Abstract
High-mobility group box 1(HMGB1) has been associated with many human cancers, but the role of HMGB1 in hepatocellular carcinoma (HCC) remains unclear. The aim of this study is to investigate the expression of HMGB1 in human HCC with regard to its clinical significance. Twelve cases of normal liver tissues, 34 cases of HCC and the corresponding liver tissue just around the tumor (LAT) were collected. Then, all the samples were subjected to clinicopathologic examination, reverse transcription–polymerase chain reaction (RT–PCR), Western-blot (WB) and immunohistochemical analysis for the expression of HMGB1. The relationships between HMGB1 mRNA expression and clinicopathologic parameters were analyzed. RT–PCR demonstrated that the expression of relative HMGB1 mRNA (HMGB1/GAPDH) was 0.854 ± 0.172; the highest in the tissue of HCC, significantly up-regulated compared with that of 0.527 ± 0.155 in LAT and of 0.405 ± 0.087 in normal liver tissues (P < 0.001). HMGB1 mRNA overexpression was significantly associated with Edmondson stage, TNM stage, vascular invasion and capsule invasion. Western-blot showed the expression of HMGB1 protein in HCC also as the highest among all the groups. Furthermore this overexpression revealed by immunostaining was predominantly localized in the nuclei of HCC; whereas, none of the stains were seen in normal liver cells and only a trace of it was detected in the cytoplasm of LAT cells. Our results suggested the overexpression of HMGB1 might be an important pathogenetic factor in HCC. The mechanisms of HMGB1 in HCC genesis, development and its possible diagnostic and prognostic roles need to be further explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), the fifth most frequent malignancy yet with an alarmingly high mortality, is one of the most common causes of cancer-related death in the world, especially in Eastern Asia and sub-Saharan Africa [1, 2]. China alone is estimated to account for more than 50% of the World’s cases [3, 4]. Despite all the current detection and aggressive treatment techniques, such like surgical resection, liver transplantation, sorafenib and radiofrequency ablation (RFA), the prognosis of HCC still shows no significant improvement and its mortality is almost as precariously high as its morbidity [2]. The poor outcome has mainly been attributed to intrahepatic and distant metastasis. Therefore, to further understand the special characteristics of HCC biology and its pathogenetic factors, discovering HCC oncogenes and metastasis genes, in order to know their mechanisms, is substantial to the development of novel strategies in the prevention and treatment of patients with HCC.
High-mobility group box 1(HMGB1), a member of the high-mobility group protein family, was originally characterized as a non-histone, nuclear DNA-binding protein [5, 6]. It has been involved in a variety of biological processes; including, transcription, DNA repair, V (D) J recombination, differentiation, development, and extracellular signaling [7]. It is also found acting as a chromatin-binding factor that bends DNA and promotes access to transcriptional protein assemble specific DNA targets [6, 8]. In addition to its nuclear role, HMGB1 also functions as an extracellular signaling molecule during inflammation, cell differentiation, cell migration, and tumor metastasis [9].
Upregulated expression of HMGB1 has been observed in many tumor types including gastric cancer [10], breast cancer [11], nasopharyngeal carcinoma [12] and SCCHN [13]. And the overexpression of HMGB1 is found related to cancer progression in various processes such as apoptosis [11], angiogenesis [14], inflammatory microenvironment [15], and invasion [16], indicating the significance of HMGB1 as a potential therapeutic target for human malignancies. Until now, there has been little information about HMGB1’s roles in HCC, in its development, invasion and metastasis, particularly in shaping its clinicopathologic characteristics. Thus, in this experiment, the expression of HMGB1 was studied using reverse transcription–polymerase chain reaction (RT–PCR), Western-blot (WB) and immunohistochemistry in the tissue samples collected from the normal liver , HCC specimens and the liver tissue around the tumor (LAT). A correlation analysis was performed to evaluate the relationship between HMGB1 expression and clinicopathological parameters of HCC patients.
Materials and Materials
Patients and Tissue Samples
12 cases fresh normal liver tissues, 34 cases fresh HCC samples and correspond liver tissue just around the tumor (LAT) (>2 cm far from the tumor edge) were obtained from the consecutive patients who underwent curative liver resection, and the patients with hepatic haemangioma or trauma between April 2010 and September 2010 at Department of General Surgery in Xiangya Hospital of Central South University, Changsha, Hunan, China. All the tumors were only single liver neoplasm; no patient had received antitumor treatment before the surgery. Each sample was divided into two portions, one was frozen in liquid nitrogen as soon as possible and preserved at −80°C until use, and the other was fixed in 4% paraform and subsequently embedded in paraffin. A prior informed consent was obtained from the patients for the collection of liver specimens in accordance with the guidelines of Central South University, and the study protocols were approved by the Ethics Committee of Central South University. All specimens were handled and made anonymous according to the ethical and legal standards. Clinical characteristics of HCC patients were given in Table 1
Reverse Transcription–Polymerase Chain Reaction (RT–PCR)
Total RNA was isolated from the frozen samples using Trizol reagent (Invitrogen, Carlsbad, USA). Purity and quantity of the RNA was assessed by ultraviolet spectrophotometry (A260/280 greater than 1.8). Complementary DNA (cDNA) was synthesized from total RNA using ReverTra Ace-α-™ First Strand cDNA Synthesis Kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The primer sequence designed by Prime5.0(ProMab, Changsha, China) which was as follows: forward sequence 5′- gagaggcaaaatgtcatcat −3′, reverse sequence 5′- gggatccttgaacttctttt −3′. The amplified target gene fragment was 250 bp. Relative quantification of target gene expression was performed using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)(ProMab) as an internal control, which primer sequence was as follows: forward sequence 5′-ACCACAGTCCATGCCATCAC-3′, reverse sequence 5′-TCCACCACCCTGTTGCTGTA-3′, and the gene fragment include 450 bp. For the first step, the following components were added to the denatured RNA to obtain the specified concentrations in a final 20 μL reaction volume: total RNA (1 μg/μL) 1 μL, 5 × RT buffer 4 μL, RNase free H2O 10 ul, dNTP (10 mmol/L) 2 ul, RNase inhibitor (10 u/μL) 1 μL, Oligo (dT) (10 pmol/μL) 1Μl, ReverTra Ace 1 μL. The reaction was performed at 25°C for 5 min, 42°C for 60 min, 70°C for 5 min, and 4°C for 5 min. In the second step, these productions were mixed with Master Mix (2×) 12.5 ul, Water nuclease-free 10.5 ul, Primer 1 ul, cDNA 1 ul. Subsequently, the Polymerase chain reaction was performed as follows: pre-denaturation at 94°C for 3 min; denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and elongation at 72°C for 45 s, total of 35 cycles, finally elongation at 72°C for 5 min. In order to keep the consistency of result, the internal control gene was amplification at the same time, and its annealing temperature was also 58°C. The PCR products were separated by electrophoresis using 1.5% agarose gels (sample volume: 10 μL, voltage: 100 V) and visualized by ethidium bromide staining for 10 min and ultraviolet illumination (Kodak, New Haven, USA). To confirm the expression of HMGB1, with GAPDH used for the internal control, 12 cases of normal liver tissues, 34 cases of HCC and corresponding liver tissue around the tumor (LAT) were used for RT–PCR. We used the gel optical density analysis software (Gel-Pro 4.0) to scan and calculate integral optical density (IOD) of strips. The relative mRNA expressions of HMGB1 were represented as ratio of HMGB1-IOD and GAPDH-IOD.
Western-Blot (WB)
In order to further study the expression of HMGB1, Western-blot was performed for analysis the protein level of HMGB1 with GAPDH used again for the internal control. 0.5 g of frozen tissue was kept on ice and ultrasonicated in the presence of 1 ml tissue lysate obtained from Total Protein Extraction Kit (ProMab). Then the crude protein was obtained by centrifugation of the homogenates in a centrifuge (Sigma, St Louis, USA) at 9000 × g for 10 min, and took the supernatant for use as protein fraction. Gel samples were prepared by mixing protein samples with gel sample buffer and boiled at 100°C for 3 min. Nuclear or cytoplasmic proteins were electrophoresed through 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in a running buffer (Bio-Rad, Hercules, USA). After SDS-PAGE, the proteins were transferred from electrophoresis to nitrocellulose membrane (Pierce, Rockford, USA). Non-specific binding sites were blocked by incubation in PBS containing 5% dried skim milk for 2 h at 37°C then incubated with the primary antibodies [Rabbit HMGB1 antibody (1:300) (Abcam, Cambridge, USA) and mouse GAPDH antibody (1:1000)(ProMab)] at room temperature for 2 h. Then the membranes were washed four times with PBST and incubated with the secondary antibody [Goat Anti Rabbit IgG/HRP (1:30000) (ProMab) and Goat Anti mouse IgG/HRP (1:50000) (ProMab)] for 1 h at room temperature. After the membranes were washed four times in PBST, enhanced chemiluminescence detection of the target protein was performed using the ECL plus Western blotting detection system (Pierce). Densitometry was performed using Kodak Molecular Imaging software. The relative protein levels of HMGB1 were represented as the density ratio vs. GAPDH (HMGB1/ GAPDH).
Immunohistochemistry
The paraffin-embedded tissues were cut at 3 μm, IHC staining was performed with a Dako EnVisionTM kit (Dakocytomation, Copenhagen, Denmark). The sections were dewaxed, incubated with methanol containing 30% H2O2 for 20 min to block endogenous peroxidase activity, immersed in 0.01 mol/L citrate buffer (pH 6.0), heated at 100°C in a microwave oven for 20 min, washed three times with distilled water and blocked with 1% bovine serum albumin (BSA) for 30 min. The sections were then incubated overnight at 4°C with Rabbit HMGB1 antibody (Abcam) (1:250 dilution). A subsequent reaction was carried out using second antibodies (1:2000 dilution) at 37°C for 30 min. Then, the sections were washed three times with phosphate buffer solution (PBS) and subsequently the color was displayed with DAB for about 5 min. Nuclei were lightly counterstained with hematoxylin. Negative controls included incubation with PBS without the primary antibody.
Statistical Analysis
The software of SPSS version 13.0 for Windows (SPSS Inc, Chicago, US) was used for the statistical analysis. Data were expressed as means ± SD. Comparisons HMGB1 between groups were made using One-way ANOVA and paired t-test when data were normally distributed. Correlation analysis was determined using the method of Pearson. P < 0.05 was considered statistically significant.
Results
Analysis of HMGB1 mRNA (RT–PCR) and HMGB1 Protein (WB)
The express of HMGB1 mRNA was detected in all the groups (Fig. 1. Left). The relative mRNA levels of the HMGB1 gene (Fig. 1. Left. b) were the highest in HCC with 0.854 ± 0.172, and the lowest, 0.405 ± 0.087, was in the normal liver tissue. The level in LAT was 0.527 ± 0.155, in the middle. The differences were significant (P < 0.001, VS normal and LAT). Western-blot analysis for HMGB1 protein showed a great match with the RT–PCR Findings (Fig. 1. Right); the same pattern was found in the expression of HMGB1 protein. The HCC group was seen with the highest protein level (0.294 ± 0.103) while the normal liver tissue showed the least HMGB1 protein expression (0.083 ± 0.052). LAT group was, again, in-between (0.134 ± 0.043). The difference was also significant (P < 0.001, VS normal and LAT). Correlation analysis between HMGB1 mRNA and protein indicated that the expression of HMGB1 mRNA had a positive correlation with HMGB1 protein (Person correlation analysis, R = 0.813, P < 0.01).
The profiles of RT–PCR and Western blot analysis. (Left. a) representative images of electrophoresis of mRNAs for HMGB1 250 bp in all the groups, GAPDH 450 bp fragment served as the internal control. (Left. b) Relative quantity of HMGB1 mRNA in all the groups (Means ± SD of IOD). (Right. a) representative images of electrophoresis of protein for HMGB1 25 KD in the groups with GAPDH 37 KD fragment used as the internal control. (Right. b) Relative quantity of HMGB1 protein in the groups (Means ± SD of IOD). Comparison of the IOD of HMGB1 mRNA and HMGB1 protein in HCC group, * vs. normal control and LAT group, P < 0.001
Correlations Between HMGB1 mRNA Expression Levels and Clinicopathologic Parameters
The association between HMGB1 mRNA expression and clinicopathologic characteristics of HCC was analyzed by the one-way ANOVA. As summarized in Table 2, HMGB1 overexpression was significantly associated with Edmondson stage, TNM stage, vascular invasion and capsule invasion. However, there were no significant relationships between HMGB1 mRNA expression and the other variables such as gender, age, tumor size, AFP.
Immunohistochemistry
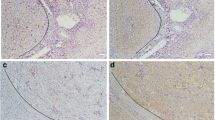
All the samples (12 cases of normal liver tissue, 34 cases of HCC and LAT) were analyzed with IHC. While there was almost no HMGB1 protein positive staining in normal liver tissue, a strong positive staining was observed in all HCC samples, predominantly in the nuclei of the carcinoma. A mild HMGB1 positive staining was presented in LAT, immunostaining was predominantly observed in cytoplasm (Fig. 2.)
Representative immunohistochemical staining for HMGB1 in normal liver tissues, HCC and LAT. Negative expression of HMGB1 in normal liver tissue (a, d). Positive expression of HMGB1 in HCC; HMGB1 immunostaining was predominantly observed in the nuclei of the carcinoma (b, e). Mild expression of HMGB1 in LAT; immunostaining was predominantly observed in cytoplasm (c, f). (Original magnification A–C, ×200; D–F, ×400. Counterstained with hematoxylin)
Discussion
HMGB1 plays dual roles as both a nuclear protein and an extracellular signaling molecule. Within the nucleus, HMGB1 binds to the minor groove of DNA, stabilizing nucleosides and facilitating the assembly of site-specific DNA-binding proteins; such as nuclear hormone/nuclear hormone receptor complexes and p53 or p73 transcriptional complexes [11, 19]. When HMGB1 releases into the extracellular environment, it binds to some specific receptors, activating a certain signaling pathway, such as MAPK and NF-κB which mediates tumor cells growth, invasion and migration [20, 21]. Nevertheless, HMGB1’s roles in HCC have yet to be thoroughly explored.
In this experiment, we have investigated HMGB1 mRNA and protein expression in human normal liver tissue, HCC and LAT. While the PT-PCR and WB analysis revealed both HMGB1mRNA and protein expressed in all the groups, HCC was with the highest upregulated expression. This suggested that HMGB1 not only regulated normal hepatic cell growth and development, but also possibly played a role in the development of HCC. And that fell in line with others observation on HMGB1’s role in other types of carcinoma; for instance, gastric cancer [10], breast cancer [11], nasopharyngeal carcinoma [12] and SCCHN [13]. Moreover, immunochemistry on the tissues not only confirmed the similar pattern of HMGB1 mRNA and protein expression, it also revealed that the positive immunostaining of HMGB1 protein was predominantly localized in the nuclei of HCC. Some studies have showed HMGB1 is actively shuttled between the nucleus and the cytoplasm, and is sometimes concentrated in cytoplasmic vesicles, such as secretory lysosomes in monocytic cells and macrophages [22, 23]. In this study whether or not HMGB1 was localized in secretory lysosomes was unknown. We speculate that HCC cells transport HMGB1 protein from the nucleus to the extracellular matrix, thus improving tumor cell survival and invasion.. In addition, with correlation analysis, the upregulated expression of HMGB1 mRNA in HCC was found significantly associated with some core clinicopathologic parameters in the patients with HCC. It was positively correlated with Edmondson stage, TNM stage, vascular invasion and capsule invasion, but not with gender, age, tumor size, AFP, HBV infection, cirrhosis or capsule formation. This suggests that the overexpression of HMGB1 mRNA was mainly associated with the invasion and metastasis of HCC; adding more backing evidence for the possibility of HMGB1 mRNA being used as a diagnostic and prognostic marker. Based on their study of the association between serum HMGB1 and HCC, Cheng et al. [24] has reported that HMGB1 may serve as a useful tumor marker and may predict the prognosis of HCC.
In conclusion, in this study, we have demonstrated the overexpression of HMGB1 in the cells of HCC. This HMGB1 overexpression was positively correlated with some core clinicopathologic parameters of the patients with HCC, suggesting that HMGB1 may play an important pathogenetic role in HCC. However, further study on the molecular mechanisms of HMGB1 in HCC genesis and its possible diagnostic and prognostic roles will be required, which may eventually lead to the development of new approaches for effective HCC therapy.
Abbreviations
- HMGB1:
-
High-mobility group box 1
- HCC:
-
Hepatocellular carcinoma
- LAT:
-
Liver tissue just around the tumor
- RT–PCR:
-
Reverse transcription–polymerase chain reaction
- WB:
-
Western-blot
References
Srivatanakul P, Sriplung H, Deerasamee S (2004) Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev 5:118–125
Yao DF, Dong ZZ, Yao M (2007) Specific molecular markers in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 6:241–247
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576
Bosch FX, Ribes J, Cleries R et al (2005) Epidemiology of hepatocellular carcinoma. Clin Liver Dis 9:191–211
Javaherian K, Liu JF, Wang JC (1978) Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 199:1345–1346
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342
Czura CJ, Wang H, Tracey KJ (2001) Dual roles for HMGB1: DNA binding and cytokine. J Endotoxin Res 7:315–321
Muller S, Scaffidi P, Degryse B et al (2001) New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J 20:4337–4340
Kostova N, Zlateva S, Ugrinova I et al (2010) The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem 337:251–258
Akaike H, Kono K, Sugai H et al (2007) Expression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancer. Anticancer Res 27:449–457
Brezniceanu ML, Völp K, Bösser S et al (2003) HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J 17:1295–1297
Wu D, Ding Y, Wang S et al (2008) Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol 216:167–175
Liu Y, Xie C, Zhang X et al (2010) Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer 46:3007–3015
Schlueter C, Weber H, Meyer B et al (2005) Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol 166:1259–1263
Dong Xda E, Ito N, Lotze MT et al (2007) High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother 30:596–606
Kuniyasu H, Oue N, Wakikawa A et al (2002) Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol 196:163–170
Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7:462–503
Greene FL, Page DL, Fleming ID et al (2002) AJCC cancer staging manual, 6th edn. Springer, New York
Thomas JO, Travers AA (2001) HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci 26:167–174
Ghavami S, Rashedi I, Dattilo BM et al (2008) S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol 83:1484–1492
Palumbo R, Galvez BG, Pusterla T et al (2007) Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol 179:33–40
Gardella S, Andrei C, Ferrera D et al (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3:995–1001
Bonaldi T, Talamo F, Scaffidi P et al (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22:5551–5560
Cheng BQ, Jia CQ, Liu CT et al (2008) Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis 40:446–452
Acknowledgements
The authors thank the patients who participated in this study, and all the staffs who assisted with the sample collection and pre-treatment in operation room.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, W., Wang, Z., Li, X. et al. High-mobility Group Box 1 is Associated with Clinicopathologic Features in Patients with Hepatocellular Carcinoma. Pathol. Oncol. Res. 18, 293–298 (2012). https://doi.org/10.1007/s12253-011-9442-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-011-9442-3