Abstract
Diffuse large B cell lymphoma (DLBCL) and plasmablastic lymphoma (PBL) represent aggressive non-Hodgkin lymphomas, particularly in the setting of HIV infection. Since the introduction of highly active antiretroviral therapy (HAART), recent studies have documented improved survival outcome in patients with AIDS-related lymphomas. This study contributes a South African perspective by correlating the HIV status and prognosis of DLBCL and PBL with differentiation profiles assessed by immunophenotyping. Analysis of the morphologic, immunophenotypic and clinicopathologic features of 52 cases of DLBCL and 9 cases of de novo PBL was performed. The overall survival of patients with PBL was poorer than that of DLBCL (logrank p value 0.002). Despite HAART, the overall survival with DLBCL and HIV infection was significantly poorer than HIV negative patients with DLBCL (p value <0.001). Profound immunosuppression was evident in the HIV positive group as the mean CD4 count was 151 cells/mm3 in DLBCL and 61 cells/mm3 in PBL. HIV positive patients were significantly younger at presentation with greater likelihood of extranodal lymphoma. When Hans’ and Muris’ algorithmic stratification of DLBCL were applied, no statistical significance was demonstrated (p values 0.188 and 0.399 respectively). However, when Bcl-2 expression occurred in germinal center-type DLBCL (Hans’ defined), improved survival was conferred by the germinal center immunophenotype (p value 0.007). The study demonstrates that DLBCL and PBL have significant potential for aggressive behaviour and poor outcome in the setting of profound immunosuppression due to HIV infection. Further studies are required to assess the effect of targeted-immunotherapy (Rituximab) in combination with recent amendment of the South African national antiretroviral treatment guidelines which has created tremendous potential for improved survival in patients with AIDS-related non-Hodgkin B-cell lymphomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B cell lymphoma (DLBCL), a malignant neoplasm of large B lymphoid cells, represents approximately 20–30 % of non-Hodgkin lymphomas in Western countries and constitutes greater percentages in developing countries [1]. Acquired immunodeficiency syndrome (AIDS) -associated lymphomas are defined as those that occur with increased frequency in patients infected with Human immunodeficiency virus (HIV) with low CD4 counts [2]. In the South African setting, DLBCL represents one of the commonest HIV-associated non-Hodgkin lymphomas. Other aggressive B-cell lymphomas included in the HIV-NHL group are plasmablastic lymphoma, Burkitt lymphoma and primary effusion lymphoma [2, 3].
At immunohistochemical and molecular levels, there have been salient prognostic subdivisions of DLBCL proposed by several investigating groups which are inclusive of Hans et al. [4], Chang et al. [5], Tumwine et al. [6], Amen et al. [7], Muris et al. [8], Choi et al. [9], Alizadeh et al. [10], and Madan et al. [11] among others.
In 1978 Banks et al. published a remarkable case report of an extramedullary lymphoid neoplasm displaying morphologic features of diffuse large B cell lymphoma of “immunoblastic sarcoma” type. This neoplasm was recognised as unique by the lack of immunohistochemical expression of B-cell surface markers and concomitant cytoplasmic expression of immunoglobulin (IgG) leading to recognition of a malignant lymphoma of plasmablastic identity [12]. Close to two decades thereafter, the term plasmablastic lymphoma (PBL) was subsequently coined by Delecluse et al. [13], following the description of an acquired immunodeficiency syndrome-associated B-lineage lymphoma with plasmacytic differentiation. Most recently, the 2008 WHO classification of tumours of haematopoietic and lymphoid tissues recognised PBL as an independent large cell non-Hodgkin mature B cell lymphoma, having been separated from the DLBCL category [14].
Aim
This study aimed to contribute a South African perspective by correlating the HIV status and prognosis of patients with DLBCL and PBL with differentiation profiles assessed by immunophenotyping. Prior to commencement, institutional ethical approval of the study was obtained (REC reference number 209/2006).
Method
Cases of large cell lymphoma comprising diffuse large B cell lymphoma and plasmablastic lymphoma were retrieved. All cases were diagnosed at the histopathology department, division of Anatomical Pathology, National Health Laboratory Service, Groote Schuur Hospital, University of Cape Town, South Africa. The cases were selected from a SNOMED-based search of the DISA computer system and an intradepartmental lymphoma database. Random selection of cases from both HIV positive and negative patients was made and tissue samples limited by the paucity of diagnostic tissue were excluded.
In total, 61 cases inclusive of 9 de novo PBL were selected. Twenty seven tumours were diagnosed in HIV positive patients, 28 in HIV negative patients and in 6 patients the HIV status was unknown.
Case selection in the HIV negative group focussed predominantly on the inclusion de novo DLBCL, not otherwise specified. However, also included in this group were two recipients of solid organ allograft transplantation (renal and cardiac), one case of T-cell rich DLBCL, one case of DLBCL possibly transformed from MALT lymphoma and a case of primary DLBCL of the central nervous system.
All the cases were diagnosed between January 2004 and December 2007, allowing for a mean follow up of 27 months from the time of diagnosis. The slides were morphologically reviewed and ancillary immunohistochemistry was performed.
Treatment and co-morbidities were documented from the clinical notes in close collaboration with the department of radiation oncology.
Morphologic Features
The morphologic features of the tumours were assessed by light microscopy using haematoxylin and eosin (H&E) stained sections, 3–4 um in thickness.
Within nodal and extranodal locations, DLBCL displayed diffusely destructive and widely permeative growth patterns. Necrosis and apoptotic debris were prominent features in some cases, particularly in HIV positive patients. The vast majority of DLBCL displayed mixed centroblastic and immunoblastic cytomorphologic features (Fig. 1a). Prominent immunoblastic morphology was evident in DLBCL occurring within the small intestine of an HIV negative patient who received previous solid organ (renal) allograft transplantation. EBV LMP-1 immunohistochemistry was positive in these tumour cells.
All cases of plasmablastic lymphoma developed de novo and displayed high grade features with sheet-like growth patterns and interspersed tingible body macrophages which imparted a starry-sky appearance (Fig. 1b). The individual tumour cells displayed blast-like cytomorphologic characteristics (Fig. 1c). Isolated cases of extra-oral plasmablastic lymphomas showed variable, intermittently subtle, degrees of plasmacytic differentiation.
The reviewed slides revealed no features of concomitant viral cytopathy, granulomatous inflammation, Castleman disease or Kaposi sarcoma.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks for each of the selected cases were retrieved from the departmental archives. A panel of immunohistochemical stains was performed, at times augmenting those done at initial histopathologic work-up and diagnosis. A total of 11, 3–4 micron, sections were cut from each representative block, floated onto silanised slides and heat fixed at 75 °C for 30 min. All immunohistochemistry (IHC) was performed on a Dako Autostainer (Universal Staining System) using the Envision Detection System. Positive and negative controls were included in each run (Table 1). Immunohistochemistry was interpreted with the aid of a scoring system in which scores of 0–4 were allocated in relation to the percentage of positively staining tumour cells (Table 2). Scores of 0–5 were used when assessing the proliferation index (Ki67). Using the cut-off limits of previous studies as guidelines, the level for interpreting CD10, Bcl-6 and Bcl-2 as positive in this study was >25 % tumour cell staining (i.e. score of at least 2). Any proportion of tumour cell immunoreactivity for CD20, CD38, CD138, MUM1, EBV LMP1 and VS38C was considered positive.
Statistical Interpretation
The data generated from the scoring system was captured in Excel-spread sheets. Information regarding age, CD4 count, IPI score, HAART, chemotherapy (CHOP) and radiation treatment was obtained. Data spread sheets were subsequently created and statistical analysis of the gathered data was conducted by a statistician using computer programme SPSS version13. Kaplan Meier curves for survival, Logrank test and the Cox regression model were used. In this study the survival time refers to the time to death and the survival time was censored. The comparison of survival curves between two groups was assessed by means of the Logrank test. Two sided p values less than 0.05 were indicative of statistical significance (Table 3).
Treatment
All patients with DLBCL and PBL were treated with CHOP therapy (cyclophosphamide, vincristine, doxorubicin/adriamycin and prednisone) without immunotherapy. Intrathecal chemotherapy was used prophylactically in patients with nasopharyngeal or paraspinal involvement and in patients with central nervous system (CNS) disease [15, 16]. Some patients in this study also received varying therapeutic combinations which included dose-reduced chemotherapy, palliative radiotherapy, involved field radiotherapy and intrathecal chemotherapy. Patients with PBL were treated with CHOP therapy and those with localised disease who had a complete response received involved field radiotherapy.
HIV positive patients who responded poorly to CHOP would receive palliative radiotherapy and/or palliative chemotherapy depending on the site and extent of residual disease.
All patients in the HIV positive group received highly active antiretroviral therapy (HAART). Some patients received HAART prior to the diagnosis of lymphoma while others commenced HAART at the time of lymphoma diagnosis. During the course of this study, the 2004 South African national antiretroviral treatment guidelines were such that first line therapy comprised stavudine with lamivudine and efavirenz or nevirapine. Second line HAART therapy comprised zidovudine, didanosine and lopinavir/ritonavir [17].
HIV negative patients who had poor response to CHOP or who relapsed would be considered for high-dose salvage chemotherapy and stem cell transplant. Due to resource limitations, Rituximab was not available to public/state patients in South Africa during the course of this study.
Co-morbidities Occurring During the Course of the Study
Opportunistic infections frequently complicate the outcome and survival of patients with lymphomas, particularly in the setting of concomitant HIV infection. During the period of our study (2004–2007), 11 patients in the HIV positive group were diagnosed with tuberculosis and one patient was diagnosed with Kaposi sarcoma. However, deaths which occurred in the HIV positive group were clinically and radiologically attributed to lymphoma rather than overwhelming opportunistic infection.
Results
The mean and median ages of patients with DLBCL in the HIV negative group were 54 and 59 years, respectively. In contrast, in the HIV positive group the overall mean and median ages were 43 and 36 years, respectively. Further stratification revealed that the mean age of HIV positive patients with DLBCL and PBL was 48 and 36 years, respectively.
In the HIV negative and HIV status unknown group, DLBCL was diagnosed in 18 females and 16 male patients. Fifteen cases were diagnosed on biopsies of lymph nodes and 18 cases on biopsies of extranodal sites. In one additional case, the distinction between nodal and extranodal involvement was uncertain as the incisional biopsy originated from the retroperitoneum. The extranodal regions included skin, kidney, testis (2 cases), nasopharynx (3), tonsil (2), adenoids (2), central nervous system (2), small intestine, spleen, bone (vertebra and clavicle) and breast.
In the HIV positive group there were ten males and eight females. Five cases were diagnosed on lymph node biopsies and 13 cases were diagnosed in extranodal topographic sites. The extranodal sites included liver (3 cases), nasopharynx (4), soft tissue, kidney, pancreas, spleen (2) and adrenal gland.
PBL was diagnosed in four male and five female patients, all of whom had HIV infection. Two cases occurred in the gingiva and floor of mouth and seven were diagnosed in extra-oral topographic regions. The latter included bone (clavicle), rectum, anus, skin, extradural T8 spinal region, scalp and suprasternal soft tissue region.
At the time of lymphoma diagnosis, the overall mean CD4 count in patients with HIV infection was 121 cells/mm3. Further stratification revealed a mean CD4 count of 151 cells/mm3 in patients with DLBCL and mean CD4 count of 61 cells/mm3 in those with PBL.
Immunohistochemistry Results
All the cases of DLBCL demonstrated diffuse expression of CD20. High proliferation indices occurred in the HIV positive and negative groups (Fig. 2a). CD10 expression, involving at least 25 % of tumour cells (score ≥2), was found in 42 % (22/52) of DLBCL cases. Similarly, Bcl-6 expression was established in 63 % (33/52) while MUM1 expression occurred in 56 % (29/52) of cases. CD38 expression was found in 98 % (51/52) of cases and EBV-LMP1 expression occurred in 5/52 cases. Bcl-2 expression (Fig. 2b), involving at least 25 % of tumour cells, occurred in 62 % (32/52) of DLBCL cases.
All cases of PBL in the study lacked CD20 expression and 8/9 cases displayed diffuse MUM1 nuclear immuno-expression. EBV LMP1 immunoexpression was detected in 3/9 cases, while VS38C, CD38 and CD138 expression was found in all PBL cases. The proliferation index in PBL was high and ranged from 76 to >95 %.
Statistical Results
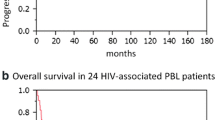
Despite antiretroviral therapy, the overall survival of HIV-positive patients with DLBCL was significantly poorer than that of HIV-negative patients (Logrank test confirmed statistically significant p value of <0.001; Fig. 3). Although the number of PBL cases was limited (n = 9), the study revealed that the overall survival of patients with plasmablastic lymphoma was significantly poorer than that of DLBCL (p value of 0.002; Fig. 4).
Kaplan Meier curves for overall survival: DLBCL occurring in HIV negative and HIV positive patients. Despite antiretroviral therapy, the overall survival of HIV-positive patients with DLBCL was significantly poorer than that of HIV-negative patients. Log-rank test confirmed statistically significant p value of <0.001
When Hans’ algorithm was used to stratify cases of DLBCL, there was no significant difference in survival between patients with germinal centre-subtype DLBCL and non-germinal centre subtypes (p value 0.188).
Similarly, when Muris’ algorithm was used to stratify cases of DLBCL, no significant difference in survival was demonstrated between group 1-type DLBCL and group 2-type DLBCL (p value 0.399). Independent assessment of CD10, Bcl-6 and MUM1 expression in DLBCL revealed no significant differences in survival (p values 0.442, 0.487, 0.124 respectively).
Furthermore, no statistical significance was found when Bcl-2 expression was assessed independently of the IPI score, CD10, Bcl-6 and MUM1 expression. However, an interesting finding was revealed when Bcl-2 expression occurred in germinal center-type DLBCL, using Hans’ algorithmic stratification, in that improved survival was conferred by the germinal center phenotype (p value 0.007; Fig. 5).
Kaplan Meier curves for overall survival in DLBCL when Bcl-2 expression occurred in the Hans-defined GCB group. No statistical significance was found when Bcl-2 expression was assessed independently of the IPI score, CD10, Bcl-6 and MUM1 expression (p value 0.371). However, an interesting finding was established when Bcl-2 expression occurred in germinal center-type DLBCL, using the Hans algorithmic stratification [9], in that improved survival was conferred by the germinal center phenotype. The accompanying statistically significant p value was 0.007
No survival difference was demonstrated in patients with low risk (0–2) and high risk IPI scores (p value 0.794).
Statistical observation revealed that CD138 expression in DLBCL involving >25 % of tumour cells occurred only in the HIV positive group and was associated with shorter survival time in this study. However no statistical significance was evident (p 0.402).
EBV-LMP1 expression was found in 3/9 cases of PBL and 5/52 cases of DLBCL. In the latter group, 4/5 EBV LMP1-positive DLBCL occurred in patients with HIV infection.
CD38 expression occurred in all cases of DLBCL in the HIV negative group and 16 cases of DLBCL in the HIV positive group. CD38 expression in DLBCL was not associated with statistically significant difference in survival (p value 0.769).
Table 3 contains predictive values of individual and combined immunohistochemical markers in DLBCL.
Discussion
The occurrence of diffuse large B cell lymphoma is amplified by the confounding co-morbidity of immunosuppression due to HIV infection, a well established risk factor for the development of NHL. The literature abounds with reported studies of various prognostic factors proven to successfully predict outcome in DLBCL. While synchronously providing a South African perspective, this study builds on and contributes to an elaboration of prognostic factors in DLBCL and PBL.
The prognostic factors are inclusive of the long-standing, internationally accepted International Prognostic Index (IPI), which combines several clinical parameters to stratify patients into (low, intermediate and high risk) prognostic groups [1, 18–21]. In contrast to several studies, the present study has not demonstrated significant survival differences between low risk and high risk IPI indices. This finding is likely due to limitations of the study sample size and heterogeneity of cases included.
Assessment of the mean and median ages at lymphoma presentation revealed that HIV positive patients were significantly younger (greater than a decade) compared to the age at presentation of DLBCL in HIV negative patients (HIV positive—mean age 43; median age 36 years; HIV negative—mean age 54; median age 59 years).
In the HIV positive group, lymphoma was diagnosed more frequently on biopsies of extranodal topographic regions than lymph node biopsies. In contrast, in the HIV negative/status unknown group, DLBCL was diagnosed in relatively equal proportions on biopsies of nodal and extranodal sites.
Several researchers have supported the positive prognostic influence of HAART on AIDS-related lymphomas. In a prospective observational study, Antinori et al. [22] established that concurrent administration of HAART and chemotherapy could significantly modify the natural history of non-Hodgkin’s lymphoma in HIV infected patients. HAART resulted in an increased response rate to chemotherapy and prolonged overall survival. In addition, HAART could possibly increase the tolerability of higher chemotherapy dose intensities. In further support thereof, Navarro et al. [23] demonstrated that patients treated with combination CHOP and HAART experienced higher rates of complete remission than those treated only with CHOP. Importantly, Besson et al. [24] found that the incidence of AIDS-related lymphomas had decreased significantly since the introduction of HAART. Patients on HAART were found to have higher CD4 counts at the time of lymphoma diagnosis and improved overall survival. A decrease in the incidence of NHL after the introduction of HAART has been further substantiated by the findings of Seaberg et al. [25] and Franceschi et al. [26].
In 2004, the South African National Antiretroviral Treatment Guidelines stipulated specific criteria for the initiation of antiretroviral therapy (ART) in patients infected with HIV. These criteria included CD4 <200 cells/mm3 irrespective of the WHO-defined disease stage or WHO stage IV AIDS-defining illness (which included lymphoma) irrespective of the CD4 count. An accompanying essential criterion was that of patient willingness and readiness to take antiviral therapy adherently [17]. In 2010, the South African National guidelines were revised to include initiation of ART when CD4 counts less than 350 cells/mm3 occurred in patients with TB and HIV co-infection and in pregnant women. In addition, the criterion for the presence of drug-resistant tuberculosis (MDR/XDR), irrespective of the CD4 count, was included [27]. In August 2011, the guidelines were further revised and synchronised with that of the World Health Organisation such that commencement of ART would be indicated when CD4 counts were 350 cells/mm3 or less [28].
The 2004 national ART guidelines were in place during the period of this study. As such, all HIV positive patients in the study received HAART, either prior to the diagnosis of lymphoma or at the time of lymphoma diagnosis. Despite HAART, a significant finding in this study was that the overall survival of patients with DLBCL and HIV infection was significantly poorer than that of patients with DLBCL in the absence of HIV infection (p value <0.001). This finding contrasts that of several studies in which higher remission and improved survival rates have been established in NHL occurring in HIV positive patients receiving HAART [22, 24, 29, 30]. Factors contributing to the disparate survival outcome in our HIV positive group include severe immunosuppression (mean CD4 121 cells/mm3 ), delayed initiation of antiviral therapy until an advanced stage of HIV infection in combination with limiting issues such as ARV non-compliance and the dreaded possibility of ARV treatment failure.
DLBCL is an aggressive malignancy and despite multidrug chemotherapeutic regimens, remissions were previously achieved in 40–60 % of cases [1, 21, 31]. Recently, further improvement in outcome of an additional 10 % has been documented in HIV negative patients due to Rituximab augmentation of chemotherapy regimens [32–34]. In the resource-limited setting of public health care in South Africa, Rituximab immunotherapy was unfortunately not available to state/public patients during the course of this study.
At immunohistochemical and molecular levels, there have been several prognostic subdivisions of DLBCL. Hans et al. [4] stratified cases of de novo DLBCL into prognostically significant subgroups with germinal center B-cell–like (GCB), activated B-cell–like (ABC) and type 3 gene expression profiles. The germinal center group of DLBCL which was CD10+ or Bcl6+/CD10-/MUM1- was associated with better overall survival relative to the non germinal center group which was CD10-/Bcl6- or CD10-/Bcl6+/MUM1+. The subclassification of DLBCL by gene expression was correlated with protein expression using immunohistochemistry. Muris et al. [8] subsequently established an interesting algorithmic stratification of DLBCL into prognostically favourable (group 1) and unfavourable groups (group 2) based on tumour expression of Bcl-2, MUM1 and CD10. Using cases of primary nodal DLBCL, this algorithmic stratification was found to be most predictive of clinical outcome in patients with intermediate to high IPI scores. When these prognostic algorithms were used in the present study, no significant differences in survival were established.
Bcl-2 is known to be an inhibitor of apoptosis. As such, it is likely that the presence of high levels of Bcl-2 protein promotes survival of tumour cells by reducing apoptosis and conferring resistance to therapeutic agents [35–37]. Several studies have supported the finding of poor prognosis when Bcl-2 expression occurs in DLBCL [4, 8, 38–41]. In the present study, when the occurrence of Bcl-2 expression in germinal center-type DLBCL was assessed using Hans’ algorithmic stratification, improved overall survival was conferred by the germinal center phenotype. Barrans et al. [41] reported that the GC phenotype was associated with an improved survival in both Bcl-2 positive and negative groups. Muris et al. [8] furthermore established that Bcl-2- positive cases of DLBCL which concomitantly expressed CD10 were found to have significantly better outcome than CD10-negative cases. These findings suggested that Bcl-2 expression alone may be insufficient to fully inhibit chemotherapy-induced apoptosis. There is corroborative evidence supportive of the understanding that CD10- expressing lymphoid cells, both neoplastic and non-neoplastic, are inclined to become increasingly prone to apoptosis. Often there is associated c-myc upregulation within these cells with resultant cellular induction into the cell cycle. This process creates the potential for improved response to apoptosis-inducing therapy in CD10-expressing DLBCL and may well account for a significant survival advantage [42–46].
Several researchers have reiterated the positive prognostic influence associated with Bcl-6 immunoexpression in DLBCL [12, 47, 48]. However, in the present study Bcl-6 expression in DLBCL was not independently associated with a positive influence on survival (p value 0.487).
Hoffman et al. [2] established that CD138 expression, in the absence of Bcl-6 and CD10, was associated with poor overall survival in AIDS-related B-cell lymphomas. In the present study, CD138 expression in DLBCL involving >25 % of tumour cells, occurred only in the HIV positive group and was associated with shorter survival time. However, no statistical significance was evident (p 0.402). Further investigation of the significance of CD138 expression in larger studies is required, as expression thereof is likely to be associated with poorer prognosis in light of an activated B cell phenotype.
Plasmablastic lymphoma (PBL) incidence accounts for approximately 2.6 % of all AIDS-associated non-Hodgkin lymphomas and occurs most often in immunocompromised adult patients with low CD4 counts [49–51] PBL has very rarely been reported in paediatric patients, most of whom are immunodeficient due to HIV infection [3, 52–55]. This tumour also rarely occurs in HIV negative and/or elderly patients with topographic sites of involvement inclusive of the oral cavity and extra-oral sites [56–58]. Epstein Barr virus (EBV) infection plays an important role in the pathogenesis of oral/extra-oral PBL and is demonstrable in 60–100 % of PBL using EBER in situ hybdridisation. However, detection of expression of EBV latency-associated proteins such as latent membrane protein-1 (LMP-1) by immunohistochemistry is very much lower [59, 60]. Interestingly, Rochford et al. [61] observed that EBER positive EBV infection, without detectable LMP-1, was associated with plasmacytic differentiation in EBV-infected B-cell lines. PBL a very aggressive NHL with poor outcome, as the estimated survival is usually less than 1 year [13, 14].
The present study included nine cases of de novo PBL occurring in HIV positive adult patients. Analysis of the age at presentation showed that HIV positive patients with PBL were significantly younger (greater than a decade) than those with DLBCL (HIV/PBL—mean age 36 years; HIV/DLBCL—mean age 48 years). A difference of 18 years was found between the mean age at presentation of HIV positive patients with PBL and HIV negative patients with DLBCL. Patients with PBL also had a significantly lower mean CD4 count, 61 cells/mm3, in contrast to the mean CD4 count of 151 cells/mm3 in patients with DLBCL. Although the number of plasmablastic lymphomas in this study is limited (n = 9), a significant difference in survival was demonstrated in that the overall survival of patients with PBL was poorer than that of DLBCL (p value 0.002). The median survival of patients with PBL was 6 months. Four of the patients with PBL were lost to follow-up.
The scenario of “lost to follow-up” was a significant limiting factor in this group of patients. In the South African milieu, loss of follow up may be attributed to low socio-economic status resulting in frequent logistic and financial challenges in accessing medical treatment, debilitating poor nutritional status, undiagnosed opportunistic diseases and the unfortunate reality of death occurring in non-hospitalised patients.
Conclusion
This study demonstrated that diffuse large B cell lymphoma and plasmablastic lymphoma have significant potential for aggressive behaviour and poor outcome in the setting of profound immunosuppression due to HIV infection.
Although the findings in this study are not novel, we have demonstrated that in the South African setting, the combination of clinical and biochemical prognostic parameters coupled with thorough morphologic assessment and immunohistochemical analysis of DLBCL and PBL has proven to yield prognostically useful information. In future, this may facilitate consideration of management stratification of HIV positive patients with DLBCL or PBL.
The welcomed recent revision of the South African National Antiretroviral Treatment Guidelines has created tremendous potential for improved survival in patients with AIDS-related malignancies. Further studies are required to assess the effect of these amendments in combination with the role of targeted-immunotherapy on survival in patients with AIDS-related B-cell lymphomas.
References
Stein H, Warnke RA, Chan WC, Jaffe ES, Chan JKC, Gatter KC, Campo E (2008) Diffuse large B cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Thiele J, Vardiman JW (eds) World Health Organisation classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon, pp 233–237
Hoffmann C, Tieman M, Schrader C, Janssen D, Wolf E, Vierbuchen M, Parwaresch R, Ernestus K, Plettenberg A, Stoehr A, Fatkenheuer G, Wyen C, Oette M, Horst HA (2005) AIDS-related B-cell lymphoma (ARL): correlation of prognosis with differentiation profiles assessed by immunophenotyping. Blood 106:1762–1769
Colomo L, Loong F, Rives S, Pittaluga S, Martínez A, López-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E (2004) Diffuse large B-cell Lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol 28:736–747
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103(1):275–282
Chang CC, McClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B, Kajdacsy-Balla A, Perkins SL (2004) Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol 28:464–470
Tumwine LK, Agostinelli C, Campidelli C, Othieno E, Wabinga H, Righi S, Falini B, Piccaluga PP, Byarugaba W, Pileri SA (2009) Immunohistochemical and other prognostic factors in B cell non Hodgkin lymphoma patients, Kampala, Uganda. BMC Clin Pathol 9:1–7
Amen F, Horncastle D, Elderfield K, Banham AH, Bower M, Macdonald D, Kanfer E, Naresh KN (2007) Absence of cyclin-D2 and Bcl-2 expression within the germinal centre type of diffuse large B-cell lymphoma identifies a very good prognostic subgroup of patients. Histopathology 51:70–79
Muris JJF, Meijer CJLM, Vos W, van Krieken JH, Jiwa NM, Ossenkoppele GJ, Oudejans JJ (2006) Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol 208:714–723
Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, Braziel RM, Geng H, Iqbal J, Lenz G, Vose JM, Hans CP, Fu K, Smith LM, Li M, Liu Z, Gascoyne RD, Rosenwald A, Ott G, Rimsza LM, Campo E, Jaffe ES, Jaye DL, Staudt LM, Chan WC (2009) A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res 15:5494–5502
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Madan R, Gormley R, Dulau A, Xu D, Walsh D, Ramesh KH, Cannizaro L, Tamas EF, Kumar P, Sparano J, LeValley A, Xue X, Bhattacharyya PK, Ioachim HL, Ratech H (2006) AIDS and non-AIDS diffuse large B-cell lymphomas express different antigen profiles. Mod Pathol 19:438–446
Banks PM, Keller RH, Li C-Y, White WL (1978) Case report malignant lymphoma of plasmablastic identity: a neoplasm with both “immunoblastic” and plasma cellular features. Am J Med 64(5):906–909
Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Huhn D, Schmidt-Westhausen A, Reichart PA, Gross U, Stein H (1997) Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 89:1413–1420
Stein H, Harris NL, Campo E (2008) Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Thiele J, Vardiman JW (eds) World Health Organisation classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon, pp 256–257
Desai J, Mitnick RJ, Henry DH, Llena J, Sparano JA (1999) Patterns of central nervous system recurrence in patients with systemic human immunodeficiency virus-associated non-Hodgkin lymphoma. Cancer 86(9):1840–1847
Sarker D, Thirlwell C, Nelson M, Gazzard B, Bower M (2003) Leptomeningeal disease in AIDS-related non-Hodgkin’s lymphoma. AIDS 17:861–865
Republic of South Africa. Department of Health. National Antiretroviral Treatment Guidelines. Jacana, 2004. Available from http://southafrica.usembassy.gov/media/2004‐doh‐art‐guidelines.pdf
Warnke RA, Strauchen JA, Burke JS, Hoppe RT, Campbell BA, Dorfman RF (1982) Morphologic types of diffuse large B-cell lymphoma. Cancer 50:690–695
Ambinder RF (2001) Epstein-Barr virus associated lymphoproliferations in the AIDS setting. Eur J Cancer 10:1209–1216
Nyman H (2010) Prognostic molecular factors and algorithms in diffuse large B-cell lymphoma. [Academic thesis] University of Helsinki
Chan JKC (2007) Tumors of the lymphoreticular system—part A: the lymph node. In: Fletcher CDM (ed) Diagnostic histopathology of tumors vol 2, 3rd edn. Churchill-Livingstone Elsevier, London, pp 1139–1288
Antinori A, Cingolani A, Alba L, Ammassari A, Serraino D, Ciancio BC, Palmieri F, De Luca A, Larocca LM, Ruco L, Ippolito G, Cauda R (2001) Better response to chemotherapy and prolonged survival in AIDS-related lymphomas responding to highly active antiretroviral therapy. AIDS 15:1483–1491
Navarro JT, Ribera JM, Oriol A (2001) Influence of highly active antiretroviral therapy on response to treatment and survival in patients with acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma treated with CHOP. Br J Haematol 112:909–915
Besson C, Goubar A, Gabarre J, Rozenbaum W, Pialoux G, Châtelet FP, Katlama C, Charlotte F, Dupont B, Brousse N, Huerre M, Mikol J, Camparo P, Mokhtari K, Tulliez M, Salmon‐Céron D, Boué F, Costagliola D, Raphaël M (2001) Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood 98:2339–2344
Seaberg EC, Wiley D, Martínez‐Maza O, Chmiel JS, Kingsley L, Tang Y, Margolick JB, Jacobson LP, Multicenter AIDS Cohort Study (MACS) (2010) Cancer incidence in the multicenter AIDS cohort study before and during the HAART era 1984–2007. Cancer 116(23):5507–5516
Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, Bouchardy C (2010) Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 103(3):416–422
Republic of South Africa. Department of Health. The South African Antiretroviral Treatment Guidelines 2010. Available from http://www.uj.ac.za/EN/CorporateServices/ioha/Documentation/Documents/ART%20Guideline.pdf
van Eeden A. New HIV treatment guidelines: 15 August 2011 [Internet] Available from http://www.samedical.org/newsroom/media-releases/archived-media-releases/new-hiv-treatment-guidelines-15-august-2011.html
Evison J, Jost J, Ledergerber B, Jost L, Strasser F, Weber R (1999) HIV-associated non-Hodgkin’s lymphoma: highly active antiretroviral therapy improves remission rate of chemotherapy. AIDS 13:732–734
Thiessard F, Morlat P, Marimoutou C, Labouyrie E, Ragnaud JM, Pellegrin JL, Dupon M, Dabis F, the Groupe d’Epidémiologie Clinique du SIDA en Aquitaine (GECSA) (2000) Prognostic factors after non-Hodgkin’s lymphoma in patients infected with the human immunodefciency virus: aquitaine cohort, France. Cancer 88:1696–1702
Coiffier B (2001) Diffuse large cell lymphoma. Curr Opin Oncol 13:325–334
Habermann TM, Weller EA, Morrison VA et al (2006) Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24:3121–3127
Pfreundschuh M, Trumper L, Osterborg A et al (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7:379–391
Sehn LH, Donaldson J, Chhanabhai M et al (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027–5033
Hockenbery D, Nunez G, Milliman C et al (1990) Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334–336
Cory S (1995) Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol 13:513–543
Reed JC (1997) Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 34:9–19
Obermann EC, Csato M, Dirnhofer S, Tzankov A (2009) BCL2 gene aberration as an IPI-independent marker for poor outcome in non-germinal-centre diffuse large B cell lymphoma. J Clin Pathol 62:903–907
Hermine O, Haioun C, Lepage E, d’Agay MF, Briere J, Lavignac C, Fillet G, Salles G, Marolleau JP, Diebold J, Reyas F, Gaulard P (1996) Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin’s lymphoma. Blood 87:265–272
Gascoyne R, Adomat S, Krajewski S, Krajewska M, Horsman DE, Tolcher AW, O’Reilly SE, Hoskins P, Coldman AJ, Reed JC, Connors JM (1997) Prognostic significance of bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 90:244–251
Barrans SL, Carter I, Owen RG, Davies FE, Patmore RD, Haynes AP, Morgan GJ, Jack AS (2002) Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood 99:1136–1143
Ohshima K, Kawasaki C, Muta H, Muta K, Deyev V, Haraoka S, Suzumiya J, Podack ER, Kikuchi M (2001) CD10 and Bcl10 expression in diffuse large B-cell lymphoma: CD10 is a marker of improved prognosis. Histopathology 39(2):156–162
Liu YJ, Johnson GD, Gordon J, Maclennan IC (1992) Germinal centres in T-cell-dependent antibody responses. Immunol Today 13:17–21
Cutrona G, Dono M, Pastorino S, Ulivi M, Burgio VL, Zupo S, Roncella S, Ferrarini M (1997) The propensity to apoptosis of centrocytes and centroblasts correlates with elevated levels of intracellular myc protein. Eur J Immunol 27:234–238
Cutrona G, Leanza N, Ulivi M, Melioli G, Burgio VL, Mazzarello G, Gabutti G (1999) Expression of CD10 by human T cells that undergo apoptosis both in vitro and in vivo. Blood 94(9):3067–3076
Cutrona G, Tasso P, Dono M, Roncella S, Ulivi M, Carpaneto EM, Fontana V, Comis M, Morabito F, Spinelli M, Frascella E, Boffa LC, Basso G, Pistoia, Ferrarini M (2002) CD10 is a marker for cycling cells with propensity to apoptosis in childhood ALL. Br J Cancer 86(11):1776–1785
Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Zehnder JL, Tibshirani R, Levy R (2001) Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood 98:945–951
Winter JN, Weller EA, Horning SJ, Krajewska M, Variakojis D, Habermann TM, Fisher RI, Kurtin PJ, Macon WR, Chhanabhai M, Felgar RE, Hsi ED, Medeiros LJ, Weick JK, Reed JC, Gascoyne RD (2006) Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood 107:4207–4213
Levine AM (2006) AIDS-related lymphoma. Semin Oncol Nurs 22:80–89
Castillo J, Pantanowithz L, Dezube BJ (2008) HIV-associated plasmablastic lymphoma: a literature review of 112 cases. Am J Hematol 83:804–809
Flaitz CM, Nichols, Walling DM, Hicks MJ (2002) Case report. Plasmablastic lymphoma: an HIV-associated entity with primary oral manifestations. Oral Oncol 38:96–102
Chabay P, De Matteo E, Lorenzetti M, Gutierrez M, Narbaitz M, Aversa L, Preciado MV (2009) Case report: vulvar plasmablastic lymphoma in a HIV-positive child: a novel extraoral localisation. J Clin Pathol 62:644–646
Radhakrishnan R, Suhas S, Kumar RV, Krishnanand G, Srinivasan R, Rao NN (2005) Plasmablastic lymphoma of the oral cavity in an HIV-positive child. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:725–731
Gogia A, Bakhshi S (2010) Letter to the editor. Plasmablastic lymphoma of oral cavity in an HIV negative child. Pediatr Blood Cancer 55:390–391
Pather S, MacKinnon D, Padayachee RS (2012) Plasmablastic lymphoma in paediatric patients: Clinicopathologic study of three cases. Ann Diagn Pathol. Accepted for publication Aug 2012
Scheper MA, Nikitakis NG, Fernandes R, Gocke CD, Ord RA, Sauk JJ (2005) Oral plasmablastic lymphoma in an HIV-negative patient: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:198–206
Lin F, Zhang K, Quiery AT, Prichard J, Schuerch C (2004) Plasmablastic Lymphoma of the cervical lymph nodes in a human immunodeficiency virus-negative patient. A case report and review of the literature. Arch Pathol Lab Med 128:581–584
Guan B, Zhang X, Hu W, Rao Q, Wang Y, Zhu Y, Wang H, Ma H, Zhou H, Zhou X (2011) Plasmablastic lymphoma of the oral cavity in an HIV-negative patient. Ann Diagn Pathol 15(6):436–440
Chetty R, Hlatswayo N, Muc R, Sabaratnam R, Gatter K (2003) Plasmablastic lymphoma in HIV + patients: an expanding spectrum. Histopathology 42:605–609
Dong HY, Scadden DT, de Leval L, Tang Z, Isaacson PG, Harris NL (2005) Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus–associated extramedullary plasmacytic neoplasm. Am J Surg Pathol 29:1633–1641
Rochford R, Hobbs MV, Garnier JL, Cooper NR, Cannon MJ (1993) Plasmacytoid differentiation of Epstein-Barr virus-transformed B cells in vivo is associated with reduced expression of viral latent genes. Proc Natl Acad Sci U S A 90:352–356
Acknowledgments
This study was made possible by a research grant (number 93874) from the National Health Laboratory Service, South Africa, which is most appreciatively acknowledged. This presentation has also been supported, in part, by a fellowship/grant from the Fogarty International Center/USNIH: grant # 3U2RTW007373 ICOHRTA and 3U2RTW007370‐05S1.
The academic inspiration of Professor Dhirendra Govender (HOD; Anatomical Pathology, UCT) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pather, S., Mohamed, Z., McLeod, H. et al. Large Cell Lymphoma: Correlation of HIV Status and Prognosis with Differentiation Profiles Assessed by Immunophenotyping. Pathol. Oncol. Res. 19, 695–705 (2013). https://doi.org/10.1007/s12253-013-9632-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9632-2