Abstract
The intestinal neoplastic transformation is a possible risk of chronic inflammatory bowel disease (IBD). Previous evidence in mice IBD provides a role for the RAS-association domain family tumor suppressor protein 1 A (RASSF1A), in the repairing process following mucosa epithelium damage, through cooperation with the HIPPO-signaling molecules p73, and YAP. HIPPO pathway which has been implicated in stem cell activity includes as key components for signal transduction the large tumor suppressor homology Ser/Thr kinases LATS1/2. The aim of this study was to assess immunohistochemically, using specific antibodies, the RASSF1A and LATS1/2 expression patterns in a cohort of patients with IBD including 52 ulcerative colitis (UC), 24 Crohn’s disease (CD) and 24 IBD unclassified (IBD-U), compared with normal intestine from non-IBD patients (control group). The relationship between subtypes of IBD and RASSF1A and LATS1/2 expression, both individually and related to p73 and YAP/pYAP(Ser127) proteins was also investigated. Quantitative analyses of the immunohistochemical findings in mucosa cells revealed a significantly decreased expression in UC and IBD-U for RASSF1A expression and a significantly elevated expression in UC, IBD-U, and CD for LATS1/2 expression compared with normal mucosa (P < 0.05). However, ROC curve analysis showed that only LATS1/2 could differentiate IBD from control group. RASSF1A expression was significantly correlated with LATS1/2 in UC with dysplasia (P < 0.0001), and p73 in UC (P < 0.001), and IBD-U (P < 0.02). The expression of all proteins did not differ significantly between subtypes of IBD (P ≥ 0.05). RASSF1A-LATS1/2 co-expression was mainly observed in IBD samples. These findings suggest that tumor suppression proteins RASSF1A and LATS1/2 may be involved in the pathogenesis of human IBD and imply a potential cooperation of RASSF1A, and HIPPO signaling pathways in human bowel inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two forms of inflammatory bowel disease (IBD). Significant differences may distinguish UC from CD. Particularly, the inflammation in UC is limited to the mucosal layer and affects with variable extension the rectum towards the cecum whereas, in CD, involves a transmural process, extending mostly to the terminal ileum and colon [1,2,3]. IBD resections with features of both UC and CD are categorized as “indeterminate” or IBD unclassified (IBD-U), and over the years remain a controversial aspect between clinicians and pathologists [4, 5]. Mucosal remodeling is the major mechanism preserving the intestine homeostasis in inflamed bowel. However, the mucosa, especially the crypts, cannot be completely reconstructed in chronic IBD. Crypt abscesses and distortions are combined with the loss of crosstalk between crypt epithelium and subepithelial myofibroblasts which results in unrestricted induction of epithelial cells apoptosis [6]. Therefore, the lack of efficient control of cell proliferation may gradually lead to the development of dysplasia and colorectal cancer (CRC) in these patients [7].
RAS association domain family protein 1 (RASSF1), a classical member of RAS effector pathways, consists of eight alternative transcripts RASSF1A-RASSF1H [8]. Only information regarding the functions of ubiquitously expressed isoforms A and C is available. RASSF1A is a 340-residue protein which mediates apoptosis, cell cycle arrest and gene stability whereas RASSF1C, a smaller isoform, although it appears to share many of the biological characteristics of RASSF1A has been implicated in unique functions not shared by RASSF1A [9]. Moreover, RASSF1A disrupts interactions among MDM2, an ubiquitin ligase that promotes p53 ubiquitination and degradation, DAXX (death-domain-associated protein) and the deubiquitinase HAUSP, in nucleus, thus contributing to the efficient p53-dependent at early time checkpoint activation in response to DNA damage [10]. In addition to these well characterized functions, RASSF1A, as a scaffolding protein, can assemble and modulate tubulin dynamics controlling cell motility and invasion [8]. Importantly, RASSF1A is frequently found inactivated by promoter methylation in a large variety of human cancers e.g. lung, breast, ovarian cancers and CRC [11] as well as in tissues of UC [12] and normal colonic mucosa [12,13,14]. Recently, RASSF1A has been shown to play an essential role for protection against intestinal inflammation through cooperation with HIPPO pathway [15].
The mammalian HIPPO signaling is involved in stem cell activity, controlling tissue size during development and normal homeostasis, and suppressing tumor growth [16, 17]. Particularly, intercellular contacts and membrane adhesion complexes mediate signal transduction through the main components of this pathway, which are highly conserved in mammals, and include the Sterile 20-like kinases MST1 and MST2 with their regulatory protein WW45 (SAV1), and the large tumor suppressor homology Ser/Thr kinases LATS1 and LATS2, with their regulatory protein MOBKL1A/B (MOB1). Once activated the MST1/2 kinases, phosphorylate LATS kinases and negatively regulate cell proliferation. Briefly, phosphorylation of LATS kinases follows a phosphorylation procedure of the transcriptional coactivators YAP at Ser 127 and TAZ at Ser 89, so as form binding sites for 14–3-3 proteins, which localize and anchor YAP/TAZ in the cytoplasm, and direct YAP for proteolytic degradation. In the absence of this inhibitory phosphorylation, these transcription factors enter to the nucleus, where they interact with transcriptional factors which promote cell proliferation [18]. On the other hand, in cells undergo apoptosis upon exposure to severe DNA damage stress, YAP binds to p73 transcription factor which is a p53-like tumor suppressor and activates the transcription of proapoptotic genes. This is mediated through phosphorylation of YAP at position Tyr357 by c-Abl protein providing higher affinity of YAP to p73 [19]. The cross-talk of RASSF1A and HIPPO pathways is triggered from stimuli outside the cell which mediate activation of the Fas and TRF death receptors. The intermediate steps encompass the RASSF1A-driven activation of MST2 by relieving it from Raf1 and association of MST2 with its substrate LATS1. This induces phosphorylation and release of YAP, allowing its translocation to the nucleus and binding to p73 and transcriptional activation of cell death genes [20]. Thus, opposite roles have been proposed for YAP: as growth promoting oncoprotein and as a tumor suppressor, depending on the cellular context [16, 17, 21].
In normal bowel, YAP is kept largely inactive and is mainly expressed in crypts modulating stem cell population numbers [22]. Recently, Barry et al. (2013) demonstrated that the cytoplasmic YAP suppresses, through inhibition of Wnt-signaling pathway, the intestinal renewal and allows progress from a proliferative progenitor/stem cell to a postmitotic, differentiated fate [23]. Further investigations have shown that NFκB-dependent intestinal inflammation results in Tyr357-phosphorylation of YAP which in turn drives colonic cell death (via p73 association) in DSS (dextran sulphate sodium)-treated RASSF1A knockout mice, leading to increased gut permeability, lack of effective epithelial repair and poor survival of the animals. On the other hand, increased translocation of YAP to nucleus followed by enhanced proliferation of crypt cells was observed in wild-type mice upon DSS treatment [15]. Moreover, elevated expression of YAP has been reported in human hyperplastic epithelium of colon [22] as well as in CRC [24, 25].
In this study, we examined the RASSF1A and LATS1/2 expression patterns in a cohort of patients with IBD compared with control intestine samples. Furthermore, the relationship between subtypes of IBD and RASSF1A and LATS1/2 expression, both individually, and related to p73, and YAP/pYAP(Ser127) proteins was studied.
Materials and Methods
Patients
The computerized files of the Department of Pathology at “Agios Andreas” Hospital, Patras were searched for IBD, UC, and CD from the January 2010 to December 2014. By careful chart review, data were collected on demographic and clinical variables, including age, gender and, IBD subtype, duration and extent of disease and, the presence or absence of dysplasia. After the diagnosis-based search the following study groups were selected: 47 cases of unequivocal UC (43 active, 4 quiescent) (mean age, 49.6; range, 21–85), 5 cases of UC with dysplasia (low grade) (mean age, 50.6; range 21–80), 24 cases of Crohn’s disease (mean age, 47.63; range, 16–88) (9 ileitis, 15 colitis), and 24 cases of IBD with term of unclassified (IBD-U) (mean age, 47.54; range, 18–85) (48 males, 52 females). Pre-existing haematoxylin and eosin (H and E) stained sections of specimens reported as showing one of the above diagnoses were reviewed and confirmed by one pathologist included in the study (EP). The corresponding tissue blocks were retrieved from archival files of the Department of Pathology at “Agios Andreas” Hospital, Patras. The control group comprised of normal gut tissue samples from non-IBD patients (n = 15; mean age, 70.53 years, range, 36–86 years, 7 males, 8 females) excluded due to colon cancer (retrieved up to 5 cm away from the tumor’s edge), postoperative ileus, and lipomatosis of the ileocecal valve which were collected from the same department, during the same period. The study population was of Greek origin. The use of the human specimens was in accordance with the University Ethics Commission. All research protocols were conducted, and patients were treated in accordance with the tenets of the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry was performed using the un-avidin-biotin complex technique named Envision (Dako Cytomation; Agilent Technologies, Inc., Santa Clara, CA, USA). Consecutive (semi-serial) 4-μm sections were cut from formalin-fixed, paraffin embedded tissue. Sections were mounted on silanized slides and allowed to dry overnight at 37 °C. After deparaffinization and rehydration, antigen retrieval was performed by microwaving the slides in 0.01 M citrate buffer (pH 6). Endogenous peroxidase activity was quenched by treatment with 1% hydrogen peroxide solution for 20 min. Incubation at room temperature with 1% bovine serum albumin (SERVA, Heidelberg, Germany) in Tris-HCL-buffered saline was performed for 10 min. Tissue sections were subsequently incubated with primary antibodies overnight at 4 °C. Primary antibodies included the following mouse monoclonal antibodies: Anti-RASSF1 (clone 8E4) (dilution 1:150) (corresponding to amino acids 1–340 of human RASSF1A), Anti-p73 (clone EP436Y) (dilution 1:100) (raised against to human p73 amino acids 50–150), and a rabbit polyclonal antibody Anti-LATS1 and LATS2 (phosphorylated at threonine 1079/1041) (dilution 1:100) which were purchased from Abcam (Cambridge, UK). Furthermore, a mouse monoclonal Anti-YAP (clone 1A12) (dilution 1:250) (which recognizes endogenous levels of total YAP protein) and a rabbit monoclonal Anti-phospho-YAP(Ser127) (clone D9W2I) (dilution 1:2000) (which recognizes endogenous levels of YAP protein only when phosphorylated at Ser127) were purchased from Cell Signaling Technology (Beverly, MA). After three rinses in buffer, the slides were incubated with the secondary antibody (Envision System). Tissue staining was visualized with 3,3΄-diaminobenzidine (DAB) as a chromogen (which yielded brown reaction products). Slides were counterstained with Mayer’s hematoxylin solution, dehydrated and mounted. To ensure antibody specificity, negative controls included the omission of primary antibody and substitution with non-immune serum. Control slides were invariably negative for immunostaining. As positive controls, human non-small cell lung carcinoma for RASSF1A [11] and YAP [26], astrocytomas for LATS1/2 [27], and urinary bladder carcinoma for p73 were used [28].
Scoring
All immunohistochemical sections were assessed blindly and independently by two observers (MA and PN), followed by joint review for resolution of any differences. The expression of proteins was determined as the mean percentage of positive mucosa epithelial cells in manually counted, with the aid of an ocular grid, ten non-overlapping, random fields (total magnification, ×400) for each case (labeling index, LI; % labeled cells). Immunopositively stained endothelial and lamina propria cells were excluded from the cell counts. The co-expression analysis was assessed for immunopositive samples, in adjacent (semi-serial) sections of each sample. In this study, a sample was designated as immunopositive when more than equal to 10% of mucosa epithelial cells were reactive. Microphotographs were obtained using a Nikon DXM 1200C digital camera mounted on a Nikon Eclipse 80i microscope and ACT-1C software (Nikon Instruments Inc., Melville, NY, USA).
Statistical Analysis
Non-parametric methods were used for statistical analysis of the results. Median comparisons were performed with Wilcoxon’s Rank-Sum test (equivalent to the Mann-Whitney U test) and the Kruskal-Wallis test. Correlation analysis was performed by utilizing Kendall’s τ (or Spearman’s ρ) rank correlation to assess the significance of associations between LIs. For assessment of diagnostic accuracy (test sensitivity and specificity), the receiver operating curves (ROC) with area under curve (AUC) analysis were generated. P values of 0.05 were considered to indicate a statistically significant difference. Statistical analyses were carried out using the SPSS package (version 17.0; SPSS, Inc., Chicago, IL, USA).
Results
Immunoexpression of RASSF1A in IBD Patients and Control Intestine Samples
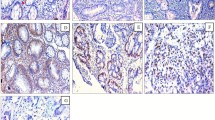
RASSF1A expression was significantly decreased in UC (P = 0.03) and IBD-U (P = 0.01) compared with mucosa of control intestine samples whereas there was not statistically difference between mucosa of control intestine samples and CD (P ≥ 0.05; Table 1). There was a positive correlation of RASSF1A expression with the female gender in UC patients (P = 0.004). The immunolocalization of RASSF1A protein expression was mixed nuclear/cytoplasmic in mucosa cells distributed in the entire epithelium. The intensity staining was moderate to strong in normal mucosa cells whereas in inflammatory intestine, the immunostaining pattern included also weak immunostained cells (Fig. 1).
Panel comparing RASSF1A, LATS1/2, p73, YAP, and pYAP(Ser127) expression in representative samples of normal intestine (a–d), UC (e–h), and CD (i–l). a Strong diffuse immunoreactivity for RASSF1A is detected in the most of mucosa cells in normal intestine. Note that cells in lamina propria are RASSF1A-immunopositive. b Weak cytoplasmic immunoreactivity in some mucosa cells for LATS1/2 in normal intestine. c, d Photomicrographs from adjacent sections of the same normal intestine sample demonstrating strong cytoplasmic immunoreactivity in mucosa cells for YAP (d) and not for pYAP(Ser127) (c). Note pYAP(Ser127)-immunopositivity in mucosal muscle layer and vessels (arrows) as internal positive control (c). e Variability of RASSF1A immunostaining is detected in UC. Note that some mucosa cells show weak to moderate immunoreactivity whereas immunostaining is absent in certain mucosa cells. Cells in lamina propria are RASSF1A-immunopositive. This specimen was LATS1/2-immunopositive and p73/pYAP(Ser127)-immunonegative. f Moderate to strong cytoplasmic immunoreactivity is found for LATS1/2 in numerous cells of mucosa (LI = 90) in this UC sample. (f, insert) Immunostaining is absent in negative control sections. g Weak to moderate cytoplasmic immunostaining for pYAP(Ser127) protein is distributed in mucosa cells of this UC specimen (LI = 60). h Mucosa cells exhibit p73 nuclear immunoreactivity (arrows) in this UC specimen (LI = 50). In addition, RASSSF1A (LI = 95) and LATS1/2 (LI = 20) expression was detected. i A high percentage of mucosa cells (LI = 95) display strong cytoplasmic and nuclear immunoreactivity for RASSF1A in this CD specimen. Increased expression for LATS1/2 (LI = 80) was also found whereas immunoreactivity for p73 and pYAP(Ser127) was not detected. j The LATS1/2-expressing cells with strong immunostaining (LI = 80) are distributed in the entire mucosa of this CD sample. (j, insert) This CD sample did not display RASSF1A immunostaining (LI = 0). k Mucosa cells of CD demonstrate cytoplasmic immunolocalization for YAP (LI = 75). l Arrows indicate p73-immunopositive cells with weak nuclear immunoreactivity in this CD sample. RASSF1A immunopositivity was detected (LI = 80). Counterstain, hematoxylin; original magnification, ×400; scale bar, 50 μm
Immunoexpression of LATS1/2 in IBD Patients and Control Intestine Samples
LATS1/2 expression was statistically increased in UC, IBD-U and Crohn’s disease samples compared with mucosa of control group (P < 0.0001; Table 1). A strong significant correlation between LATS1/2 and RASSF1A expression in UC samples with dysplasia was observed (Spearman correlation = 0.506, P < 0.0001). Moderate to strong immunoreactivity for LATS1/2 expression was primarily localized within the cytoplasm of mucosa cells distributed in the entire epithelium and, in some cases, in cells in lamina propria, and enteric nervous system (Fig. 1). ROC curve analysis showed that LATS1/2 could differentiate ulcerative colitis from control samples with an AUC of 0.798 (95% CI 0.679–0.917), IBD-U from control samples with an AUC of 0.854 (95% CI 0.735–0.973), and Crohn’s disease from control samples with an AUC of 0.890 (95% CI 0.786–0.994) (Fig. 2).
Receiver operating characteristic (ROC) curve analysis using examined LATS1/2 for discriminating IBD patients from healthy individuals. AUC (the area under ROC curve) estimation for the examination of LATS1/2 proteins in discrimination of the UC (a), CD (b) and IBD-U (c) patients from the healthy individuals
Immunoexpression of p73 in IBD Patients and Control Intestine Samples
Weak to strong p73 staining was confined to the nuclear area of mucosa cells distributed, in most cases, in the entire epithelium (Fig. 1). Expression of p73 did not differ significantly between control intestine and IBD samples (P ≥ 0.05; Table 1). The expression of p73 was significantly correlated with RASSF1A in UC (Spearman correlation = 0.440, P < 0.001) and IBD-U (Spearman correlation = 0.464, P < 0.02).
Immunoexpression of YAP and pYAP(Ser127) in IBD Patients and Control Intestine Samples
The immunolocalization of YAP and pYAP(Ser127) in epithelial cells was primarily cytoplasmic (Fig. 1). Immunoreactivity of YAP showed the same distribution pattern as pYAP(Ser127) did and a significant strong correlation between YAP and pYAP(Ser127) expression was found (P < 0.0001). However, YAP nuclear immunolocalization was also noticed in a few epithelial cells of some UC samples. Expression of YAP and pYAP(Ser127) did not differ significantly between control intestine and IBD samples (P ≥ 0.05; Table 1). In mucosa of control group, a strong inverse correlation between pYAP(Ser127) and RASSF1A expression was observed (Spearman correlation = −0.804, P < 0.0001). YAP expression was significantly correlated with p73 expression in UC (Spearman correlation = 0.274, p = 0.01).
No significant difference was detected for all proteins examined between subtypes of IBD (P ≥ 0.05). Co-expression of RASSF1A with LATS1/2 and p73/or pYAP(Ser127) was found in IBD and control samples (Table 2). A few (3%) IBD samples demonstrated RASSF1A and p73/or pYAP(Ser127) co-expression, being LATS1/2 immunonegative. Finally, although the median age of the cohort of healthy controls was higher than that of IBD patients, there was no significant correlation between age at the time of biopsy and expression of RASSF1A, LATS1/2, p73, and YAP proteins (P ≥ 0.05).
Discussion
Previous studies report expression of RASSF1A [12,13,14] and the members of HIPPO pathway YAP and, p73 in human normal mucosa [25, 29]. In this study, expression profile of RASSF1A and HIPPO signaling pathway phosphorylated kinases LATS1/2, and p73, and YAP proteins was analyzed in patients with IBD compared with control intestine. RASSF1A expression decreased significantly in UC and IBD-U compared with control samples. It is worth noting that, expression of RASSF1A was similar in CD and control intestine. Previous knowledge shows that promoter hypermethylation of RASSF1A exists in a vast number of cancerous and precancerous colorectal tissues [30]. Notably, RASSF1A methylation has been reported in an important percentage of matching normal colonic mucosa tissues available for CRC [12, 13, 31] suggesting that silencing of RASSF1A, in sites proximal to tumor, may play a role in colorectal cancer. Abouzeid et al. (2011) also reported RASSF1A hypermethylation in 26% (5/19) of UC samples included in their study [12]. According to the above data, RASSF1A–immunonegativity found in certain control and IBD intestine samples of our study may be the result of promoter silencing due to possible methylation. Moreover, in the present study, RASSF1A immunolocalization in mucosa cells, mainly in IBD samples, was cytoplasmic as well as nuclear. There is emerging evidence implicating RASSF1A with MDM2 in the nucleus promoting MDM2 self-ubiquitination in cell-cycle p53 checkpoint control in response to DNA damage [10]. Considering that RASSF1A mediates RAS-dependent apoptosis, the nuclear immunolocalization may indicate a specific role in proapoptotic events to protect mucosal cells. Additionally, a positive correlation of RASSF1A expression with the female gender in UC patients was found. This observation confirms previous results showing higher frequency of loss of RASSF1A expression in men compared with women [14].
LATS1/2 expression was significantly increased in UC, IBD-U as well as CD compared with control mucosa. LATS1 and LATS2 kinases are classically found as key molecules in the HIPPO pathway, but they are also implicated in the regulation of cytoskeletal dynamics and migration, protein stability, transcriptional activity, and maintenance of genetic stability [32]. Investigations in human tumors showed decreased expression of LATS1 in gliomas, breast, gastric, and colorectal cancers, due to hypermethylation of LATS1 gene promoter [27, 33] whereas, overexpression of LATS1 inhibited tumorigenicity in vivo by inducing apoptosis [34]. Furthermore, in vitro experiments in glioma cells revealed that forced expression of LATS1 retarded cell cycle progression [27]. Consequently, the elevated expression of LATS1/2 kinases, independently of IBD subtype, may indicate a possible role of these proteins in protection of mucosal cell integrity. Interestingly, even though a high percentage of inflammatory bowel disease samples co-expressed LATS1/2 and RASSF1A proteins, only in UC samples with dysplasia, LATS1/2 expression levels were significantly associated with RASSF1A expression. Moreover, curve analysis revealed that the elevated expression of LATS1/2 proteins in mucosa cells could be used to distinction of IBD patients, independently of disease activity and type, from healthy individuals. Since endoscoping healing after a therapy treatment is not necessarily paralleled by histological healing in IBD and the histological features of IBD are variable in time [35], the management of IBD patients requires the identification of sensitive and specific molecular markers to distinguish long standing IBD patients from normal as well as assessment of different types and disease activity of IBD.
It is well known that the impact of RASSF1A on HIPPO signaling may modulate different apoptotic pathways recruiting many proteins to promote cell death [36]. The interaction between RASSF1A and HIPPO pathways has been shown to potentiate apoptosis through p73 activation in an experimental model of chemically induced colitis in mice [15]. In this study, a proportion of RASSF1A-LATS1/2-immunopositive IBD and control intestine samples displayed co-expression of p73. Furthermore, the nuclear expression levels of p73 coincided significantly with RASSF1A expression in UC and IBD-U samples. The above facts indicate a potential cooperation of RASSF1A and HIPPO signaling pathway in human inflamed intestine. Importantly, a few IBD samples demonstrated RASSF1A and p73/or pYAP(Ser127) co-expression, being LATS1/2 immunonegative suggesting that further investigation is needed for the molecular mechanisms underlying RASSF1A and HIPPO cross-talk in human IBD.
Finally, previous reports have shown the lack of response of some UC and CD patients with the anti-TNF agents such as infliximab [37, 38]. As the intestinal inflammation involves both loss of epithelial cells accompanied with increased epithelial proliferation, as a repair mechanism, inhibiting possible disproportionate crosstalk between RASSF1A and HIPPO signaling may aid in designing future treatment schemes for patients with chronic bowel inflammation.
References
Maher MM (2012) Inflammatory bowel disease: review and future view. Front Biosci (Elite Ed) 4:1638–1647
Conrad K, Roggenbuck D, Laass MW (2014) Diagnosis and classification of ulcerative colitis. Autoimmun Rev 13(4–5):463–466
Laass MW, Roggenbuck D, Conrad K (2014) Diagnosis and classification of Crohn's disease. Autoimmun Rev 13(4–5):467–471
Feakins RM (2014) Ulcerative colitis or Crohn's disease? Pitfalls and problems. Histopathology 64(3):317–335
Martland GT, Shepherd NA (2007) Indeterminate colitis: definition, diagnosis, implications and a plea for nosological sanity. Histopathology 50(1):83–96
Okayasu I (2012) Development of ulcerative colitis and its associated colorectal neoplasia as a model of the organ-specific chronic inflammation-carcinoma sequence. Pathol Int 62:368–380
Rogler G (2014) Chronic ulcerative colitis and colorectal cancer. Cancer Lett 345:235–241
Donninger H, Vos MD, Clark GJ (2007) The RASSF1A tumor suppressor. J Cell Sci 120:3163–3172
Overmeyer JH, Maltese WA (2011) Death pathways triggered by activated Ras in cancer cells. Front Biosci (Landmark Ed) 16:1693–1713
Song MS, Song SJ, Kim SY, Oh HJ, Lim DS (2008) The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2–DAXX–HAUSP complex. EMBO J 27:1863–1874
Agathanggelou A, Cooper WN, Latif F (2005) Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res 65:3497–3508
Abouzeid HE, Kassem AM, Abdel Wahab AH, El-mezayen HA, Sharad H, Abdel Rahman S (2011) Promoter hypermethylation of RASSF1A, MGMT, and HIC-1 genes in benign and malignant colorectal tumors. Tumour Biol 32:845–852
Sakamoto N, Terai T, Ajioka Y, Abe S, Kobayasi O, Hirai S, Hino O, Watanabe H, Sato N, Shimoda T, Fujii H (2004) Frequent hypermethylation of RASSF1A in early flat-type colorectal tumors. Oncogene 23(55):8900–8907
Cao D, Chen Y, Tang Y, Peng XC, Dong H, Li LH, Cheng K, Ge J, Liu JY (2013) Loss of RASSF1A expression in colorectal cancer and its association with K-ras status. Biomed Res Int 2013:976765
Gordon M, El-Kalla M, Zhao Y, Fiteih Y, Law J, Volodko N, Anwar-Mohamed A, El-Kadi AO, Liu L, Odenbach J, Thiesen A, Onyskiw C, Ghazaleh HA, Park J, Lee SB, Yu VC, Fernandez-Patron C, Alexander RT, Wine E, Baksh S (2013) The tumor suppressor gene, RASSF1A, is essential for protection against inflammation-induced injury. PLoS One 8(10):e75483
Hiemer SE, Varelas X (2013) Stem cell regulation by the Hippo pathway. Biochim Biophys Acta 1830(2):2323–2334
Ramos A, Camargo FD (2012) The Hippo signaling pathway and stem cell biology. Trends Cell Biol 22(7):339–346
Hong W, Guan KL (2012) The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol 23(7):785–793
Levy D, Adamovich Y, Reuven N, Shaul Y (2008) Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell 29(3):350–361
Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O'neill E (2007) RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27(6):962–975
Li VS, Clevers H (2013) Intestinal regeneration: YAP-tumor suppressor and oncoprotein? Curr Biol 23(3):R110–R112
Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D (2010) The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24(21):2383–2388
Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD (2013) Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493(7430):106–110
Avruch J, Zhou D, Bardeesy N (2012) YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle 11(6):1090–1096
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, Wen W, Zhu Q (2013) Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One 8(6):e65539
Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X (2010) Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 101:1279–1285
Ji T, Liu D, Shao W, Yang W, Wu H, Bian X (2012) Decreased expression of LATS1 is correlated with the progression and prognosis of glioma. J Exp Clin Cancer Res 31:67
Puig P, Capodieci P, Drobnjak M, Verbel D, Prives C, Cordon-Cardo C, Di Como CJ (2003) p73 expression in human normal and tumor tissues: loss of p73 expression is associated with tumor progression in bladder cancer. Clin Cancer Res 9:5642–5651
Guan M, Peng HX, Yu B, Lu Y (2003) p73 Overexpression and angiogenesis in human colorectal carcinoma. Jpn J Clin Oncol 33(5):215–220
Fernandes MS, Carneiro F, Oliveira C, Seruca R (2013) Colorectal cancer and RASSF family--a special emphasis on RASSF1A. Int J Cancer 132(2):251–258
Wagner KJ, Cooper WN, Grundy RG, Caldwell G, Jones C, Wadey RB, Morton D, Schofield PN, Reik W, Latif F, Maher ER (2002) Frequent RASSF1A tumour suppressor gene promoter methylation in Wilms' tumour and colorectal cancer. Oncogene 21(47):7277–7282
Visser S, Yang X (2010) LATS tumor suppressor: a new governor of cellular homeostasis. Cell Cycle 9(19):3892–3903
Wierzbicki PM, Adrych K, Kartanowicz D, Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I, Celinski K, Gach T, Kulig J, Korybalski B, Kmiec Z (2013) Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol 19(27):4363–4373
Xia H, Qi H, Li Y, Pei J, Barton J, Blackstad M, Xu T, Tao W (2002) LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene 21(8):1233–1241
Villanacci V, Antonelli E, Geboes K, Casella G, Bassotti G (2013) Histological healing in inflammatory bowel disease: a still unfulfilled promise. World J Gastroenterol 19(7):968–978
Fausti F, Di Agostino S, Sacconi A, Strano S, Blandino G (2012) Hippo and rassf1a pathways: a growing affair. Mol Biol Int 2012:307628
Arijs I, Li K, Toedter G, Quintens R, Van Lommel L, Van Steen K, Leemans P, De Hertogh G, Lemaire K, Ferrante M, Schnitzler F, Thorrez L, Ma K, Song XY, Marano C, Van Assche G, Vermeire S, Geboes K, Schuit F, Baribaud F, Rutgeerts P (2009) Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut 58(12):1612–1619
Arijs I, Quintens R, Van Lommel L, Van Steen K, De Hertogh G, Lemaire K, Schraenen A, Perrier C, Van Assche G, Vermeire S, Geboes K, Schuit F, Rutgeerts P (2010) Predictive value of epithelial gene expression profiles for response to infliximab in Crohn's disease. Inflamm Bowel Dis 16(12):2090–2098
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nterma, P., Panopoulou, E., Papadaki-Petrou, E. et al. Immunohistochemical Profile of Tumor Suppressor Proteins RASSF1A and LATS1/2 in Relation to p73 and YAP Expression, of Human Inflammatory Bowel Disease and Normal Intestine. Pathol. Oncol. Res. 26, 567–574 (2020). https://doi.org/10.1007/s12253-018-00575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-00575-z