Abstract
Integrin-linked kinase (ILK) is an unique intracellular serine/threonine kinase and adapter protein. When dysregulated, it has been associated with increased cell proliferation, anchorage-independent cell growth, evasion of apoptosis, angiogenesis, invasion of surrounding tissues, downregulation of E-cadherin expression, nuclear translocation of β-catenin and metastasis, all features of tumoral malignancy. The objective of the present work was to evaluate the expression of ILK in clear cell renal carcinomas (CCRC) as a possible prognostic indicator. ILK immunoexpression was evaluated in a tissue microarray (TMA) with 45 human CCRCs. In addition, the apoptotic and proliferative indices and the immuno-expression of β-catenin and E-cadherin were also evaluated. E-cadherin expression was significantly decreased in tumors with positive ILK expression in relation to those with negative immunoexpression (p = 0.011). ILK immunostaining was significantly increased in high-grade in comparison to low-grade CCRCs (p = 0.0008). ILK expression was also associated with increased proliferative index (p = 0.020), tumor size >7.0 cm (p = 0.018) and with renal vein and capsule invasion (p = 0.003 and p = 0.00). Finally, tumors stage I and II (noninvasive) presented significantly reduced ILK immunoexpression when compared to stage III (locally invasive) (p = 0.0028). ILK immunoexpression in CCRC increases with loss of intercellular adhesion, nuclear grading, increased proliferative index and Robson stage. Altogether, our data suggest a possible role for ILK in the progression of CRCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Integrin-linked kinase (ILK) is a serine/threonine kinase that interacts with β-integrin cytoplasmic domain and functions as a scaffolding protein in the formation of multiprotein complexes connecting integrins to the actin cytoskeleton, therefore modulating intracelullar signaling pathways originated by those connections [1, 2]. Its kinase activity is stimulated by cell adhesion to extracellular matrix (ECM) [3] or by growth factors and chemokines through tyrosine kinase receptors in a phosphatidylinositol 3-kinase (Pi3K)-dependent maner [4]. ILK has been shown to directly phosphorylate several proteins, resulting in several oncogenesis-related processes, such as, increased cell proliferation, anchorage and growth-factor independence, evasion of apoptosis, angiogenesis, invasion of surrounding tissues and metastasis [5]. Accordingly, ILK has been shown to modulate the localization and transcriptional activity of β-catenin, the kinase activity of PKB/Akt, the expression of E-cadherin and the activity of several transcription factors [5–10].
ILK expression and activity are increased in many types of cancer, such as prostate, colon, gastric, ovarian cancers, malignant melanomas, Ewing’s sarcoma, primitive neuroectodermal tumour, non-small cell lung cancer, basal cell carcinoma, ductal pancreatic adenocarcinoma, hepatocellular carcinoma, laryngeal carcinomas and bladder cancer [11–26].
The clear cell renal carcinoma (CCRC) is the most common histologic variant of renal cell carcinoma, accounting for approximately 70 % of cases and is well-known for a variable and unpredictable biological behavior. In order to correlate its histologic findings with outcome, numerous studies on tumour microvascular density, cell proliferation and apoptosis have been performed with conflicting results [27, 28]. The overall survival rate after nephrectomy for patients with CCRC is around 50 %. Numerous pathological variables have been investigated to help predicting survival. Among prognostic factors determining patient outcome, ie., nuclear grade, tumor size, sarcomatoid differentiation, infiltrative margin and vascular invasion, the tumor stage is the most important. However, CCRCs in the same stage or grade may exhibit distinct biological behavior. Therefore, other indicators that might help the improvement of prognostic evaluation of CCRC are required.
In the present study, we analyzed ILK expression by immunohistochemistry in a series of CCRCs. We also evaluated E-cadherin and β-catenin expression as well as the apoptotic and cellular proliferative indices. Potential correlations among those elements and their relationship to prognostic and progression parameters of those tumors were addressed.
Materials and Methods
Renal Cell Carcinoma Tissue Microarray Construction
TMA included archival, formalin-fixed, paraffin-embedded surgical specimens of 45 CCRCs from the Pathology Department of Federal University of São Paulo (UNIFESP/EPM) and Salomão & Zoppi Laboratory, São Paulo city, dated between 1997 and 2001. The study was approved by the institutional Committee on Research and Ethics. Hematoxylin and eosin (H&E) stained microslides were reviewed and graded according to the Fuhrman system. Core biopsies of 1.0 mm in diameter were taken from each donor block and arrayed into a recipient paraffin block in spaces of 1.2 mm using a tissue microarrayer (Beecher Instruments, Sun Prairie, Wisconsin, USA), as described by Kononen et al. [29]. There were two cores for each tumor in the array, resulting in two histological spots on the corresponding slides. Controls were 5 cores of normal renal tissue, which was represented in the array by two cores each.
Immunohistochemistry
Antibody staining was performed on 5 μm histological sections of the receptor block (three for each antibody). All sections were deparaffinized in xylene and rehydrated in graded ethanols before antigen retrieval for 30 min with microwave trearment in 0.1 M citrate buffer (pH 6.0). After incubation with 0.3 % hydrogen peroxide in methanol and incubation with normal blocking serum, sections were incubated with primary antibody: anti-E-cadherin (Dako – clone NCH-38 – cat # M3612) 1:100; anti-β-catenin (BD Biosciences – clone 14), 1:600; anti-PCNA (Dako clone PC10 – cat # M 0879), 1:500; anti-ILK (BD Biosciences – cat # 611802) 1:150 at 3 °C overnight. Immunodetection was performed with the LSAB kit+, Peroxidase – Universal, cat # K 0690 (Dako) using diaminobenzidine as chromogen. Positive controls for immunostaining were formalin-fixed, paraffin-embedded specimens of human colonic mucosa (E-cadherin), colon carcinoma (β-catenin), breast carcinoma (PCNA), and kidney (ILK). For negative controls, blocking solution was instead of the primary antibody. All sections were counterstained with Harris’s hematoxylin.
Immunohistochemical Evaluation
Staining patterns, including cell distribution (membrane, cytoplasmic, and nuclear pattern), extent and intensity of stain were evaluated independently for each specimen by three investigators. For ILK, the evaluation was categorical, and staining was classified as negative (<10 % of the neoplasm stained), patchy,(heterogeneously positive with less than 50 % of the neoplasm stained), or uniformly positive (involving more than 90 % of the neoplasm). Comparisons were made between negative/patchy and uniform staining pattern [11]. For E-cadherin e β-catenin, a 0–4 scale was used: negative; focal (<10 %), regional (10–50 %) e diffuse (>50 %). Tumors with negative or patchy staining were considered negative. Tumors presenting regional and difuse staining were categorized as positive.
Assessment of Proliferation and Apoptosis
Proliferative index was assessed by immunostaining with mouse monoclonal antibody to PCNA by evaluating all cells in each core. Apoptotic index was assessed by TUNEL staining (kit ApopTag® Plus Peroxidase, Intergen, New York, EUA) by counting in each core all cells showing terminal deoxynucleotidyl transferase positivity. Counting was performed with the Image-tool for Windows program. An average of 1218 ± 197 cells was counted per section for PCNA positivity. An average of 509 ± 84 cells was counted per section for terminal deoxynucleotidyl transferase positivity. The proliferative and apoptotic indices were then calculated by the following equation: (number of stained nuclei/total number of nuclei counted × 100).
Statistical Analyses
For statistical analyses, the samples were classified in accordance with Fuhrman nuclear grade (low grade = grade 1 and 2, high grade = grade 3 and 4), size of the tumor (<7 cm and >7 cm, <4 cm and >4 cm), presence or absence of capsule invasion, presence or absence of renal vein invasion and Robson stage (I–II and III). Relationships between staining patterns and each tissue group were evaluated by Fisher’s exact test. Differences between groups with respect to proliferative index (i.e., PCNA staining) and apoptotic fraction (TUNEL staining) were calculated using Mann Whitney test. A p value inferior to 0.05 was accepted as significant.
Results
Correlation between ILK Expression and Tumor Severity
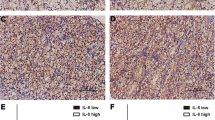
There was a statistically significant correlation between ILK expression and Fuhrman’s nuclear grade, with high grade CCRCs presenting higher levels of ILK than low grade tumors (p = 0.0008) (Fig. 1a and b). Furthermore, there was a simultaneous and significant increase in proliferative index and ILK expression in high grade in comparison to low grade CCRCs (p = 0.002 and p = 0.0008) (Table 1). In addition, tumors with ILK expression positive showed increased proliferation index in relation to tumors with ILK expression negative (p = 0.020) (Table 2).
Renal vein invasive and renal capsule invasive tumors showed significantly higher expression of ILK when compared to those that did not present invasion (p = 0.00 and p = 0.003). And tumors larger than 7.0 cm presenting higher levels of ILK in relation CCRCs smaller than 7.0 cm (p = 0.018). Finally, tumors stage I and II (noninvasive) presented significantly reduced ILK immunoexpression when compared to stage III (locally invasive) (p = 0.0028) (Table 3).
Correlation between ILK Expression and E-cadherin Expression
CCRC group presented significantly reduced E-cadherin expression when compared to controls (p = 0.005) (Fig. 1e and f, Table 5). When related to Fuhrman’s grade, E-cadherin expression was also significantly diminished in high-grade in relation to low grade carcinomas (p = 0.046) (Table 1). E-cadherin expression was significantly decreased in tumors with positive ILK expression in relation to those with negative immuno-expression (p = 0.011) (Table 2). Finally, tumors with negative E-cadherin expression showed lower apoptotic index than positive E-cadherin expression (p = 0.0099) (Table 4).
Correlation between ILK Expression and β-catenin Expression
β-catenin immunoexpression presented membranous pattern of staining and was significantly higher in ILK immunopositive CCRCs in comparison to the negative ones (p = 0.012) (Fig. 1c and d, Table 4). β-catenin expression was significantly decreased in CCRCs (p = <0.020) in relation to controls (Table 5).
Discussion
So far, there is no report in the literature, to our knowledge, using ILK immunoexpression as a prognostic indicator in patients with CCRC. We used the tissue microarray technique, which permit the simultaneous evaluation of a large number of tissue samples and a high level of immunohistochemistry staining standardization, since all tissue samples are pretreated and stained in identical conditions [29–32].
In normal human kidney tubular cell staining, primarily in the distal and proximal tubules, was present, but not in the glomerulus. As a whole, ILK immuno-expression was significantly lower in CCRCs as compared to the control group. Accordingly, Haase et al. [33] concluded that the mean ILK expression in renal cell carcinomas is reduced by 35 % compared with normal kidney tissue, which is statistically significant. The explanation for this apparent contradiction is that CCRC cells present cytoplasm almost totally occupied with droplets of lipids due to deficient glycogenolysis and lipolysis, making its comparison with other histologic types flawed Therefore, we decided to compare ILK immunoreactivity in relation to nuclear grade.
More realistically, our results showed that high-grade CCRCs presented ILK expression significantly higher in relation to low grade CCRCs. Consistently, several studies demonstrated increased ILK immunostaining in high-grade primary human prostatic, ovarian, colonic and no small cell pulmonary carcinomas in comparison to low-grade tumors [11, 14, 16, 19].
CCRCs with positive ILK expression presented significant increase in proliferation index (p = 0,002) in relation to tumors with negative ILK expression. Compatibly, ILK expression was also specifically related to the increase in proliferative index that contributes to the net gain of prostatic carcinomas cells during progression [11].
CCRCs larger than 7.0 cm and CCRCs invading the renal vein and capsule presented ILK immunoexpression significantly higher than tumors smaller than 7.0 cm and that did not invade it, which was suggestive of involvement of ILK in tumor dissemination. Finally, stage III tumors (locally invasive) showed increased ILK expression in relation to stage I and II tumors (noninvasive), In gastric carcinoma significant association was detected between ILK mRNA expression and presence of nodal metastasis and strong expression of ILK protein was significantly associated with deep invasion of tumor cells in gastric wall [15]. The levels of ILK expression correlated strongly with tumour invasion and stage, and were significantly higher in metastatic human colon cancer [14]. Furthemore, ILK expression is significantly associated with melanoma thickness and with lymph node invasion [17]. In non-small cell lung cancer, ILK expression was significantly associated with T status, lymph node metastasis and stage [19]. Matsui Y et al. reported that ILK is overexpressed in invasive bladder cancer and plays an important role in the EMT of bladder cancer via the control of E-cadherin and MMP-9 expression [26].
Loss of cell–cell adhesion mediated by the β-catenin/E-cadherin complex proteins is well documented in various human malignancies. Down-regulation of E-cadherin is a critical step in the induction of epithelial-mesenchymal transition (EMT), a process considered crucial to cancer invasion and metastasis [34]. In addition, the poor prognostic implications of decreased expression of these proteins have been reported in various carcinomas, including those arising from kidney [35–37]. In the present study, E-cadherin expression was significantly decreased in CCRC in relation to control and was also significantly diminished in high-grade in relation to low grade carcinomas. However it was not correlated with tumor size, capsule or vein invasion as also reported by Slaton et al. [37] in their study of 46 cases of CRCCs.
E-cadherin expression was significantly decreased in tumors with positive ILK expression in relation to those with negative immuno-expression. Normally, loss of cell adhesion leads to apoptosis. However, we found that tumors with negative E-cadherin expression showed lower apoptotic index than positive E-cadherin expression, reflecting both decrease in intercellular adhesion and evasion of apoptosis. These two aspects might be vinculated to ILK activity, since it has been shown that ILK down-regulates E-cadherin expression [10] and may inhibit apoptosis via PKB/Akt signaling pathway. As a matter of fact, inhibition of ILK leads to apoptosis in anaplastic thyroid cancer, pancreatic adenocarcinoma and glioblastomas [38–40]. Recently, Bravou et al. [14] demonstrated that in human colon cancer progression, ILK overexpression correlates with activation of β-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. Moreover, ILK overexpression can inhibit MTA-3 expression, resulting in the stimulation of the E-cadherin transcriptional repressor, Snail [10].
β-catenin is involved in intercellular adhesion and function as a key molecule in the Wnt signalling pathway [41]. In our study, β-catenin expression in CCRCs presented membrane staining pattern and was significantly lower than in control group. We could not detect nuclear accumulation of β-catenin, suggesting that Wnt pathway activation is not a frequent phenomenon in CRCC. Furthermore, β-catenin expression was significantly higher in ILK immunopositives CCRCs in compariso to negatives CCRCs. Accordingly, Guo et al. [42] concluded that β-catenin expression is generally down-regulated in low nuclear grade CCRCs. Conversely, it appears to be preserved in those with high nuclear grades. Kim et al. [43], studied β-catenin expression in 52 RCCs, and demonstrated that it was mainly localized in cell membrane. Five of 22 CCRCs showed β-catenin accumulation in the cytoplasm. None displayed positive β-catenin immunostaining in the nuclei and only one case presented mutation in β-catenin exon 3. In a recent study, analyzing 38 samples of RCCs, it was demonstrated that β-catenin gene was not overexpressed at the mRNA level and protein level and no alterations was found in β-catenin exon 3 [44].
In relation to apoptosis, the CCRC group presented increased apoptosis index when compared to control group and high grade tumors presented lower apoptosis index and higher proliferative index in relation to low grade. This finding implies an oncogenetic mechanism leading to both stimulation of cellular cycle progression and promotion of apoptosis evasion. As a matter of fact, progression from well to less differentiated renal cell carcinoma requires decrease of apoptotic rate, as well as increase in proliferative activity [45]. ILK could be responsible for this effect, since it is already known that it has anti-apoptotic activity through PKB/Akt signaling pathway as well as proliferative activity through stimulation of the cell cycle [5–10, 14].
Altogether the present data indicate that ILK immuno-expression in CRCCs is related to loss of intercellular adhesion, to the differentiation grade and keeps positive correlation with the proliferative index, renal capsule and renal vein invasion, tumor size and Robson stage. ILK immuno-expression presented a direct correlation with the progression of the tumor in CCRCs, and opens up new perspectives for a targeted molecular therapy of CCRCs with specific pharmacologic inhibitors of members of the ILK pathway.
References
Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L et al (1996) Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379:91–96
Dedhar S, Williams B, Hannigan G (1999) Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol 9:319–323
Wu C, Keightley SY, Leung-Hagesteijn C et al (1998) Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem 273:528–536
Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A 95:11211–11216
Hannigan G, Troussard AA, Dedhar S (2005) Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer 5:51–63
Wu C, Dedhar S (2001) Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 155:505–510
Persad S, Attwell S, Gray V et al (2000) Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A 97:3207–3212
Novak A, Hsu S-C, Leung-Hagesteijn C et al (1998) Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc Natl Acad Sci U S A 95:4374–4379
D’Amico M, Hulit J, Atullah DF et al (2000) The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3B and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem 75:32649–32657
Oloumi A, McPhee T, Dedhar S (2004) Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta 169:1–15
Graff JR, Deddens JA, Konicek BW et al (2001) Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res 7:1987–1991
Marotta A, Tan C, Gray V et al (2001) Dysregulation of integrin-linked kinase (ILK) signaling in colonic polyposis. Oncogene 20:6250–6267
Marotta A, Parhar K, Owen D, Dedhar S, Salh B (2003) Characterisation of integrin-linked kinase signalling in sporadic human colon cancer. Br J Cancer 88:1755–1762
Bravou V, Klironomos G, Papadaki E, Taraviras S, Varakis J (2006) ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol 208:91–99
Ito R, Oue N, Zhu X et al (2003) Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch 442:118–123
Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA (2003) Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol 201:229–237
Dai DL, Makretsov N, Campos EI et al (2003) Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res 9:4409–4414
Chung DH, Lee JI, Kook MC et al (1998) ILK (beta1-integrin-linked protein kinase): a novel immunohistochemical marker for Ewing’s sarcoma and primitive neuroectodermal tumour. Virchows Arch 433:113–117
Takanami I (2005) Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer 5:1
Okamura M, Yamaji S, Nagashima Y et al (2007) Prognostic value of integrin beta1-ILK-pAkt signaling pathway in non-small cell lung cancer. Hum Pathol 38:1081–1091
Watzka SB, Rauscher-Pötsch I, Stubenberger E et al (2010) Immunoreactivity of integrin-linked kinase in primary non-small-cell lung cancer and survival after curative resection. Eur J Cardiothorac Surg 38:254–259
Papanikolaou S, Bravou V, Gyftopoulos K, Nakas D, Repanti M, Papadaki H (2010) ILK expression in human basal cell carcinoma correlates with epithelial-mesenchymal transition markers and tumour invasion. Histopathology 56:799–809
Schaeffer DF, Assi K, Chan K et al (2010) Tumor expression of integrin-linked kinase (ILK) correlates with the expression of the E-cadherin repressor snail: an immunohistochemical study in ductal pancreatic adenocarcinoma. Virchows Arch 456:261–268
Peroukides S, Bravou V, Varakis J, Alexopoulos A, Kalofonos H, Papadaki H (2008) ILK overexpression in human hepatocellular carcinoma and liver cirrhosis correlates with activation of Akt. Oncol Rep 20:1337–1344
Goulioumis AK, Bravou V, Varakis J, Goumas P, Papadaki H (2008) Integrin-linked kinase cytoplasmic and nuclear expression in laryngeal carcinomas. Virchows Arch 453:511–519
Matsui Y, Assi K, Ogawa O et al (2012) The importance of integrin-linked kinase in the regulation of bladder cancer invasion. Int J Cancer 130:521–531
Yoshino S, Kato M, Okada K (2000) Clinical significance of angiogenesis, proliferation and apoptosis in renal cell carcinoma. Anticancer Res 20:591–594
Kirkali Z, Yorukoglu K, Ozkara E, Kazimoglu H, Mungan U (2001) Proliferative activity, angiogenesis and nuclear morphometry in renal cell carcinoma. Int J Urol 8:697–703
Kononen J, Bubendorf L, Kallioniemi A et al (1998) Tissue microarrays for high-through put molecular profiling of tumor specimens. Nat Med 4:844–847
Kallakuri BVS, Sheehan CE, Winn-Deen E et al (2001) Decreased expression of catenins (α and β), p120, CTNM and E-cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer 92:2786–2795
Rao JY, Seligson D, Visapaa H et al (2002) Tissue microarray analysis for cytoskeletal actin-associated biomarkes gelsolin and cadherin in urothelial carcinoma. Cancer 95:1247–1257
Skacel M, Skilton B, Pettay JD, Tubbs RR (2002) Tissue microarrays: a powerful tool for high-throughput an analisis of clinical specimens—a review of the method whit validation data. Appl Immunohistochem Mol Morphol 10:1–6
Haase M, Gmach CC, Eke I, Hehlgans S, Baretton GB, Cordes N (2008) Expression of integrin-linked kinase is increased in differentiated cells. J Histochem Cytochem 56:819–829
Thiery JP (2004) Epithelial-mesenchymal transition: twist in development and metastasis. Cell 1014:1125–1136
Katagiri A, Watanabe R, Tomita Y (2000) E-cadherin expression in renal cell cancer and its significance in metastasis and survival. Br J Cancer 85:801–804
Pecina-Slaus N, Gall-TroSelj K, Slaus M et al (2004) Genetic changes of the E-cadherin and APC tumour suppressor genes in clear renal cell carcinoma. Pathology 36:145–151
Slaton JW, Inoue K, Perrotte P et al (2001) Expression levels of genes that regulate metastasis and angiogenesis correlate with advanced pathological stage of renal cell carcinoma. Am J Pathol 158:735–743
Younes MN, Kim S, Yigitbasi OG et al (2005) Integrin-linked kinase is a potential therapeutic target for anaplastic thyroid cancer. Mol Cancer Ther 4:1146–1156
Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE (2005) RNA interference demonstrates a novel role for integrin-linked kinase as a determinant of pancreatic adenocarcinoma cell gemcitabine chemoresistance. Clin Cancer Res 11:3433–3438
Edwards LA, Thiessen B, Dragowska WH et al (2005) Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene 24:3596–3605
Han L, Pamukcu R, Thompson J (2002) β-catenin signaling: therapeutic strategies in oncology. Cancer Biol Ther 1:621–625
Guo L, Kuroda N, Miyazaki E et al (2001) The complementary role of beta-catenin in diagnosing various subtypes of renal cell carcinomas and its up-regulation in conventional renal cell carcinomas with high nuclear grades. Oncol Rep 8:521–526
Kim YS, Kang YK, Kim JB, Han SA, Kim KI, Paik SR (2000) β-catenin expression and mutational analysis in renal cell carcinomas. Pathol Int 50:725–730
Shiina H, Igawa M, Breault J et al (2003) The human T-cell factor-4 gene splicing isoforms, WNT pathway and apoptosis in renal cell carcinoma. Clin Cancer Res 9:2121–2132
Hindermann W, Berndt A, Wunderlich H, Katenkamp D, Kosmehl H (1997) Quantitative evaluation of apoptosis and proliferation in renal cell carcinoma. Correlation to tumor subtype, cytological grade according to thoenes-classification and the occurrence of metastasis. Pathol Res Pract 193:1–7
Acknowledgments
The authors would like to thank Mrs. Ivonete Barbosa for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engelman, M., Grande, R.M., Naves, M.A. et al. Integrin-Linked Kinase (ILK) Expression Correlates with Tumor Severity in Clear Cell Renal Carcinoma. Pathol. Oncol. Res. 19, 27–33 (2013). https://doi.org/10.1007/s12253-012-9554-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-012-9554-4