Abstract
As important regulators of chromatin, histone deacetylases (HDACs) are involved in silencing tumor suppressor genes. HDAC2, a member of HDACs, has been demonstrated to be implicated in the development and progression of various human malignancies; however, its expression and role in human primary gallbladder carcinoma (PGC) are not fully understood. Therefore, we conducted this study to address this problem. The subjects were 136 patients underwent resection for PGC. Immunostainings for HDAC2 were performed on these archival tissues. The correlation of HDAC2 expression with clinicopathological characteristics including survival was analyzed. HDAC2 was positively expressed in the nucleus of tumor cells in 86.0 % (117/136) of PGC but not in the normal epithelium of the gallbladder. Additionally, there was a significant difference in the incidence of positive nodal metastasis between high and low HDAC2 expression groups (P = 0.001). The incidences of advanced clinical stage (P = 0.005) and pathologic T stage (P < 0.001), and higher histologic grade (P < 0.001) were respectively higher in the high HDAC2 expression group than in the low group. Moreover, univariate and multivariate analyses revealed the high HDAC2 expression to be an independent prognostic factor for both overall and disease-free survival of patients with PGC. High HDAC2 expression was correlated with a high incidence of lymph node metastasis and aggressive tumor progression of PGC. It also was an independent prognostic factor for poorer overall and disease-free survival in patients. Therefore, detection of HDAC2 expression may help us screen patients at increased risk of malignant behavior for PGC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary gallbladder carcinoma (PGC) represents one of the most common biliary tract carcinomas in China. The complete surgical resection at the early stage is the most effective therapy to cure this disease. However, many PGC patients initially present with unresectable tumors, because there are no typical symptoms or signs at the early stages. Thus, the clinical outcome of PGC patients remains poor, with a 30 % 5-year survival rate for lesions confined to the gallbladder mucosa and a 10 % 1-year survival rate for advanced disease [1, 2]. Currently, the clinic-pathologic TNM staging system has been served as the standard for determining prognosis of patients with PGC, but it is probably an imprecise predictor of the prognosis of an individual patient because of the heterogenicity of this tumor [3, 4]. Therefore, it is important to identify effective prognostic biomarkers for PGC in order to better predict the individual prognosis at the time of the surgery and greatly facilitate therapeutic decision-making.
Histone deacetylases (HDACs) are major enzymes involved in the acetylation of histones and non-histone proteins, which plays an important role in the regulation of gene expression and cell signaling [5]. The modification of acetylation has already been demonstrated to be implicated in tumor progression and metastasis [6]. Especially in tumor cells, HDACs can change the nucleosomal conformation via post-translational deacetylation of the core histones [7]. The aberrant activation of HDACs may result in transcriptional repression of various genes mainly involved in the regulation of tumor cells’ proliferation, differentiation, angiogenesis and invasion, as well as migration and metastasis [8]. Recent studies are focusing on the potential of HDAC inhibitors which are a group of recently discovered targeted anti-cancer agents [9, 10]. In tumor cells, HDAC inhibitors may lead to cell cycle inhibition, activation of extrinsic and intrinsic apoptotic pathways, autophagic cell death, and mitotic cell death. A wide body of literature provides evidence for effective treatment of different tumor cells using HDAC inhibitors in vitro and in vivo. To our interests, Xu et al. [11] in 2008 found that one HDAC inhibitor trichostatin A could inhibit the growth of cholangiocarcinoma and gallbladder carcinoma cell lines in vitro and in vivo; Yamaguchi et al. [12] in 2009 demonstrated another HDAC inhibitor suberoylanilide hydroxamic acid treatment to be able to arrest cell growth of gallbladder carcinoma cell lines in vitro; in 2012, Kitamura et al. [13] further confirmed the therapeutic effect of another histone deacetylase inhibitor PCI-24781 on BK5.erbB2 mice with PGC. In humans, there are 18 HDAC isoforms which are generally divided into four classes based on sequence homology to yeast counterparts [14]. HDAC2, together with HDAC1 and HDAC3, belongs to class I [15]. Evidence has shown that HDAC2 plays a role in the development and progression of various human malignancies, such as breast cancer, ovarian and endometrial carcinomas, oral cancer, lung cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, prostate cancer and renal cell cancer [16–20]. However, its expression and role in human PGC are not fully understood. Therefore, we conducted this study to address this problem.
Materials and Methods
Patients and Tissue Samples
The study was approved by the Research Ethics Committee of Department of general surgery, Tangdu Hospital, Fourth Military Medical University, Xi’an, P.R.China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Prospectively collected data of 136 patients (60 men and 76 women), who underwent surgery for PGC between November 1997 and November 2006, were reviewed. The mean age of the patients was 66 years (range, 30–87 years). A curative resection (R0) was defined as negative resection margins by light microscopical examination. For each patient prospectively registered clinicopathological variables were extracted from the electronic clinical records: demographic data (age, gender), presenting symptoms, biochemistry, and surgical therapy. All the pathology slides were reviewed by two pathologists with special attention for tumor growth pattern and differentiation, the pathologic margin status, the presence of lymphovascular invasion, perineural invasion and the total number and status of regional and distant lymph nodes harvested. Tumor stage was classified according to the American Joint Committee on Cancer system. For histologic grading, the PGC specimens were examined by routine hematoxylin and eosin staining. The specimens were graded into well (G1), moderately (G2), poorly differentiated (G3), and undifferentiated (G4) adenocarcinoma according to the World Health Organization classification. The clinicopathological features of the patients are summarized in Table 1.
Follow-up data were recorded from the patient’s medical records and completed by a telephone survey performed on July 2011. Overall survival (OS) was defined as the time (months) from the date of surgery to the date of death by PGC. Disease-free survival (DFS) was defined as the time (months) from the date of surgery to the date of the first recurrence confirmed by imaging modalities. Median post-operative follow-up was 12.8 months (range: 22 days~126.3 months). During the follow-up period, 32 patients (23.5 %) were still alive, but 104 patients (76.5 %) died. The overall mean ± SEM survival time of the 136 patients was 29.6 ± 1.8 months. There was no perioperative mortality.
Immunohistochemical Staining and Assessment
For immunohistochemical staining, formalin-fixed paraffin-embedded tissue was cut into 4-μm sections. Commercially available rabbit anti-human polyclonal antibody against HDAC2 (1:500 dilution; #ab12169, Abcam plc, Cambridge, UK) was used. The specificity of the primary antibody against HDAC2 has been demonstrated by the previous study of Quint et al [18]. Immunohistochemical staining was carried out on sections using the avidin-biotin method and a commercially available kit (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA). Deparaffinized sections were treated with methanol containing 3 % hydrogen peroxide for 10 min before conducting antigen retrieval using a microwave oven at 95 °C for 5 min and cooling at 25 °C for 2 h. After washing with PBS, blocking serum was applied for 10 min. The sections were incubated with anti-HDAC2 polyclonal antibody overnight at 4 °C. After washing in PBS, a biotin-marked goat anti-rabbit secondary antibody was applied for 10 min followed by a peroxidase-marked streptavidin for an additional 10 min. The reaction was visualized by using 3, 3’-diaminobenzidine tetrahydrochloride. The nuclei were counterstained with hematoxylin. Positive and negative immunohistochemistry controls were routinely used. Reproducibility of staining was confirmed by reimmunostaining via the same method in multiple, randomly selected specimens.
Immunoreactivity was assessed by two investigators who were blinded to clinicopathologic data. Discrepancies were resolved by simultaneous reexamination of the slides by both investigators using a double-headed microscope. The percentage scoring of immunoreactive tumor cells was as follows: 0 (0 %), 1 (1–10 %), 2 (11–50 %) and 3 (>50 %). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). A final immunoreactivity scores (IRS) was obtained for each case by multiplying the percentage and the intensity score. The expression levels of HDAC2 were further analyzed by classifying IRS values as low (based on a IRS value less than 5 that was the median of IRS values of HDAC2 in all PGC specimens) and as high (based on a IRS value greater than 5). The protocol was done in triplicates.
Statistical Analysis
SPSS13.0 software for Windows (SPSS Inc, USA) was used for statistical analysis. Continuous variables were expressed as \( \overline{X}\pm s \). Group comparisons of categorical variables were evaluated using the Fisher’s exact or Pearson’s chi-square test. Kaplan-Meier method was used for the question of survival. Chiquest trend test and Cox regression analysis were performed for ordinal datum and the multivariate analysis, respectively. The p values of less than 0.05 were considered to be statistically significant.
Results
Expression and Cellular Localization of HDAC2 in PGC
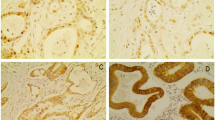
HDAC2 immunohistochemical expression was successfully detected for PGC samples from 136 patients with PGC. The positive immunostaining of HDAC2 was localized in the cellular nuclei of tumor cells in PGC samples (Fig. 1a) which was consistent with previous studies [18–20], and varied in intensity and extent of staining in different tumors. However, HDAC2 immunostaining was undetectable in the normal epithelium of the gallbladder (Fig. 1b). Additionally, HDAC2 was positively expressed in 86.0 % (117/136) tumor samples. Among these, 78.6 % (92/117) of cases displayed high expression of HDAC2.
Correlation Between HDAC2 Protein Expression and Clinicopathological Characteristics of PGC
Table 1 summarized the correlation between HDAC2 protein expression and various clinicopathological characteristics of PGC patients. There was a significant difference in the incidence of positive nodal metastasis between the high and low HDAC2 expression groups (P = 0.001). The PGC tissues with high HDAC2 expression more frequently showed positive nodal metastasis. The incidences of advanced clinical stage (P = 0.005) and pathologic T stage (P < 0.001), and higher histologic grade (P < 0.001) were respectively higher in the high HDAC2 expression group than in the low group. The PGC tissues with high HDAC2 expression tended to show advanced clinical and pathologic T stage, and higher histologic grade. There was no significant association with age, gender, or tumor size (Table 1).
Prognostic Value of HDAC2 Expression in PGC
To evaluate the prognostic value of HDAC2 protein expression in PGC, the adequate clinical follow-up information of all 136 PGC patients were reviewed. The OS rates of low and high HDAC2 expression groups were respectively 47.4 % (9/19) and 12.8 % (15/117), and the DFS rates of low and high HDAC2 expression groups were respectively 52.6 % (10/19) and 14.5 % (17/117). Figure 2 showed the survival curves according to HDAC2 immunohistochemical expression. The analysis with Kaplan-Meier method clearly showed that PGC patients with high HDAC2 expression had both increased OS and DFS compared with patients with low HDAC2 expression (P < 0.001 and 0.008, Fig. 2a and b, respectively).
Overall survival (a) and disease-free survival (b) in 136 patients with human primary gallbladder carcinoma (PGC) according to the immunohistochemical expression of HDAC2. The analysis with Kaplan-Meier method clearly showed that PGC patients having tumors with strong HDAC2 expression (+++) had respectively decreased overall (P < 0.001) and DFS (P = 0.008) compared with patients with negative (-), weak (+) or moderate (++) HDAC2 expression
Then, we estimated the clinical significance of various prognostic factors that might influence survival and recurrence. As summarized in Table 2, the univariate analysis suggested that advanced pathologic T stage (P = 0.008), advanced clinical stage (P < 0.001), nodal metastasis (P = 0.001), distant metastasis (P = 0.001), and high HDAC2 expression (P < 0.001) were statistically significant risk factors affecting OS of patients with PGC. In case of DFS, advanced pathologic T stage (P = 0.02), advanced clinical stage (P = 0.001), nodal metastasis (P = 0.001), and high HDAC2 expression (P = 0.008) were statistically significant risk factors.
Furthermore, we evaluated the independent prognostic impacts of these various factors. As summarized in Table 3, the multivariate analysis using the Cox proportional hazards model shown that advanced clinical stage (hazard ratio [HR], 8.862; 95 % confidence interval [CI], 1.328–26.523; P = 0.008) and high HDAC2 expression (HR, 8.221; 95 % CI, 1.293–23.128; P = 0.008) were independent risk factors predicting short OS. In case of DFS, advanced clinical stage (HR, 3.391; 95 % CI, 1.183–9.923; P = 0.02) and high HDAC2 expression (HR, 8.695; 95 %CI, 1.248–27.079; P = 0.008) were independent risk factors predicting short DFS (Table 3).
Discussion
It is of great challenge for the clinicians on the management of PGC to accurate risk stratification which may play an important role in decision making of patient tailored strategy. In the present study, we detected the immunohistochemical expression of the HDAC2 protein in a large number of PGC tissues. There are four points of our findings. Firstly, the HDAC2 protein was overexpressed in tumor cells of PGC tissues, but not expressed in normal epithelium of gallbladder; Secondly, the up-regulation of HDAC2 protein in PGC tissues was significantly correlated with advanced tumor progression and aggressive clinicopathological features; Thirdly, the results of Kaplan-Meier analyses shown that PGC tissues with high HDAC2 expression tend to have decreased OS and DFS rates. Finally, both univariate and multivariate analyses clearly demonstrated that the high HDAC2 expression was a statistically significant risk factor affecting OS and DFS of patients with PGC, suggesting that HDAC2 expression could be a useful marker to predict patient survival.
HDACs are enzymes that catalyze the removal of acetyl moieties from lysine residues [21]. They were initially identified as histone deacetylating enzymes and regulators of chromatin structure. In recent years, HDACs have been demonstrated to be involved in various biological processes, including proliferation and cell survival [22]. Especially, HDACs function as negative regulators of gene expression and play important roles in tumorigenesis and tumor progression. The inhibition of HDACs’ activity may lead to suppression of cell proliferation and apoptosis in neoplastic transformed but not in normal cells. Thus, HDACs are promising targets for anti-tumor drugs. According to molecular structure, enzymatic activity, localization and expression pattern, HDACs are divided into four classes: class-I (HDAC1, 2, 3 and 8 have homology to yeast RPD3); class-IIa (HDACs 4, 5, 7 and 9 have homology to yeast HDA1); class-IIb (HDACs 6 and 10 have two catalytic sites) and class-IV (HDAC11, has conserved residues shared with both class-I and class-II deacetylases) [23]. Among the class I enzymes, HDAC1 and HDAC2 are shown to be highly homologous. Both are ubiquitously expressed with nuclear localization and are components of large protein complexes with transcriptional repression activity, participating in the regulation of cell cycle, differentiation and development [24, 25]. Especially, HDAC2 has a rather specific anti-apoptotic function in tumor cells. Huang et al. [26] reported that HDAC2 knockdown in tumor cells may lead to a more differentiated phenotype and increased apoptosis caused by augmented levels of p21. In breast cancer cells, Harms et al. [27] also confirmed that HDAC2 knockdown may induce the binding activity of p53 resulting in the inhibition of cell proliferation and cellular senescence. In recent years, increasing evidences have found the overexpression of HDAC2 in the oncogenic process of various human malignancies. For instance, the increased HDAC2 expression was detected in colonic, liver, gastric, pancreatic and cervical cancers [16–20]; Zhu et al. [28] further demonstrated that HDAC2 expression was increased by the loss of APC in human colorectal cancer; Chang et al. [29] found that HDAC2 expression elevated distinctly from epithelial dysplasia to oral squamous cell carcinoma, and cancer of advanced stage, with larger tumor size or positive lymph node metastasis, had higher level of HDAC2 protein expression. In agreement with these results, our data also showed that the nuclear HDAC2 immunostaing was greatly increased in PGC tissues compared with normal epithelium tissues of gallbladder. To our knowledge, this is the first study to show expression and localization of HDAC2 protein in PGC tissues. Moreover, we also found that PGC tissues at advanced stage, with positive nodal metastasis, and in higher histological grade, had higher HDAC2 immunohistochemical expression. These findings suggest that the prevalent expression of HDAC2 in cancer cells may be closely related to cell migration and invasion potential.
On the other hand, increasing numbers of studies suggest that expression of HDAC2 is associated with tumor prognosis in several types of cancer. For instance, Chang et al. [29] demonstrated that oral squamous cell carcinoma patients with high HDAC2 expression had significantly shorter overall survival than those with low HDAC2 expression; In prostate cancer patients, HDAC2 was associated with PSA relapse-free survival; Quint et al. [18] indicated that HDAC2 overexpression in hepatocellular carcinoma was associated with poor outcome, both in the general patient population, comparing all cases (HDAC low vs. high expression) as well as in low-grade (G1) and early-tumor stage (pT1/2) patients. Consistent with these findings, our data also confirmed that the increased HDAC2 expression in PGC was associated with poor overall and disease-free survival in patients. Therefore, HDAC2 expression may be served as an adjuvant marker of unfavourable clinical outcome in PGC patients.
In conclusion, high HDAC2 expression was correlated with a high incidence of lymph node metastasis and aggressive tumor progression of PGC. It also was an independent prognostic factor for poorer overall and disease-free survival in patients. Therefore, detection of HDAC2 expression may help us screen patients at increased risk of malignant behavior for PGC. However, the precise mechanism that HDAC2 is involved in the tumorigensis and tumor progression of PGC remains unclear. The further prospective analysis would be worth doing.
References
Misra S, Chaturvedi A, Misra NC, Sharma ID (2003) Carcinoma of the gallbladder. Lancet Oncol 4:167–176
Chen Y, Chen Y, Yu G, Ding H (2011) Lymphangiogenic and angiogentic microvessel density in gallbladder carcinoma. Hepatogastroenterology 58:20–25
Araida T, Higuchi R, Hamano M, Kodera Y, Takeshita N, Ota T, Yoshikawa T, Yamamoto M, Takasaki K (2009) Should the extrahepatic bile duct be resected or preserved in R0 radical surgery for advanced gallbladder carcinoma? results of a Japanese Society of Biliary Surgery Survey: a multicenter study. Surg Today 39:770–779
Kokudo N, Makuuchi M, Natori T, Sakamoto Y, Yamamoto J, Seki M, Noie T, Sugawara Y, Imamura H, Asahara S, Ikari T (2003) Strategies for surgical treatment of gallbladder carcinoma based on information available before resection. Arch Surg 138:741–750
Davies GF, Ross AR, Arnason TG, Juurlink BH, Harkness TA (2010) Troglitazone inhibits histone deacetylase activity in breast cancer cells. Cancer Lett 288:236–250
Brandl A, Heinzel T, Kramer OH (2009) Histone deacetylases: salesmen and customers in the post-translational modification market. Biol Cell Auspices Eur Cell Biol Organ 101:193–205
Ishdorj G, Graham BA, Hu X, Chen J, Johnston JB, Fang X, Gibson SB (2008) Lysophosphatidic acid protects cancer cells from histone deacetylase (HDAC) inhibitor-induced apoptosis through activation of HDAC. J Biol Chem 283:16818–16829
Mehdi O, Françoise S, Sofia CL, Urs G, Kevin Z, Bernard S, Igor S, Anabela CD, Dominique L, Eric M, Ali O (2012) HDAC gene expression in pancreatic tumor cell lines following treatment with the HDAC inhibitors panobinostat (LBH589) and trichostatine (TSA). Pancreatology 12:146–155
Spiegel S, Milstien S, Grant S (2012) Endogenous modulators and pharmacological inhibitors of histone deacetylases in cancer therapy. Oncogene 31:537–551
Lee JH, Jeong EG, Choi MC, Kim SH, Park JH, Song SH, Park J, Bang YJ, Kim TY (2010) Inhibition of histone deacetylase 10 induces thioredoxin-interacting protein and causes accumulation of reactive oxygen species in SNU-620 human gastric cancer cells. Mol Cells 30:107–112
Xu LN, Wang X, Zou SQ (2008) Effect of histone deacetylase inhibitor on proliferation of biliary tract cancer cell lines. World J Gastroenterol 14:2578–2581
Yamaguchi J, Sasaki M, Sato Y, Itatsu K, Harada K, Zen Y, Ikeda H, Nimura Y, Nagino M, Nakanuma Y (2010) Histone deacetylase inhibitor (SAHA) and repression of EZH2 synergistically inhibit proliferation of gallbladder carcinoma. Cancer Sci 101:355–362
Kitamura T, Connolly K, Ruffino L, Ajiki T, Lueckgen A, Digiovanni J, Kiguchi K. (2012) The therapeutic effect of histone deacetylase inhibitor PCI-24781 on gallbladder carcinoma in BK5.erbB2 mice. J Hepatol. In press
Willis-Martinez D, Richards HW, Timchenko NA, Medrano EE (2010) Role of HDAC1 in senescence, aging, and cancer. Exp Gerontol 45:279–285
Schüler S, Fritsche P, Diersch S, Arlt A, Schmid RM, Saur D, Schneider G (2010) HDAC2 attenuates TRAIL-induced apoptosis of pancreatic cancer cells. Mol Cancer 9:80
Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ, Xie HJ, Chang YG, Kim MG, Park H, Lee JY, Nam SW (2012) HDAC2 overexpression confers oncogenic potential to human lung cancer cells by deregulating expression of apoptosis and cell cycle proteins. J Cell Biochem 113:2167–2177
Patani N, Jiang WG, Newbold RF, Mokbel K (2011) Histone-modifier gene expression profiles are associated with pathological and clinical outcomes in human breast cancer. Anticancer Res 31:4115–4125
Quint K, Agaimy A, Di Fazio P, Montalbano R, Steindorf C, Jung R, Hellerbrand C, Hartmann A, Sitter H, Neureiter D, Ocker M (2011) Clinical significance of histone deacetylases 1, 2, 3, and 7: HDAC2 is an independent predictor of survival in HCC. Virchows Arch 459:129–139
Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn CO, Heidecke CD, Friess H, Büchler M, Evert M, Lerch MM, Weiss FU (2012) Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 61:439–448
Ramsey MR, He L, Forster N, Ory B, Ellisen LW (2011) Physical association of HDAC1 and HDAC2 with p63 mediates transcriptional repression and tumor maintenance in squamous cell carcinoma. Cancer Res 71:4373–4379
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370:737–749
Khier H, Bartl S, Schuettengruber B, Seiser C (1999) Molecular cloning and characterization of the mouse histone deacetylase 1 gene: integration of a retrovirus in 129SV mice. Biochim Biophys Acta 1489:365–373
Brunmeir R, Lagger S, Seiser C (2009) Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53:275–289
Yamaguchi T, Cubizolles F, Zhang Y, Reichert N, Kohler H, Seiser C, Matthias P (2010) Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev 24:455–469
Jurkin J, Zupkovitz G, Lagger S, Grausenburger R, Hagelkruys A, Kenner L, Seiser C (2011) Distinct and redundant functions of histone deacetylases HDAC1 and HDAC2 in proliferation and tumorigenesis. Cell Cycle 10:406–412
Huang BH, Laban M, Leung CH, Lee L, Lee CK, Salto-Tellez M, Raju GC, Hooi SC (2005) Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ 12:395–404
Harms KL, Chen X (2007) Histone deacetylase 2 modulates p53 transcriptional activities through regulation of p53-DNA binding activity. Cancer Res 67:3145–3152
Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Göttlicher M (2004) Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell 5:455–463
Chang HH, Chiang CP, Hung HC, Lin CY, Deng YT, Kuo MY (2009) Histone deacetylase 2 expression predicts poorer prognosis in oral cancer patients. Oral Oncol 45:610–614
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Source of support
This work was supported by the National Natural Science Foundation of China (no.81172287)
Xilin Du and Huadong Zhao equally contribute to this work.
Rights and permissions
About this article
Cite this article
Du, X., Zhao, H., Zang, L. et al. Overexpression of Histone Deacetylase 2 Predicts Unfavorable Prognosis in Human Gallbladder Carcinoma. Pathol. Oncol. Res. 19, 397–403 (2013). https://doi.org/10.1007/s12253-012-9592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-012-9592-y