Abstract
Goblet cell carcinoid (GCC) of the appendix is currently classified as a neuroendocrine tumor, together with typical carcinoid (TC) of the appendix. However, whether GCC is a variant of TC or a mucin-producing adenocarcinoma is still controversial. To get a better understanding, we investigated the clinicopathological features of 55 Chinese patients (26 GCCs and 29 TCs), and explored the histochemical properties and expression profiles of CK7, CK20, P63, CEA, CgA, NSE, CD56, and Ki67 in 37 out of these 55 patients (18 GCCs and 19 TCs). Our results showed GCC had a male predominance, older age involvement, significantly larger tumor size, and significantly more frequency of mesoappendix infiltration than TC. Alcian blue/PAS stains were positive in all the GCC cases, while negative in all the TC cases. CK7 and CK20 expressions were significantly more frequent in GCC (P = 0.03 and 0.00001, respectively). However, P63 expression was detected in none of the GCC cases but in 6 TC cases (P = 0.02). Although the expression of CgA was similar, strong expression (3+) was significantly more frequent in TC (P = 5.7 × 10−11). Also, NSE and CD56 expressions were significantly more frequent in TC (P = 0.02 and 1.26 × 10−4, respectively). CEA expression was significantly more frequent in GCC (P = 2.4 × 10−6). Finally, Ki67 index was low in GCC (4.7%), but significantly higher than TC (0.9%) (P = 5.4 × 10−6). Taken together, these distinct features support that GCC differs from TC, a classical neuroendocrine tumor, and harbors an immunophenotype of adenocarcinoma. Therefore, the term “low grade adenocarcinoma with neuroendocrine differentiation” might be more appropriate for GCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the WHO classifications of neuroendocrine tumors of the appendix, [1] carcinoid tumors of the appendix are divided into 4 subtypes: carcinoid (typical carcinoid, TC); tubular carcinoid; mixed carcinoid-adenocarcinoma; goblet cell carcinoid (GCC). Among them, GCC merits special attention since it is characterized by a combined phenotype of both endocrine and glandular differentiation, leading to confusion of its nomenclature and clinical treatment. It was once considered to be similar to TC. Moreover, it has been designated as adenocarcinoid, mucinous carcinoid, amphicrine (endo-exocrine) neoplasm, and microglandular carcinoma [2]. Several studies indicated that it might be a more aggressive variant of TC, [3–6] whereas others considered it to be a low-grade adenocarcinoma with neuroendocrine differentiation, most likely to arise from crypt cells [7, 8]. But conventional genetic abnormalities of colorectal adenocarcinoma, e.g., mutations of K-ras, p53, and DPC-4, were not encountered in GCC [9, 10].
To date, we are unaware of any reports regarding the expression profiles of these proteins in Chinese patients in English literature. This study was therefore designed to investigate the clinicopathological features in 55 Chinese patients (26 GCCs and 29 TCs), and to explore the histochemical properties and the expression profiles of CK7, CK20, P63, CEA, CgA, NSE, CD56 and Ki67 in 37 of these 55 patients (18 GCCs and 19 TCs) in order to get a better understanding of GCC.
Materials and Methods
Twenty-six GCCs and twenty-nine TCs of the appendix were collected from the archives of Department of Pathology, West China Hospital of Sichuan University as well as several multiple cooperative hospitals in Sichuan province, China. Clinical data includes age, gender, clinical manifestation, tumor size, and tumor invasion. Clinical follow-up data were available in 18 GCC and 15 TC patients, from 1991 to 2009.
The diagnoses of these tumors were established by histological analysis in conjunction with the finding of immunoreactivity for endocrine markers according to the criteria of the WHO Classification of Tumors. Consensus diagnosis was made by two experienced pathologists. Formalin-fixed, paraffin-embedded tissue sections were cut at 4 um and deparaffinized. Histochemistry includes alcian blue/PAS stains that can identify mucin. Cells staining blue were identified as alcian blue positive and pink as PAS positive. Immunostaining was performed with a broad panel of antibodies (Table 1). The staining was performed manually by using a streptavidin-biotin complex method following relevant antigen retrieval techniques according to standard protocols. (Table 1) Appropriate tissues were used as positive and negative controls. For all the markers except Ki67, the positive tumor cells were semiquantitatively graded and therefore divided into four groups: 0 (negative), <10% (1+), 10–50% (2+), and >50% (3+). Proliferative index was defined as the average percentage of cells with Ki-67 positive expression to tumor cells by counting 100 tumor cells in each of 10 randomly consecutive high-power fields (400×). Positive is defined as the presence of yellow-brown stained cytoplasm for CK7, CK20, CgA, NSE and CEA, yellow-brown stained cell membrane for CD56, and yellow-brown stained nuclei for Ki67 and P63.
Overall survival was determined from the time of the initial diagnosis to the time of death or date of last follow-up. Surviving patients were censored at the date of last contact. Statistical analysis was carried out using SPSS Version 16.0. The Pearson chi-square test or Fisher’s exact test and Student’s t test were used to analyze the data and a P value less than 0.05 was considered statistical significance.
Results
Most of the patients manifested signs and symptoms of right lower quadrant pain or vague abdominal pain, followed by lower abdominal mass. These tumors were frequently incidental findings associated with acute appendicitis. In addition, in six patients of TC, tumors were identified unexpectedly during an operation for other diseases, e.g., hysteromyoma, ovarian mucinous cystadenoma.
Goblet Cell Carcinoid
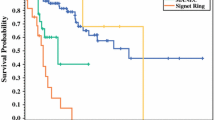
The clinicopathological features of the GCC cases were summarized in Table 2. There were 19 males and 7 females (a male/female ratio = 2.71/1). Ages ranged from 31 to 70 (54.6 ± 11.6), with a median age of 58. The favored site was the apex (54%) of the appendix. The diameter of these tumors ranged from 5 to 60 mm (16.0 ± 13.3). All tumors except in two patients extended beyond muscularis propria, infiltrating either serosa or mesoappendiceal fat. The detailed follow-up data were available for 18 patients with a mean follow-up time of 55.9 months (range, 33–102 months).
Under light microscope, for all the GCCs, tumors diffusely infiltrated submucosa and muscularis propria of the appendiceal walls, with intact overlying mucosa. They all consisted of tight solid nests or tubules of tumor cells with peripheral nuclei, but occasionally had small lumina. Most of the neoplastic cells took on a goblet cell or signet-ring-like morphology with a small compressed nucleus and abundant intracytoplasmic mucin. (Figure 1a) All of the tumor cells presented mild to intermediate atypia, but confluent tumor necrosis could not be identified. Mitotic figures ranged from 0 to 2 per 10 high power field (HPF). Paneth cells and mucin lakes were found in 2 and 5 cases, respectively. Within mucin lakes were found central lumina that resembled normal crypts.
a Tumor cells with peripheral nuclei consisted of tight solid nests (H&E stain, magnification × 400). Note that most of the neoplastic cells took on goblet morphology with abundant intracytoplasmic mucin. b GCC stained positive for alcian blue/PAS (magnification × 400). c, GCC showed mild or intermediate expression of CK7 (SP × 450). d CK20 was intermediately or strongly expressed in GCC (SP × 450)
Histochemistry was available in 18 GCC cases. All the cases were positive for alcian blue/PAS (Fig. 1b).
Immunophenotypic analyses were available in the same 18 cases of the GCC patients as histochemistry. (see Table 3). Eight GCC cases (43%) expressed CK7 and all the 18 GCCs consistently demonstrated intermediate to strong positivity for CK20 staining. (Figure 1c & d) However, none of them was positive for P63. The expression of CgA was weakly or intermediately positive in most of the GCCs (16/18) (Fig. 2a), only 2 out of 18 cases were negative for CgA. Moreover, 13 and 8 out of the 18 cases were positive for NSE and CD56, respectively. In those two CgA negative cases, both NSE and CD56 expressions were positive. Conversely, all the cases strongly expressed the epithelial marker, CEA. (Figure 2c) Immunostaining for the proliferative marker Ki67 showed immunoreactivity ranging from 1.7% to 12.7% (mean, 4.7%) (Fig. 2d).
Typical Carcinoid
Among the 29 TC cases, nine were males and twenty were females, with a male/female ratio of 0.45/1 (see Table 2). Their ages (35.7 ± 15.2) ranged from 11 to 68, with a median age of 32. Moreover, the diameter of these tumors (6.8 ± 3.4) ranged from 2 to 14 mm. Among the 29 patients, seven were affected by mesentery fat invasion, 9 by serosa invasion, 3 by musularis propria invasion, and the rest 10 by invasion localized in submucosa. Clinical and follow-up data were obtained from 15 cases, with a mean survival period of 72.9 months (range, 33–146 months).
Under light microscope, TC showed their classical morphology. Small polyhedral neoplastic cells arranged in a nest-like or rosette-like morphology, with stippled round or oval nuclei. Not infrequently, tumor cells formed adenoid tubules.
Nineteen of the 29 TC cases available for histochemistry were negative for alcian blue/PAS.
Among the 29 cases of TC, the same 19 cases as for histochemistry were available for immunohistochemical staining (see Table 3). All the 19 cases displayed strong positivity for CgA (Fig. 2b) and intermediate to strong expressions of NSE and CD56. CK 20 and CK7 expressed in 6 and 2 cases, respectively. In addition, six cases were positive for P63, presenting a nuclear staining pattern. Only 5 cases showed weak to intermediate staining for CEA. The proliferative index ranged from 0.4% to 2.4% (mean, 0.9%).
Comparison between GCC and TC
GCC has a significant male predominance compared to TC (P = 0.002), with male/female ratios of 19/7 and 9/20, respectively. Meanwhile, there were significant differences between GCC and TC regarding age involvement (P = 3.24 × 10−6), with the former being older. With regard to tumor size, GCC is significantly larger than TC (P = 0.002).Moreover, GCC is significantly more frequent to infiltrate the mesoappendix than TC (P = 0.024).
GCC showed significantly higher expressions of CK7 and CK20 than TC (P = 0.03 and 1.0 × 10−5, respectively). In contrast, P63 expression was significantly more prevalent in TC than GCC (P = 0.02). Although two carcinoids were similar with regard to CgA positivity, strong expression (3+) of CgA favors TC (P = 5.7 × 10−11). NSE and CD56 expressions were both significantly less frequent in GCC than in TC (P = 0.02 and 1.26 × 10−4, respectively). Furthermore, there was significantly higher frequency of CEA expression in GCC (P = 2.4 × 10−6). Finally, GCC (4.74 ± 2.53%) showed a significantly higher Ki67 index compared to TC (0.91 ± 0.60%) (P = 5.36 × 10−6).
Discussion
Appendix is a predilection site for neuroendocrine tumors, harboring approximately 19% of all the carcinoids. On the other hand, carcinoids are the most common tumors in the appendix, accounting for 50–77% of all the neoplasms in the appendix [1]. GCC, coined by Subbuswamy et al. [11] in 1974, is a rare tumor of the appendix. It displays a dual-differentiation between adenocarcinoma and carcinoid. It is still controversial whether GCC should be categorized as a low-grade adenocarcinoma or a subgroup of the neuroendocrine tumors, as is reflected by its differing designations and clinical interventions. Therefore, studies differed pertaining to its pathogenesis, biological behavior, and clinical management.
In our study, GCC showed a significant male predominance and older age involvement, compared to TC. Moreover, GCC showed a more aggressive behavior than TC, as reflected by their significantly larger tumor sizes and significantly more frequency of mesoappendix infiltration. These different clinicopathological features suggest GCC and TC are two different entities.
Our results showed that alcian blue and PAS stains were positive in all the GCC cases,while negative in all the TC cases, indicating that alcian blue/PAS stains could be used to differentiate GCC from TC. Moreover, given that ordinary adenocarcinoma was negative for alcian blue/PAS, identification of mucin by alcian blue/PAS would help to differentiate GCC from ordinary adenocarcinoma.
Immunohistochemical staining of CK7 and CK20 has been utilized to help determine the epithelial nature of some undefined tumor or the primary lesion of a metastatic malignancy. For example, a strong expression of CK7 favors a primary tumor of ovary or lung, and CK20 positivity implies a primary colorectal adenocarcinoma. Our results showed CK7 and CK20 expressions were significantly higher in GCC. Moreover, there are 60 GCCs and 58 TCs if we combine our present study with several seminal studies [7, 8, 12] on expressions of CK7 and CK20 in GCC. Among them, CK20 stained positive in all the GCC cases (60/60, 100%) and in only a fraction of TC cases (10/58, 17.2%). The consistent CK20 expression in GCCs implies that GCC shares some common immunophenotypic features with conventional colon adenocarcinoma. CK7 expressed in over half of the GCCs (33/60, 55%) but in only two cases of TC (2/58, 3.4%), which is consistent with our study. Moreover, over half of the GCC cases showed co-expressions of CK7 and CK20, a phenomenon observed in primary colorectal adenocarcinoma, but not in TCs. Therefore, the expression profiles of CK7 and CK20 suggest that GCC is more likely to be histogenetically related to colorectal adenocarcinoma.
In addition to CK7 and CK20, we explored the expressions of several well-known neuroendocrine markers, CgA, NSE and CD56. Although GCC was similar to TC regarding CgA positivity, strong CgA expression favored TC, with weak expression in over 70% GCC cases and strong in all the TC cases. Moreover, NSE and CD56 expressions were both significantly more frequent in TC. Therefore, based on the distinct expressions of these neuroendocrine markers, we suggest that GCC harbors less neuroendocrine component than TC.
Yet, there were still two GCC cases that were negative for CgA. In these two CgA negative cases, other neuroendocrine markers, NSE and CD56, justified their neuroendocrine differentiation and to differentiate from mucinous adenocarcinoma. Moreover, alcian blue/PAS stains helped to identify mucin, thereby differentiating GCC from ordinary adenocarcinoma. Thus, in clinical practice, especially for CgA negative cases, we suggest alcian blue/PAS and other neuroendocrine markers, e.g., NSE and CD56, be performed additionally to confirm the diagnosis of GCC.
Compared to TC, GCC had a significantly more frequency of CEA (an epithelial marker) expression, suggesting the epithelium-derived nature of GCC. In details, all the GCC cases were strongly positive for CEA, which was in agreement with prior studies [8, 13]. Whereas, only 5 TC cases showed mild to moderate CEA positivity.
Although P63 staining has been used in the diagnosis of various tumors, only a few studies have investigated its expression pattern in neuroendocrine tumors [14, 15]. It was reported that P63 stained positive in TC, while consistently negative in colorectal adenocarcinoma [15]. In our study, 6 of 19 TC cases expressed P63 protein, implying some potential roles of P63 in TC. While P63 stained negative in all the GCC cases, which is consistent with colorectal adenocarcinoma.
Proliferative index correlates with mitotic activity and has been utilized as a marker for proliferation. The prognostic value of Ki-67 has been already established in pancreatic and gastric neuroendocrine tumors and its high index suggests an aggressive behavior [8, 16]. Although a Ki-67 greater than 3% was reported in high-grade GCC, [17] no definite evidence could be found regarding its prognostic roles in GCC. In our study, the Ki-67 index is significantly higher in GCC than that in TC, suggesting a more aggressive behavior of GCC, which is in accord with the findings mentioned above that GCC had significantly larger tumor size and significantly more frequency of mesoappendix involvement.
Finally, GCC presented a male predominance (a male/female ratio of 2.7/1) in our study. However, McCusker et al. [18] conducted a review of 227 diagnosed GCC patients and found GCC occurred with equal proportion with regard to genders (1.08/1). Since it has been demonstrated that the site distribution of colorectal carcinoids differs apparently among different human races, [19] we therefore assume that male is more susceptible to appendiceal GCC among Chinese.
In summary, we investigated the clinicopathological characteristics and explored the expression patterns of CK7, CK20, P63, CEA, CgA, NSE, CD56 and Ki67 in the appendiceal GCCs and TCs among Chinese patients. The clinicopathological features differed regarding gender ratio, age involvement, tumor size, and tumor invasion.Furthermore, the mucin histochemical stains and the immunohistochemical features supported the notion that GCC differs from TC, a classical neuroendocrine tumor, and harbors an immunophenotype of adenocarcinoma. Therefore, based on our present study, GCC should not be regarded as a neuroendocrine tumor. The term “low grade adenocarcinoma with neuroendocrine differentiation” might be more appropriate to present its clinicopathological features and biological behavior. However, further studies with larger sample size offering more accurate understanding of GCC are urgently needed.
Abbreviations
- GCC:
-
Goblet Cell Carcinoid
- TC:
-
Typical Carcinoid
- HPF:
-
High Power Field
References
Capella C, Solcia E, Sobin LH, Arnold R (2000) Endocrine tumors of the appendix. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the digestive system. In: Hamilton SR, Aaltonen LA (ed) IARC Press, Lyon, pp 99–101
Pahlavan PS, Kanthan R (2005) Goblet cell carcinoid of the appendix. World J Surg Oncol 3:36
Kanthan R, Saxena A, Kanthan SC (2001) Goblet cell carcinoids of the appendix: immunophenotype and ultrastructural study. Arch Pathol Lab Med 125(3):386–90
Park K, Blessing K, Kerr K, Chetty U, Gilmour H (1990) Goblet cell carcinoid of the appendix. Gut 31:322–324
Deans GT, Spence RA (1995) Neoplastic lesions of the appendix. Br J Surg 82:299–306
Butler TA, Houshiar A, Lin F, Wilson SE (1994) Goblet cell carcinoid of the appendix. Am J Surg 168:685–687
Alsaad KO, Serra S, Schmitt A, Perren A, Chetty R (2007) Cytokeratins 7 and 20 immunoexpression profile in goblet cell and classical carcinoids of appendix. Endocr Pathol 18(1):16–22
van Eeden S, Offerhaus GJ, Hart AA et al (2007) Goblet cell carcinoid of the appendix: a specific type of carcinoma. Histopathology 51(6):763–73
Ramnani DM, Wistuba II, Behrens C et al (1999) K-ras and p53 mutations in the pathogenesis of classical and goblet cell carcinoids of the appendix. Cancer 86(1):14–21
Stancu M, Wu TT, Wallace C et al (2003) Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol 16(12):1189–98
Subbuswamy SG, Gibbs NM, Ross CF, Morson BC (1974) Goblet cell carcinoid of the appendix. Cancer 34(2):338–44
Kende AI, Carr NJ, Sobin LH (2003) Expression of cytokeratins 7 and 20 in carcinomas of the gastrointestinal tract. Histopathology 42(2):137–40
Toumpanakis C, Standish RA, Baishnab E, Winslet MC, Caplin ME (2007) Goblet cell carcinoid tumors (adenocarcinoid) of the appendix. Dis Colon Rectum 50(3):315–22
Wang BY, Gil J, Kaufman D et al (2002) P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol 33(9):921–6
Owens SR, Greenson JK (2007) Immunohistochemical staining for p63 is useful in the diagnosis of anal squamous cell carcinomas. Am J Surg Pathol 31(2):285–90
Sokmensuer C, Gedikoglu G, Uzunalimoglu B (2001) Importance of proliferation markers in gastrointestinal carcinoid tumors: a clinicopathologic study. Hepatogastroenterology 48(39):720–3
Li CC, Hirowaka M, Qian ZR, Xu B, Sano T (2002) Expression of E-cadherin, b-catenin, and Ki-67 in goblet cell carcinoids of the appendix: an immunohistochemical study with clinical correlation. Endocr Pathol 13(1):47–58
McCusker ME, Cote TR, Clegg LX, Sobin LH (2002) Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 94(12):3307–12
Konishi T, Watanabe T, Muto T, Kotake K, Nagawa H (2006) Site distribution of gastrointestinal carcinoids differs between races. Gut 55(7):1051–2
Acknowledgement
We thank Kaixuan Yang, Ping Hua, Zhirong Yang, and Ling Wu for their providing the specimens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y., Long, H., Wang, W. et al. Clinicopathological Features and Immunoexpression Profiles of Goblet Cell Carcinoid and Typical Carcinoid of the Appendix. Pathol. Oncol. Res. 17, 127–132 (2011). https://doi.org/10.1007/s12253-010-9291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-010-9291-5