Abstract
Regional hypoxia caused by accelerated cell proliferation and overgrowth is an important characteristic of neoplasm. Hypoxia can cause a series of changes in gene transcription and protein expression, thereby not only inducing tumor cell resistance to radiotherapy and chemotherapy but also promoting tumor invasion and metastasis. This study aimed to investigate the relationship between HIF-1α expression and cellular apoptosis, angiogenesis and clinical prognosis in rectal carcinoma. In 113 rectal carcinoma cases, cellular apoptosis was analyzed by the in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, whereas the levels of HIF-1α expression, VEGF expression, microvessel density (MVD) and lymphatic vessel density(LVD) were examined by immunohistochemical staining. HIF-1 expression was detected in 67 of 113 rectal carcinoma cases (59.3 %). A positive correlation was found among HIF-1α expression, cellular apoptosis and angiogenesis. The 5-year survival rate in the HIF-1α-negative group was significantly higher than that in the HIF-1α-positive group (81.34 % versus 50 %, P < 0.05). According to the Cox regression analysis, HIF-1α expression, VEGF expression and cellular apoptosis index were independent risk factors for clinical prognosis in rectal carcinoma. Aberrant HIF-1α expression correlates with apoptosis inhibition, angiogenesis and poor prognosis in rectal carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoplastic development and metastasis are associated with aberrant cell proliferation, apoptosis and angiogenesis [1, 2]. Regional hypoxia caused by accelerated cell proliferation and overgrowth is an important characteristic of neoplasm. Hypoxia can cause a series of changes in gene transcription and protein expression, thereby not only inducing tumor cell resistance to radiotherapy and chemotherapy but also promoting tumor invasion and metastasis [3]. Hypoxia inducible factor 1 (HIF-1) is a heterodimeric transcription factor formed by the α subunit (HIF-1α) dimerizing with the constitutively expressed β subunit, which subsequently binds to hypoxia response elements in the promoters of target genes [4, 5]. Protein expression of HIF-1α in tumor cells is regulated by a variety of stimuli. It is involved in regulating energy metabolism [6] and oxygen transport under anaerobic conditions, thereby facilitating the adaptation of tumor cells to anaerobic conditions. Therefore, HIF-1α is the key regulator maintaining intracellular oxygen balance in humans [7]. As one of the most important transcription factors mediating hypoxia-induced cellular responses, HIF-1 can promote tumor angiogenesis and glycolysis [8], thereby playing a critical role in the adaptation of tumor cells to hypoxia. Vascular endothelial growth factor (VEGF) is an important signaling factor regulating angiogenesis and cellular responses by binding to tyrosine kinase receptors (VEGFRs) on the cell surface [9], inducing VEGFR dimerization, which in turn activates VEGF by transphosphorylation. In the present study, we sought to investigate the expression of HIF-1α in rectal carcinoma and its potential impact on tumor cell apoptosis, angiogenesis and clinical prognosis.
Materials and Methods
Patients and Samples
A total of 113 rectal adenocarcinoma cases were included in this study. The recruited patients had undergone curative tumor resection, without chemotherapy or radiotherapy before surgery, at the First Affiliated Hospital of Chongqing Medical University from 2004 to 2007. These patients were composed of 11 cases of well-differentiated adenocarcinoma, 81 cases of moderately differentiated adenocarcinoma and 21 cases of poorly differentiated adenocarcinoma. There were 61 males and 52 females, with the mean age of 52.5 years. In addition, 10 normal rectal tissue samples were used as negative control.
Immunohistochemical Staining for HIF-1α and VEGF
Immunohistochemical staining was performed using the standard streptavidin-peroxidase method and the S-P Histostain-Plus Kit (Beijing Zhongshan Biotechnology CO., LTD.), according to the manufacturer’s instructions. A rabbit antibody against the protein (HIF-1α and VEGF, diluted at 1:100, Boster Biological Technology LTD.) was used as the primary antibody for HIF-1α and VEGF detection. Scoring criteria for HIF-1α and VEGF expression were as follows: samples with <10 % positively stained tumor cells were considered negative for the corresponding protein expression; otherwise, the expression was considered positive.
Immunohistochemical Staining for Microvessel Density (MVD) and Lymphatic Vessel Density(LVD)
After deparaffinization and rehydration, tumor tissue sections were subjected to immunohistochemical staining using the same method as for HIF-1α antigen staining. A rabbit antibody against the CD34 (Beijing Zhongshan Biotechnology CO., LTD.) and mouse monoclonal antibody against podoplanin (Santa Cruz) were used. For MVD and LVD determination, three areas in the sections were randomly selected and examined under 200-fold magnification using the point-counting method. The average count was recorded and expressed as the number of vessels per square millimeter in each case.
In Situ Detection of Apoptosis
Cellular apoptosis in the tumor tissue sections were determined by the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, using an in situ cell death detection kit, POD (Boehringer Mannheim). After deparaffinization and rehydration, the sections were digested with proteinase K (20 μg/ml, AMERSCO) for 20 min at room temperature and were washed with PBS. After blocked with methanol containing 3 % hydrogen peroxide for 10 min, the sections were washed with PBS again and mixed with a permeabilisation solution (0.1 % Triton X-100, 0.1 % sodium citrate) for 2 min on ice. The TUNEL reaction mixture was pipetted onto the sections, which were then incubated at 37 °C for 1 h. The reaction was terminated by washing the sections with PBS, after which converter-POD was added onto the slides. Finally, the slides were washed with PBS, stained with diaminobenzdine and counterstained with hematoxylin. The cell nucleus stained yellow-brown was considered positive for the corresponding protein expression. The apoptotic index (AI) was expressed as the ratio of positively stained tumor cells to all tumor cells. For each case, 1000 tumor cells randomly selected in 5 areas were counted under 400-fold magnification.
Statistical Analysis
All statistical analysis was performed using the SPSS 11.0 software package(version 11.0, SPSS Inc., Chicago, IL). Differences in tumor cell AI and MVD between the two groups dichotomized by HIF-1α expression and VEGF expression were analyzed using the t test. Clinicopathologic factors associated with the expression of HIF-1α and VEGF were analyzed using the χ 2 test, whereas the factors associated with AI, MVD and LVD were analyzed using t test. Survival curves were plotted according to the Kaplan-Meier method and were examined using the log-rank test. Cox proportional hazard regression analysis was conducted to determine predictive factors for prognosis. Differences with P < 0.05 were considered statistically significant.
Results
Expression of HIF-1α and VEGF in Rectal Carcinoma
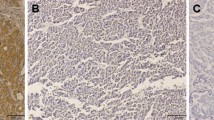
By immunohistochemistry, HIF-1α expression was detected positive, i.e., yellow-brown, in the cytoplasm of tumor cells in 67 of 113 cases (59.3 %, Fig. 1b). VEGF expression was detected positive, i.e., brown or yellow-brown, in the cytoplasm and on the envelope of tumor cells in 55 of 113 cases (48.7 %, Fig. 1d). In contrast, the expression of HIF-1α and VEGF in normal tissue samples was negative (P < 0.01, Fig. 1a, c).
Correlation Among HIF-1α Expression, AI, MVD and LVD
In the 113 cases, the means of AI, MVD and LVD were 16.87 % ± 6.05 % (range: 9.0 %–31.2 %, Fig. 2a) and 28.1 ± 5.7 (range: 16–66, Fig. 2b) and 9.77 ± 4.84(range: 2–21, Fig. 2c), respectively. The mean of AI in HIF-1α positive cases (n = 67) was (13.60 ± 3.59)%, which was significantly lower than the number of (21.06 ± 6.02)% in HIF-1α negative cases (n = 46; P < 0.01). On the contrary, the mean of MVD in HIF-1α positive cases was significantly higher than that in HIF-1α negative cases (31.57 ± 7.72 versus 27.17 ± 5.16, P < 0.05). And there was no significant difference for the mean of LVD between HIF-1α positive cases and HIF-1α negative cases (10.58 ± 3.92 versus 9.69 ± 5.15, P > 0.05).
Microvessels, Cellar apoptosis and Lymphatic vessels in rectal carcinoma tissues. (a, c, e × 100;b, d, f × 400.). a and b The microvessels in rectal carcinoma tissues. The microvessels stained by CD34 were shown in brownish yellow. Red arrows in picture B point at the microvessel. c and d The cellar apoptosis in rectal carcinoma tissues. The cellar apoptosis were stained by TUNEL and shown in brownish yellow. Blue arrows in picture D point at the apoptotic bodies. e and f The lymphatic vessels in rectal carcinoma tissues. The lymphatic vessels stained by Podoplanin were shown in brownish yellow. Black arrows in picture F point at the lymphatic vessel
Correlation Among VEGF Expression, AI, MVD and LVD
The mean of AI in VEGF positive cases (n = 55) was (15.34 ± 2.31)%, which was significantly lower than the number of (23.22 ± 3.51)% in VEGF negative cases (n = 58; P < 0.01). On the contrary, the mean of MVD in VEGF positive cases was significantly higher than that in VEGF negative cases (34.07 ± 8.97versus24.06 ± 5.91, P < 0.01). And there was no significant difference for the mean of LVD between VEGF positive cases and VEGF negative cases (10.13 ± 5.42versus 10.67 ± 4.87, P > 0.05).
Correlation Between Clinicopathological Factors and HIF-1α Expression, VEGF Expression, AI, MVD and LVD
The correlation between clinicopathological factors and HIF-1α expression, VEGF expression, AI, MVD and LVD were analyzed. As shown in Table 1, differences in age, sex and differentiation degree did not significantly affect HIF-1α expression, VEGF expression, AI, MVD or LVD(P > 0.05). In contrast, differences in lymph node metastasis and Duke’s classification significantly affected these five indices (P < 0.05). In particular, distant metastasis significantly correlated with positive VEGF expression and MVD (P < 0.05).
Analysis of Prognostic Factor in Patients with Rectal Carcinoma
Kaplan-Meier curves in patients with rectal carcinoma categorized according to HIF-1α and VEGF expression are shown in Figs. 3 and 4. The overall 5-year survival rate in HIF-1α positive patients was significantly lower than that in HIF-1α negative patients (50 % versus 81.34 %, P < 0.05). Similarly, the overall 5-year survival rate in VEGF positive patients was significantly lower than that in VEGF negative patients (37.93 % versus 86.67 %, P < 0.01).
For the analysis of correlation among AI, MVD and prognosis, the patients were dichotomized by the cutoff of 17 % for AI and of 29.6 for MVD, which provided a more sensitive parameter for detecting differences in survival of these patients. The overall 5-year survival rates in the high AI group (≥17.0 %, 88 %) and the low MVD group (≤28.1, 77.27 %) were significantly higher than those in the low AI group (<17 %, 38.77 %) and the high MVD group (>28.1, 31.82 %), respectively (Figs. 5 and 6; P < 0.05).
In addition, using the variables of age, sex, differentiation degree, distant metastasis, lymph node metastasis, Duke’s classification, HIF-1α expression, VEGF expression, AI, MVD and LVD, the multivariate Cox proportional hazard model demonstrated that distant metastasis, lymph node metastasis, HIF-1α expression, VEGF expression and AI were independent predictors for overall survival (P < 0.05).
Discussion
At the early stage of solid tumor development, tumor cell proliferation depends on the nutrition obtained from dispersion. When the diameter of a solid tumor exceeded 1–2 mm, dispersion would not be able to satisfy the need for tumor cell survival and growth, thereby leading to regional hypoxia, which in turn induces a series of changes in gene transcription and protein expression in response to the hypoxic environment. The most direct and obvious change is the significantly increased angiogenesis, which can increase local blood supply and help removing the large number of metabolic products from the tumor mass. Hypoxia is a characteristic feature of the microenvironment of solid tumor, which plays an important role in neoplastic development and metastasis [10]. Tumor angiogenesis and the adaptation of tumor cells to hypoxia are the key contributing factors to tumor progression [11]. Therefore, new blood vessels would be required for preventing tumor cell necrosis. Angiogenesis plays a crucial role in promoting tumor cell proliferation, invasion and metastasis, which is considered an independent prognosis factor for multiple types of neoplastic tumors. Hypoxia can induce transcriptional activation of a series of genes that are specifically associated with angiogenesis and hypoxia-induced metabolic alterations, which in turn facilitates tumor cell adaptation to the hypoxic environment. HIF-1α plays an important role in this process, thereby promoting tumorigenesis and neoplastic development [12]. Hypoxia is the most prominent factor for HIF-1α expression induction. In addition, HIF-1α can activate the expression of its downstream target vascular endothelial growth factor, thereby promoting tumor cell proliferation [13]. Disturbing the balance between cell proliferation and apoptosis could affect neoplastic development. Many previous investigations in colorectal carcinoma have focused on proliferation and apoptosis [14–16]. Moreover, several reports have reported a significant increase in MVD in colorectal carcinoma.
As a transcriptional factor, the heterodimer HIF-1α recognizes and binds to the consensus sequence of 5′-(A/G)CGTG-3′, also named hypoxia-responsive elements (HREs) to activate the transcriptional activity of target genes [5, 17]. Increasing evidence has implicated the function of HIF-1α in tumor cell growth and metastasis. Immunohistochemistry analysis has demonstrated HIF-1α overexpression in many types of cancer compared with the respective normal tissues, including colon, breast, gastric, lung, skin, ovarian, pancreatic, prostate and renal carcinomas [18, 19]. In the present study, we found that HIF-1α expression in rectal carcinoma tissue was significantly elevated compared to that in normal tissue, and that HIF-1α expression level correlated with the status of lymph node metastasis and tumor stages, consistent with the observations by Simiantonaki et al. [20].
HIF-1α is known to stimulate angiogenesis by activating the transcription of several growth factors, including VEGF, evidenced by the abnormal vascular development in HIF-1α-knockout mice [21]. In a following study, Tsuzuki Y et al. reported that HRE-/- ES tumors produced the same level of VEGF as VEGF-/- ES tumors, indicating the role of HIF-1α/HRE in transcriptional regulation of VEGF in tumor cells [22]. Moreover, it has been reported that HIF-1α can not only induce VEGF transcriptional activation but also increase the stability of VEGF mRNA, thereby elevating the VEGF protein level [23]. The elevation of VEGF expression, especially VEFG-A expression, can promote proliferation of tumor-derived vascular epithelial cells through multiple signaling pathways, including PI3K, MAPK, Ras and PLC, thereby facilitating tumor angiogenesis [24], which in turn promote tumor growth, invasion and metastasis. In the present study, we found that VEGF expression was elevated in 48.7 % of the rectal carcinoma samples and correlated with the status of lymph node metastasis, distant metastasis and tumor stages, further indicating that the HIF-1α-VEGF signaling pathway mediated by HIF-1α is closely related to neoplastic development and metastasis.
In this study, we showed that apoptosis inhibition by HIF-1α was a predictive indicator of poor prognosis and short survival rate in rectal carcinoma. The mean AI in HIF-1α positive cases was significantly lower than that in HIF-1α negative cases, which was associated with lymph node metastasis and Duke’s classification. In addition, the prognosis of patients with high AI was more promising than that of the patients with low AI. These data suggested that aberrant HIF-1α expression might result in apoptosis inhibition, thereby decreasing the sensitivity to apoptosis in rectal carcinoma development and metastasis. But the occurrence of apoptosis inhibition depends on the severity of hypoxia. Some researches showed that the anti-apoptotic effect was observed with mild hypoxia because HIF-1α was activated with the dimerization of HIF-1α and ARNT, leading to higher gene transcription. On the other hand, severe hypoxia leads to cell death at least partially via the stabilization of p53 by HIF-1α, and HIF-1α was degraded finally. Suzuki et al. [25] demonstrated that two different forms of HIF-1α were responsible for these two totally different functionalities of HIF-1α. Phosphorylated HIF dimerizes with ARNT while dephosphorylated HIF-1α associates with p53 and induces apoptosis via Bax over-expression. HIF-1α could prevent apoptosis of tumor cells through decreasing Bax/Bcl-2 ratio and reducing caspase 3 activity [26]. Therefore, HIF-1α overexpression could further destabilize cancer cells, thereby promoting metastasis. This argument was supported by the multivariate Cox proportional hazard model, which determined AI as a significant independent predictor of overall survival in our examined rectal carcinoma patients (P < 0.05).
HIF-1α expression, which can regulate VEGF transcription and activation, positively correlates with VEGF expression in rectal cancer. The expression of VEGF and its receptors Flt-1 and Flk-1 were closely associated with angiogenesis in colorectal cancer. VEGF can promote colorectal tumor cell invasion through autocrine, thereby playing an important role in tumor cell infiltration and metastasis [27]. HIF promotes tumor angiogenesis and cell proliferation in the presence of wild-type P53 [28]. As one of the most important findings in this study, the significant decrease of AI was accompanied by a significant increase of MVD in HIF-1α positive cases. Moreover, the 5-year survival rate of the patients with low MVD was significantly higher than that of the patients with high MVD (P < 0.05). These data suggested that HIF-1α might play a role in protecting endothelium and accelerating cell proliferation through anti-apoptosis during cell cycle progression, which in turn suggested that inhibiting HIF-1α expression could promote endothelial apoptosis and inhibit metastasis.
Research shows that VEGF induced formation of lymphatic vessels and promoted the expansion of lymphatic vessels [29], and in breast cancer the expression of VEGF associated with LVD [30]. In the present study, we found that the expression of LVD has no significant difference between the positive group and negative group of HIF and VEGF expression, but has correlation with tumor lymphatic metastasis. We believe that tumor lymphangiogenesis providing an indispensable way for tumor metastasis, and resulting in lymph node metastasis. Because the tumor lymphatic microvessel consists of only a single cell, no basal layer, and when the interstitial pressure increases, the elastic wire in extracellular matrix will expand, and the tumor cells can enter the lymphatic then metastasis to lymph nodes. Gao et al. [31] studies show, the expression of VEGF has no correlation with LVD in the gastrointestinal tract tumor, consistent with the results of our findings. Which suggested that VEGF could not stimulate the formation of lymphatic vessels. Lee et al. [32] studies shown that there has some heterogeneity in different lymphatic endothelial cells, and its heterogeneity depends on various factors (including anatomy and organ-specific vascular bed). We believe that the inconsistent conclusions may be caused by the heterogeneity of different organs of lymphatic endothelial cell. The differences of the immune function and other aspects of biolog of lymphatic endothelial cells in different organs leads to different stimulating factors required for lymphatic hyperplasia in different organs. The VEGF could stimulate the lymphatic formation vessels in breast cancer, but could not in gastrointestinal tumors. In addition, the sensitivity and specificity of the lymphatic markers, and the numbers of experimental samples may also cause differences in this result. So, selecting more samples in different tumors to research in this area will help to clarify this problem.
In conclusion, our findings in this study indicate that HIF-1α expression positively correlates with tumor cell apoptosis, angiogenesis, low survival rate and unfavorable prognosis in rectal carcinoma. Further investigations are warranted to explore the potential of HIF-1α as a target candidate for anti-angiogenesis therapy.
References
Gupta SC, Kim JH, Prasad S et al (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29(3):405–434
de Krijger I, Mekenkamp LJ, Punt CJ et al (2011) MicroRNAs in colorectal cancer metastasis. J Pathol 224(4):438–447
Arvelo F, Cotte C (2009) Hypoxia in cancer malignity. Invest Clin 50(4):529–546
Greijer AE, van der Groep P, Kemming D et al (2005) Up-regulation of gene expression by hypoxia is medi-ated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol 206:291–304
Belozerov VE, Van Meir EG (2005) Hypoxia inducible factor-1: a novel target for cancer therapy. Anticancer Drugs 16(9):901–909
Mucaj V, Shay JE, Simon MC (2012) Effects of hypoxia and HIFs on cancer metabolism. Int J Hematol 95(5):464–670
Semenza GL (2000) HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 88(4):1474–1480
Yeung SJ, Pan J, Lee MH (2008) Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci 65(24):3981–3999
Rapisarda A, Melillo G (2012) Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res 114:237–267
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358(19):2039–2049
Merritt WM, Sood AK (2007) Markers of angiogenesis in ovarian cancer. Dis Markers 23(5–6):419–431
Menrad H, Werno C, Schmid T et al (2010) Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology 51(6):2183–2192
Ardyanto TD, Osaki M, Nagahama Y et al (2008) Down-regulation of cobalt-induced HIF-1alpha expression correlates with cell proliferation and apoptosis in human gastric carcinoma cells. Oncol Rep 19(2):339–343
Hao X, Du M, Bishop AE et al (1998) Imbalance between proliferation and apoptosis in development of colorectal carcinoma. Virchows Arch 433:523–527
Benchabane H, Ahmed Y (2009) The adenomatous polyposis coli tumor suppressor and Wnt signaling in the regulation of apoptosis. Adv Exp Med Biol 656:75–84
Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S et al (2012) TGF-beta signalling in colon carcinogenesis. Cancer Lett 314(1):1–7
Liu W, Shen SM, Zhao XY et al (2012) Targeted genes and interacting proteins of hypoxia inducible factor-1. Int J Biochem Mol Biol 3(2):165–178
Zhong H, De Marzo AM, Laughner E et al (1999) Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 59:5830–5835
Koh MY, Spivak-Kroizman TR, Powis G (2010) HIF-1alpha and cancer therapy. Recent Results Cancer Res 180:15–34
Simiantonaki N, Taxeidis M, Jayasinghe C et al (2008) Hypoxia-inducible factor 1 alpha expression increases during colorectal carcinogenesis and tumor progression. BMC Cancer 8:320
Kotch LE, Iyer NV, Laughner E et al (1999) Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 209:254–267
Tsuzuki Y, Fukumura D, Oosthuyse B et al (2000) Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha–> hypoxia re-sponse element–> VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res 60:6248–6252
Rey S, Semenza GL (2010) Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86(2):236–242
Duffy JP, Eibl G, Reber HA et al (2003) Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer 2:12
Suzuki H, Tomida A, Tsuruo T (2001) Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene 20(41):5779–5788
Piret JP, Mottet D, Raes M et al (2002) Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol 64(5–6):889–892
Price DJ, Miralem T, Jiang S et al (2001) Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ 12(3):129–135
Bos R, van Diest PJ, van der Groep P et al (2004) Expression of hypoxia-inducible factor-1alpha and cell cycle proteins in invasive breast cancer are estrogen receptor related. Breast Cancer Res 6(4):R450–R459
Nagy JA, Vasile E, Feng D et al (2002) Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 196(11):1497–1506
Mohammed RA, Green A, El-Shikh S et al (2007) Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer 96(7):1092–1100
Gao Y, Zhong WX, Mu DB et al (2008) Distributions of angiogenesis and lymphangiogenesis in gastrointestinal intramucosal tumors. Ann Surg Oncol 15(4):1117–1123
Lee S, Choi I, Hong YK (2010) Heterogeneity and plasticity of lymphatic endothelial cells. Semin Thromb Hemost 36(3):352–361
Acknowledgments
The work was Supported by Chongqing Municipal Natural Science Foundation (Grant No.cstc2013jcyjA10057).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, L., Tao, L., Dawei, H. et al. HIF-1α Expression Correlates with Cellular Apoptosis, Angiogenesis and Clinical Prognosis in Rectal Carcinoma. Pathol. Oncol. Res. 20, 603–610 (2014). https://doi.org/10.1007/s12253-013-9738-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9738-6