate timing and validation of the real targets - especially the mTORC1 and C2 activity - in these cases.
References
Tsimberidou D (2015) AM: targeted therapy in cancer. Cancer Chemother Pharmacol 76(6):1113–1132
Herzig DO, Tsikitis VL (2015) Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol 111(1):96–102
Kalia M (2015) Biomarkers for personalized oncology: recent advances and future challenges. Metabolism 64:S16–S21
Dietel M, Jöhrens K, Laffert MV, Hummel M, Bläker H, Pfitzner BM, Lehmann A, Denkert C, Darb-Esfahani S, Lenze D, Heppner FL, Koch A, Sers C, Klauschen F, Anagnostopoulos I (2015) A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: a review focusing on clinical relevance. Cancer Gene Ther 22(9):417–430. https://doi.org/10.1038/cgt.2015.39
Chong CR, Jänne PA (2013) The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 19(11):1389–1400
Khamisipour G, Jadidi-Niaragh F, Jahromi AS, Zandi K, Hojjat-Farsangi M (2016) Mechanisms of tumor cell resistance to the current targeted-therapy agents. Tumour Biol:1–19
Zhao Y, Butler EB, Tan M (2013) Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4(3):e532
Francipane MG, Lagasse E (2014) mTOR pathway in colorectal cancer: an update. Oncotarget 5(1):49–66
Chiarini F, Evangelisti C, McCubrey JA, Martelli AM (2015) Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci 36(2):124–135
Francipane MG, Lagasse E (2015 Nov 26) Therapeutic potential of mTOR inhibitors for targeting cancer stem cells. Br J Clin Pharmacol 82:1180–1188. https://doi.org/10.1111/bcp.12844.
Sticz T, Molnár A, Márk Á, Hajdu M, Nagy N, Gy V, Micsik T, Kopper L, Sebestyén A (2016) mTOR activity and its prognostic significance in human colorectal carcinoma depending on C1 and C2 complex related protein expression. Clin Pathol 70:410–416. https://doi.org/10.1136/jclinpath-2016-203913
Huang Z, Wu Y, Zhou X, Qian J, Zhu W, Shu Y, Liu P (2015) Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review. Future Oncol 11(11):1687–1699. https://doi.org/10.2217/fon.15.70
Lamming DW (2016) Inhibition of the mechanistic target of rapamycin (mTOR)-rapamycin and beyond. Cold Spring Harb Perspect Med 6(5). https://doi.org/10.1101/cshperspect.a025924
Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA (2013 Sep 16) Epigenetic and genetic features of 24 colon cancer cell lines. Oncogene 2:e71. https://doi.org/10.1038/oncsis.2013.35
Roohi A, Hojjat-Farsangi M (2016) Recent advances in targeting mTOR signaling pathway using small molecule inhibitors. J Drug Target 3:1–13 PubMedPMID: 27632356
Spindler KL, Sorensen MM, Pallisgaard N, Andersen RF, Havelund BM, Ploen J, Lassen U, Jakobsen AK (2013) Phase II trial of temsirolimus alone and in combination with irinotecan for KRAS mutant metastatic colorectal cancer: outcome and results of KRAS mutational analysis in plasma. Acta Oncol 52(5):963–970
Harada K, Miyake H, Kumano M, Fujisawa M (2013 [Epub ahead of print]) Acquired resistance to temsirolimus in human renal cell carcinoma cells is mediated by the constitutive activation of signal transduction pathways through mTORC2. Br J Cancer 109:2389–2395
Sebestyén A, Márk Á, Hajdu M, Nagy N, Molnár A, Végső G, Barna G, Kopper L (2015) Rapamycin can restore the negative regulatory function of transforming growth factor beta 1 in high grade lymphomas. Cytokine 73(2):219–224
Márk Á, Hajdu M, Váradi Z, Sticz TB, Nagy N, Csomor J, Berczi L, Varga V, Csóka M, Kopper L, Sebestyén A (2013) Characteristic mTOR activity in Hodgkin-lymphomas offers a potential therapeutic target in high risk disease--a combined tissue microarray, in vitro and in vivo study. BMC Cancer 13:250. https://doi.org/10.1186/1471-2407-13-250
Gao F, Huang C, Zhang Y, Sun R, Zhang Y, Wang H, Zhang S (2016) Combination targeted therapy of VEGFR inhibitor, sorafenib, with an mTOR inhibitor, sirolimus induced a remakable response of rapid progressive Uterine PEComa. Cancer Biol Ther 17(6):595–598
Martin R, Desponds C, Eren RO, Quadroni M, Thome M, Fasel N (2016) Caspase-mediated cleavage of raptor participates in the inactivation of mTORC1 during cell death. Cell Death Discov 2:16024. https://doi.org/10.1038/cddiscovery.2016.24.eCollection
Weigelt B, Warne PH, Downward J (2011) PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitor drugs. Oncogene 30(29):3222–3233
Wang Z, Martin D, Molinolo AA, Patel V, Iglesias-Bartolome R, Degese MS, Vitale-Cross L, Chen Q, Gutkind JS (2014) mTOR co-targeting in cetuximab resistance in head and neck cancers harboring PIK3CA and RAS mutations. J Natl Cancer Inst 106(9). https://doi.org/10.1093/jnci/dju215
Li B, Gao S, Wei F, Bellail AC, Hao C, Liu T (2012) Simultaneous targeting of EGFR and mTOR inhibits the growth of colorectal carcinoma cells. Oncol Rep 28(1):15–20
Leisching GR, Loos B, Botha MH, Engelbrecht AM (2015) The role of mTOR during cisplatin treatment in an in vitro and ex vivo model of cervical cancer. Toxicology 335:72–78
Liu J, Zhang L, Zhang X, Xing X (2015) Rapamycin enhanced the antitumor efficacy of oxaliplatin in cisplatin-resistant ovarian cancer cells A2780cis both in vitro and in vivo. J Chemother 27(6):358–364. https://doi.org/10.1179/1973947815Y.0000000021
Costello BA, Borad MJ, Qi Y, Kim GP, Northfelt DW, Erlichman C, Alberts SR (2014) Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Investig New Drugs 32(4):710–716. https://doi.org/10.1007/s10637-014-0096-3
Im-Aram A, Farrand L, Bae SM, Song G, Song YS, Han JY, Tsang BK (2013) The mTORC2 component Rictor contributes to cisplatin resistance in human ovarian Cancer cells. PLoS One 8(9):e75455
Kim A, Lee JE, Lee SS, Kim C, Lee SJ, Jang WS, Park S (2013) Coexistent mutations of KRAS and PIK3CA affect the efficacy of NVP-BEZ235, a dual PI3K/MTOR inhibitor, in regulating the PI3K/MTOR pathway in colorectal cancer. Int J Cancer 133:984–996
Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin J, Feng GK, Zhu XF (2013) Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 has a therapeutic potential and sensitizes cisplatin in nasopharyngeal carcinoma. PLoS One 8(3):e59879
Hecht JR, Reid TR, Garrett CR, Beck JT, Davidson SJ, Mackenzie MJ, Brandt U, Rizvi S, Sharma S (2015) Phase I study of everolimus, cetuximab and irinotecan as second-line therapy in metastatic colorectal cancer. Anticancer Res 35(3):1567–1573
Severyn B, Nguyen T, Altman MD, Li L, Nagashima K, Naumov GN, Sathyanarayanan S, Cook E, Morris E, Ferrer M, Arthur B, Benita Y, Watters J, Loboda A, Hermes J, Gilliland DG, Cleary MA, Carroll PM, Strack P, Tudor M, Andersen JN (2016) Development of a high-throughput gene expression screen for modulators of RAS-MAPK signaling in a mutant RAS cellular context. J Biomol Screen 21(9):989–997. https://doi.org/10.1177/1087057116658646
Pohl M, Schmiegel W (2016) Therapeutic strategies in diseases of the digestive tract- 2015 and beyond targeted therapies in Colon Cancer today and tomorrow. Dig Dis 34(5):574–579. https://doi.org/10.1159/000445267
Kim ST, Kim SY, Klempner SJ, Yoon J, Kim N, Ahn S, Bang H, Kim KM, Park W, Park SH, Park JO, Park YS, Lim HY, Lee SH, Park K, Kang WK, Lee J (2016) Rapamycin-insensitive companion of mTOR (RICTOR) amplification defines a subset of advanced gastric Cancer and is sensitive to AZD2014-mediated mTORC1/2 inhibition. Ann Oncol:mdw669. https://doi.org/10.1093/annonc/mdw669
McCoach CE, Bivona TG, Blakely CM, Doebele RC (2016) Neoadjuvant oncogene-targeted therapy in early stage non-small-cell lung Cancer as a strategy to improve clinical outcome and identify early mechanisms of resistance. Clin Lung Cancer 17(5):466–469. https://doi.org/10.1016/j.cllc.2016.05.025
Chen DH, Zhang XS (2015) Targeted therapy: resistance and re-sensitization. Chin J Cancer 34(11):496–501
Acknowledgements
The project was supported by OTKA project (K84262) and Semmelweis University Scientific and Innovation Founds (STIA-KF-17); and by Bolyai fellowship (590/2015 - Hungarian Academy of Sciences) of the corresponding author (A. Sebestyén). The related research works of Z.Hujber and T. Dankó were also supported by New National Excellence Programs (ÚNKP-17-3; ÚNKP-17-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights:
- Activity of mTOR complexes in colon carcinoma cells correlates to mTORI sensitivity
- mTORIs promote anti-tumour effects of EGFRIs in therapy resistant colon carcinomas
- Additional markers and agents related to mTOR activity for personalised treatments
Rights and permissions
About this article
Cite this article
Sticz, T., Molnár, A., Dankó, T. et al. The Effects of Different mTOR Inhibitors in EGFR Inhibitor Resistant Colon Carcinoma Cells. Pathol. Oncol. Res. 25, 1379–1386 (2019). https://doi.org/10.1007/s12253-018-0434-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0434-4