Abstract
The presence of cancer stem-like cells (CSCs) has been demonstrated to be associated with tumor metastasis, chemoresistance, and rapid recurrence of various tumors. The impact of CSC-related markers in the metastasis and prognosis of ovarian cancer has not been well established. In this study, the protein expression of musashi-1 and ALDH1 was measured using immunohistochemistry. Results demonstrated that the percentage of positive musashi-1 and ALDH1 expression were significantly higher in ovarian serous adenocarcinomas, mucinous adenocarcinomas and clear cell adenocarcinomas than in cystadenomas and normal tissues. The percentage of positive musashi-1 and ALDH1 expression were significantly lower in patients identified with clinical stage I or II ovarian adenocarcinomas without lymph node metastasis compared to patients with clinical stage III or IV tumors and lymph node metastasis. The expression of musashi-1 and ALDH1 was found to be highly consistent in ovarian adenocarcinomas. Univariate Kaplan-Meier analysis showed a negative correlation between musashi-1 or ALDH1 expression and overall survival. Multivariate Cox regression analysis showed that positive expression of musashi-1 or ALDH1 in ovarian adenocarcinoma was an independent predictor of poor prognosis. Our study suggested that musashi-1 and ALDH1 expression are closely related to metastasis of ovarian adenocarcinoma. The positive expression of musashi-1 and ALDH1 might be a poor-prognostic factor of ovarian adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the fifth most common gynecologic malignancy in women and the leading cause of death among gynecologic malignancies worldwide [1]. This disease is characterized by few early symptoms and poor prognosis [2, 3]. More than 60 % of women diagnosed with ovarian cancer have stage III or stage IV cancer in which tumor cells have already spread beyond the ovaries. Currently, surgical treatment is the primary treatment for well-differentiated and localized tumors. For patients with advanced disease, the standard treatment is a combination of reduction surgical treatment and chemotherapy. However, the usefulness of chemotherapy is debatable. Many patients with ovarian cancer that initially respond well to chemotherapy eventually relapse with drug-resistant disease [4]. Although many studies have tried to investigate the mechanisms responsible for chemoresistance, the mechanism has not been elucidated.
Cancer stem-like cells (CSC) were first identified by Dr. John Dick in 1999 [5]. Usually, CSCs are quiescent and generate tumors through the differentiation of stem-like cells into multiple cell types [6]. Notably, current studies point to the presence of a small subpopulation of CSCs, which are resistant to current chemotherapy and radiation regimens and may be responsible for relapse and metastasis of cancers [7]. Benson et al. study found that the remaining tumor cells after anti-cancer therapy have a distinct molecular phenotype compared to tumor cells that were sensitive to the therapy [8], which supports the theory that CSCs escape chemotherapy and allow the tumor to grow back after an initial remission [9]. However, there is currently no widely accepted CSC marker for ovarian cancer.
Aldehyde dehydrogenase (ALDH1) is a cytosolic enzyme responsible for oxidizing a variety of intracellular aldehydes to carboxylic acids [10] and is widely distributed in a variety of tissues. Recently, ALDH1 activity has been employed as a marker of stem-like cells in many cancers including cervical and breast cancers [9]. Also, high activity of ALDH1 is associated with poor prognosis in breast, bladder and prostate cancer patients [9, 11]. Moreover, ALDH1 activity was demonstrated to be significantly higher in metastatic breast tumor cells that escaped chemotherapy [12]. ALDH1 expression was found to be significantly increased after chemotherapy while high ALDH1 phenotype is a good predictive marker for chemotherapy resistance and poor prognosis [13]. ALDH1 also plays an important role in tumor cell proliferation, invasion, and metastasis [14, 15]. However, the role of ALDH1 in ovarian cancer remains controversial. Chang et al study suggests that ALDH1 is a favorable prognostic factor in ovarian carcinoma [16]. Therefore, other CSC markers need to be identified and ALDH1’s role as a CSC marker in ovarian cancer requires further investigation.
The potential of CSCs to escape anti-tumor therapy is probably related to the regulation of gene expression by some key molecules [17]. One candidate gene is musashi-1. Musashi-1 was first identified as a RNA-binding protein required for asymmetric division of sensory neuron precursor cells [18]. Musashi-1 expression was then found in mouse small intestine and in human colon crypt stem cells [19, 20]. Recently, musashi-1 expression was identified in a variety of cancers, including endometrial carcinoma [21]. The expression of musashi-1 in these malignancies was considered a marker of their progression, metastasis, and prognosis. In addition, musashi-1 has been shown to activate the Notch signaling pathway by suppressing the translation of the Notch inhibitor m-Numb [22]. Therefore, musashi-1 is not only a marker of cancer stem cells but also functions as a signaling molecule involved in the protection of stem cells from chemo- or radiotherpy. However, the expression of musashi-1 in ovarian cancer has not been investigated.
In this study, the expressions of musashi-1 and ALDH1 in surgically resected specimens including adenocarcinoma, mucinous cystadenoma tissues, and normal ovarian tissues were examined using immunohistochemistry. The correlation of musashi-1 and ALDH1 expressions with the behavior and prognosis of adenocarcinoma, clinical manifestations, and survival were evaluated.
Materials and Methods
Case Selection
Eighty ovarian adenocarcinomas, 30 ovarian cystadenomas, and 10 normal ovarian tissues were collected between 1996 and 1999. All diagnoses were based on morphological criteria, immunohistochemical staining, and clinical findings. Patients of all 80 adenocarcinomas were not given chemotherapy before surgical resection. The histopathologic subtypes of the 80 adenocarcinomas included 40 serous cystadenocarcinomas, 25 mucinous cystadenocarcinomas, and 15 clear cell adenocarcinoma. The histopathologic grading of the 80 adenocarcinoma patients revealed that 20 patients had stage I (G1), 28 patients had G2 and 32 patients had G3, respectively. TNM staging was determined using the FIGO (International Federation of Gynecology and Obstetrics) staging system. According to the FIGO system, 14 cases had stage I, 15 had stage II, 32 had stage III, and 19 had stage IV. Among the 80 patients with adenocarcinomas, 50 patients had regional lymph node metastasis, while 30 had no metastasis. Among the 80 adenocarcinomas, 29 developed in the left ovary, 26 in the right ovary, and 25 developed bilaterally. Survival information of 80 patients with ovarian adenocarcinomas was obtained through letters and phone calls, of which 38 patients survived less than 5 years, 27 patients survived 5–10 years, and 15 patients survived longer than 10 years. No case was given preoperative chemotherapy. Sixty-five cases received postoperative chemotherapy, including 30 cases for PC regimen (platinum + cyclophosphamide) and 35 cases for TP regimen (cisplatin + paclitaxel). The histopathologic subtypes of the 30 ovarian cystadenomas included 15 serous cystadenomas and 15 mucinous cystadenomas. The study was approved by the Ethics Committee of Xiangya hospital, Central South University. Signed informed consent forms were obtained from all subjects who participated in the study. The Code of Ethics of the World Medical Association (Declaration of Helsinki) was followed.
EnVision Immunohistochemistry
Four-micrometer-thick sections were cut from routinely paraffin-embedded tissues. EnVision™ Detection Kit was purchased from Dako Laboratories (CA, USA). The staining of musashi-1 and/or ALDH1 was carried out according to the manufacture’s protocol. Rabbit anti-human musashi-1 and ALDH1 primary antibodies were purchased from Cruz Biotechnology (Santa Cruz, CA, USA). Briefly, the sections were deparaffinized and then incubated with peroxidase inhibitor (3 % H2O2) in the dark for 15 min, followed by EDTA-trypsin digestion for 15 min. The sections were then incubated with primary antibody for 60 min, followed by second antibody for 30 min, and washed with PBS for 3 × 5 min. Solution A was added to the sections for 30 min followed by DAB staining and hematoxylin counter-staining. The slides were dehydrated with different concentrations (70–100 %) of alcohol, soaked in xylene for 3 × 5 min, and finally mounted with neutral balsam. The positive controls were positive sections of musashi-1 and ALDH1 in hepatocellular carcinoma provided by Cruz Biotechology Agent (Beijing Zhongshan Golden Bridge Biotechology Co., Ltd, Beijing, China). The positive controls were positive sections of musashi-1 and ALDH1 in hepatocellular carcinoma provided by Cruz Biotechology Agent (Beijing Zhongshan Golden Bridge Biotechology Co., Ltd, Beijing, China). The negative controls used 5 % fetal bovine serum in place of primary antibodies. The positive case was determined mainly by the percentage of positive stained cells with a cutoff value of 10 %. To determine the percentage, the positively and negatively stained cells were counted from 10 random fields and the percentage of positive cells was calculated. The case with positive cells ≥10 % was considered positive.
Statistical Analysis
Data was analyzed using the statistical package for the Social Sciences Version 13.0 (SPSS 13.0). The relationship of musashi-1 or ALDH1 expression with histology or clinical factors was analyzed using χ2 or Fisher’s exact test. Kaplan-Meier and time series test (log-rank test) were used for univariate survival analysis. Cox proportional hazards model was used for multivariate analysis and to determine the 95 % confidence interval.
Results
Musashi-1 and ALDH1 Expression in Ovarian Adenocarcinomas, Mucinous Cystadenomas and Normal Ovarian Tissues
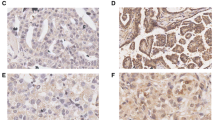
Immunohistochemistry showed that both musashi-1 (Fig. 1) and ALDH1 (Fig. 2) positive reactions were mainly localized in the cytoplasm. The percentage of cases with positive musashi-1 or ALDH1 expression was significantly higher in ovarian serous adenocarcinomas (57.5 %, 50.0 %, respectively), mucinous adenocarcinomas (56.0 %, 52.0 %, respectively) and clear cell adenocarcinomas (66.7 %, 53.3 %, respectively) than in cystadenoma tissues (6.7 %, p < 0.001) and normal tissues (0 %, p < 0.001) (Table 1). This suggests that both musashi-1 and ALDH1 could be used to evaluate pre-malignancy changes. Of the 47 musashi-1 positive cases, ALDH1 was positively expressed in 31 cases, suggesting high consistency in expression between these two markers (p < 0.001).
Immunohistochemistry of musashi-1 expression. Immunohistochemistry showed that positive reaction was mainly localized in the cell cytoplasm. a) Positive musashi-1 expression was observed in serous adenocarcinoma. b) Positive musashi-1 expression in mucinous adenocarcinoma. c) Positive musashi-1 expression in clear cell adenocarcinoma. d) Negative musashi-1 expression was observed in cystadenoma
Immunohistochemistry of ALDH1 expression. Immunohistochemistry showed that positive reaction was mainly localized in the cell cytoplasm. a) Positive ALDH1 expression was observed in clear cell adenocarcinoma. b) Positive ALDH1 expression was observed in serous cystoadenoma. c) Positive ALDH1 expression was observed in mucinous cystoadenoma. d) Negative ALDH1 expression was observed in clear cell adenocarcinoma
The Association of Musashi-1 and ALDH1 Expressions with Clinicopathological Characteristics of Ovarian Adenocarcinoma
As shown in Table 2, expression of musashi-1 and ALDH1 was significantly lower in patients with clinical stage I and II tumors with no lymph node metastasis than patients with clinical stage III and IV tumor with lymph node metastasis of adenocarcinoma (p < 0.001 or p = 0.004). Expression of musashi-1 was significantly lower in cases with G1 than in cases with G3 (p = 0.009). Also, expression of ALDH1 was significantly lower in hemilateral cases than in bilateral cases (p = 0.043). Musashi-1 and ALDH1 expression exhibited no significant association with histopathologic subtypes and other clinicopathological characteristics, such as the sex and age.
The Correlation Between Musashi-1 and ALDH1 Expression with Survival in Patients with Ovarian Adenocarcinoma
Survival information of 80 patients with adenocarcinoma was obtained through letters and phone calls. Of which, 38 patients survived less than 5 years (2 patients died in the first year, 36 died in 2nd, 3rd, and 4th years, respectively, 10 died in 5th year), 27 patients survived 5–10 years (10 patients died in the 5th year, 6 in the 6th year, 6 in the 7th year, 3 in the 8th year, 1 in the 9th year, and 1 in the 10th year), and 15 patients survived longer than 10 years. The Kaplan-Meier survival analysis revealed that the histological grades (p = 0.000), lymph node metastasis (p = 0.000), clinical stage (p = 0.000) and tumor location in the ovary (p = 0.005) were significantly associated with the average survival time in the ovarian adenocarcinoma patients. The average survival time of musashi-1 or ALDH1 positive patients was significantly shorter than patients with negative musashi-1 or ALDH1 expression (PMusashi-1 = 0.000, PALDH1 = 0.000) (Table 3, Fig. 3a and b). In addition, the average survival time of patients having both musashi-1 and ALDH1 positive expression was significantly lower than patients with both musashi-1 and ALDH1 negative expression (Table 3, Fig. 3c). Cox multivariate analysis showed that histological grades, lymph node metastasis, clinical stage as well as musashi-1 or ALDH1 positive expression negatively correlated with overall survival and positively correlated with mortality. This suggests that histological grading, lymph node metastasis, clinical stage, and musashi-1 and ALDH1 expressions are risk factors and have strong impact on prognosis of ovarian cancer (Table 4).
Musashi-1 or ALDH1 expression and survival in patients with ovarian adenocarcinoma. a) Kaplan-Meier plots of overall survival in patients with ovarian adenocarcinoma and with musashi-1 positive and negative expression. b) Kaplan-Meier plots of overall survival in patients with ovarian adenocarcinoma and with ALDH1 positive and negative expression. c) Kaplan-Meier plots of overall survival in patients with ovarian adenocarcinoma and with musashi-1 (+)ALDH1(+) and musashi-1(−)ALDH1(−) expression
Discussion
Cancer stem-like cells have been widely identified in ovarian cancers. However, the prognostic impact of the cancer stem cell-related markers has not been widely identified in ovarian adenocarcinomas. In this study, we measured the expression of ALDH1 together with musashi-1 in 80 ovarian adenocarcinomas. We then compared the expression of the two markers in ovarian adenocarcinomas with their expression in ovarian benign lesions, and normal tissues of the ovary. Normal ovarian tissues were found not to express musashi-1 and ALDH1, benign lesions were found to express very low levels of musashi-1 and ALDH1, and adenocarcinomas were found to express high levels of the two markers. Musashi-1 and ALDH1 levels significantly correlated with tumor metastases and clinical outcomes of adenocarcinomas. The elevated musashi-1 and ALDH1 protein levels are also important poor-prognostic factors independent of other clinicopathological factors. To our knowledge, this is the first report to identify musashi-1 in ovarian cancer and document the correlation of musashi-1 and ALDH1 expression in patients with ovarian cancer. Therefore, our study provided a chance to validate the role of CSC--related markers in the progression and prognosis of ovarian cancers.
A previous study demonstrated that ALDH1 was detected in 48.9 % of ovarian carcinomas [16], but it is a favorable prognostic factor. However, ALDH1 activity has been identified as an effective CSC marker and predictor of poor prognosis in several tumors [9]. In this study, 58.8 and 51.3 % of ovarian adenocarcinomas positively expressed musashi-1 and ALDH1, respectively. Positive musashi-1 and ALDH1 expression significantly correlated with lymph node metastasis and clinical stage, suggesting that musashi-1 and ALDH1-positive tumor cells may be responsible for metastasis and worsening clinical outcomes of ovarian cancers. Unitivariate Kaplan-Meier analysis showed that positive expression of musashi-1 or ALDH1 was associated with decreased overall survival of ovarian adenocarcinomas. Multivariate Cox regression analysis showed that positive musashi-1 or ALDH1 expression was an independent poor-prognostic factor in ovarian adenocarcinoma (Table 4). This finding suggested that musashi-1 and ALDH1 are poor-prognostic factors for ovarian cancer.
Cancer cells with positive ALDH1 expression have been demonstrated to be resistant to chemotherapy in breast cancer [13] and lung cancer [23]. This might be due to the fact that once CSCs are treated with chemotherapy, ALDH expression levels increased, thereby allowing CSCs to acquire the ability to become drug resistant [11]. However, the role of ALDH1 in ovarian cancer was conflicting. Chang et al. study revealed that ALDH1 is a favorable prognostic factor in ovarian endometrioid adenocarcinomas [16]. Penumatsa et al. study found that a low level of ALDH1 was expressed in poorly differentiated malignant serous ovarian cancer [24]. Interestingly, Wang et al., study using tissue microarray of epithelial ovarian cancer samples revealed that patients with higher ALDH1 expression had poor overall survival. However, their results did not support ALDH1 alone as an ovarian cancer stem cell marker [25]. In contrast, Deng et al. study revealed that serous ovarian cancer with a high percentage of ALDH1 positive cells was associated with poor clinical outcomes [26]. A recent immunohistochemical study demonstrated that higher ALDH1 levels are related to poorer prognosis of patients with ovarian serous adenocarcinoma and clear cell adenocarcinoma [27]. Moreover, ALDH1 has been demonstrated to be a poor prognostic factor in other gynecologic malignancies in women. For example, high ALDH1 expression was an independent factor for poor prognosis of uterine endometrioid adenocarcinoma [28]. It is currently unclear whether the inconsistent findings are histopathologic-type specific or comes from the differences in antibody specificity. Our study demonstrated that high ALDH1 expression correlated with short overall survival time in ovarian adenocarcinomas (40 serous adenocarcinomas and 25 mucinous adenocarcinomas), which was not associated with the histopathological subtype of ovarian adenocarcinomas.
Interestingly, our study revealed that ALDH1 expression was consistent with musashi-1 expression in ovarian adenocarcinomas. Musashi-1 is involved in the fate decisions of stem cells, including the maintenance of the stem cell state, differentiation and tumorigenesis [29, 30]. Tumors with high musashi-1 expression are more likely to recur and have poor prognosis [11, 31, 32]. In addition, musashi-1 is an important molecule involved in the protection of stem cells. In this study, ALDH1 was positively expressed in 26 cases of 37 musashi-1 positive cases, suggesting a high consistency between these two markers in ovarian cancer (P = 0.000). The average survival time of musashi-1 or ALDH1 positive patients was significantly lower than patients with negative musashi-1 or ALDH1 expression (Pmusashi-1 = 0.000, PALDH1 = 0.000) (Table 3, Fig. 3a and b). In addition, the average survival time of patients having musashi-1 and ALDH1 expression was significantly lower than patients with neither musashi-1 nor ALDH1 expression (Table 3, Fig. 3c). With the knowledge that chemotherapeutics only interfere with rapidly growing cells, musashi-1 and ALDH1-positive CSCs might be spared, leading to tumor metastasis and rapid recurrence of tumor in ovarian cancers after chemotherapy.
By considering that cancer stem-like cells confer resistance to chemotherapy possibly through the modification of gene expression of key markers, targeting these markers will sensitize the chemotherapy of ovarian cancer. However, whether a gene could serve as a marker of stem/progenitor cells is dependent on its abundance in both the tumor and its corresponding normal tissues [26]. Consistent with previous studies, ALDH1 is expressed in a limited number of cells of ovarian adenocarcinomas and not expressed in normal ovary tissues. Also, the percentage of ALDH1 positive expression in benign cystadenoma is low (6.7 %). Therefore, ALDH1 could be used as a stem cell marker for ovarian adenocarcinomas. Interestingly, musashi-1 exhibited a similar expression pattern to ALDH1 in tumor, benign and normal ovarian tissues. The highly consistent expression between musashi-1 and ALDH1 suggested that they are similar markers for stem/progenitor cells in ovarian cancers.
In conclusion, positive expressions of musashi-1 and ALDH1 in ovarian adenocarcinoma are important cancer stem cell markers that are associated with clinical biological behavior and prognosis.
References
Van Simaeys D, López-Colón D, Sefah K, Sutphen R, Jimenez E, Tan W (2010) Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PLoS One 5:e13770
Hartge P (2010) Designing early detection programs for ovarian cancer. J Natl Cancer Inst 102:3–4
Willmott LJ, Fruehauf JP (2010) Targeted therapy in ovarian cancer. J Oncol 2010:740472
Hassan MK, Watari H, Christenson L, Bettuzzi S, Sakuragi N (2011) Intracellular clusterin negatively regulates ovarian chemoresistance: compromised expression sensitizes ovarian cancer cells to paclitaxel. Tumour Biol 32:1031–1047
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Yen TH, Wright NA (2006) The gastrointestinal tract stem cell niche. Stem Cell Rev 2:203–212
Dave B, Chang J (2009) Treatment resistance in stem cells and breast cancer. J Mammary Gland Biol Neoplasia 14:79–82
Douville J, Beaulieu R, Balicki D (2009) ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev 18:17–25
Benson DM Jr, Panzner K, Hamadani M, Hofmeister CC, Bakan CE, Smith MK, Elder P, Krugh D, O’Donnell L, Devine SM (2010) Effects of induction with novel agents versus conventional chemotherapy on mobilization and autologous stem cell transplant outcomes in multiple myeloma. Leuk Lymphoma 51:243–251
Riveros-Rosas H, Julian-Sanchez A, Pina E (1997) Enzymology of ethanol and acetaldehyde metabolism in mammals. Arch Med Res 28:453–471
Ma I, Allan AL (2011) The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev 7:292–306
Sladek NE, Kollander R, Sreerama L, Kiang DT (2002) Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: a retrospective study, rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer Chemoth Pharm 49:309–321
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15:4234–4241
Alison MR, Guppy NJ, Lim SM, Nicholson LJ (2010) Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose. J Pathol 222:335–354
Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky SH, Birnbaum D, Viens P, Wicha MS (2010) Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res 16:45–55
Chang B, Liu G, Xue F, Rosen DG, Xiao L, Wang X, Liu J (2009) ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol 22:817–823
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A 100:15178–15183
Okabe M, Imai T, Kurusu M, Hiromi Y, Okano H (2001) Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature 411:94–98
Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H (2003) Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71:28–41
Nishimura S, Wakabayashi N, Toyoda K, Kashima K, Mitsufuji S (2003) Expression of musashi-1 in human normal colon crypt cells: a possible stem cell marker of human colon epithelium. Dig Dis Sci 48:1523–1529
Götte M, Greve B, Kelsch R, Müller-Uthoff H, Weiss K, Kharabi Masouleh B, Sibrowski W, Kiesel L, Buchweitz O (2011) The adult stem cell marker musashi-1 modulates endometrial carcinoma cell cycle progression and apoptosis via notch-1 and p21(WAF1/CIP1). Int J Cancer 129:2042–2049
Okano H, Imai T, Okabe M (2002) Musashi: a translational regulator of cell fate. J Cell Sci 115:1355–1359
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL (2009) Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 7:330–338
Penumatsa K, Edassery SL, Barua A, Bradaric MJ, Luborsky JL (2010) Differential expression of aldehyde dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian tumors. J Ovarian Res 3:28
Wang YC, Yo YT, Lee HY, Liao YP, Chao TK, Su PH, Lai HC (2012) ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am J Pathol 180:1159–1169
Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang Y, Montone K, Bützow R, Coukos G, Zhang L (2010) Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One 5:e10277
Kuroda T, Hirohashi Y, Torigoe T, Yasuda K, Takahashi A, Asanuma H, Morita R, Mariya T, Asano T, Mizuuchi M, Saito T, Sato N (2013) ALDH1-high ovarian cancer stem-like cells can be isolated from serous and clear cell adenocarcinoma cells, and ALDH1 high expression is associated with poor prognosis. PLoS One 8:e65158
Rahadiani N, Ikeda J, Mamat S, Matsuzaki S, Ueda Y, Umehara R, Tian T, Wang Y, Enomoto T, Kimura T, Aozasa K, Morii E (2011) Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid adenocarcinoma and its clinical implications. Cancer Sci 102:903–908
Todaro M, Francipane MG, Medema JP, Stassi G (2010) Colon cancer stem cells: promise of targeted therapy. Gastroenterology 138:2151–2162
Todaro M, Perez Alea M, Scopelliti A, Medema JP, Stassi G (2008) IL-4-mediated drug resistance in colon cancer stem cells. Cell Cycle 7:309–313
Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, Held-Feindt J (2008) Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol 86:31–45
Moreira AL, Gonen M, Rekhtman N, Downey RJ (2010) Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol 23:889–895
Conflicts of Interest
The authors declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Px., Li, Qy. & Yang, Z. Musashi-1 Expression is a Prognostic Factor in Ovarian Adenocarcinoma and Correlates with ALDH-1 Expression. Pathol. Oncol. Res. 21, 1133–1140 (2015). https://doi.org/10.1007/s12253-015-9943-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-015-9943-6