Abstract
Regulatory T cells (Tregs) have been implicated as inhibitors of antitumor immune reactions. However, data on the relevance of their prevalence at tumor sites in influencing disease outcome are controversial. The aim of our study was to investigate the role in tumor progression and the prognostic impact of the density of lymphocytes expressing FOXP3, a transcription factor expressed predominantly by CD4+CD25+ Tregs, in primary cutaneous melanoma. We examined the infiltration of FOXP3+ cells by immunohistochemistry in tumor samples from 97 patients and evaluated in relation to patient and tumor parameters. The degree of infiltration by FOXP3+ cells did not show correlation with the thickness of melanomas. Moreover, no associations were found with metastasis formation during the 5-year follow-up period, patient survival, or any other clinicopathologic parameters studied. These results suggest that the presence of FOXP3+ lymphocytes in primary tumors is not of prognostic importance in human cutaneous melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human solid tumors are often infiltrated by CD4+ and CD8+ lymphocytes, and numerous tumor-associated antigens have been discovered that can stimulate these cells after presentation by antigen-presenting cells in the context of the appropriate MHC molecules, inducing tumor-specific cytokine production or tumor cell lysis [1]. However, tumor progression is frequently seen in the presence of substantial lymphocytic infiltration, suggesting that this immune response is apparently incapable of controlling tumor growth. Escape mechanisms that might explain the lack of effectiveness of antitumor immune reactions include down-regulation of tumor-associated antigens (TAA) or MHC class I molecules, insufficient presentation of antigenic peptides, lack of costimulation, or inhibition of tumor-reactive T cells by immune suppressive factors or cells (reviewed in ref. [2]).
Numerous suppressor cell types have been identified that could contribute to the decrease in antitumor immune functions, including, among others, regulatory T cells, myeloid-derived suppressor cells and plasmacytoid dendritic cells [3]. The best characterized cell type is CD4+CD25+FOXP3+ regulatory T (Treg) cells, which comprise a functionally unique population of T lymphocytes controlling proliferation and function of other CD4+ as well as of CD8+ T cells, thus mediating peripheral tolerance. Natural Tregs arise in the thymus, but adaptive Tregs may also develop by conversion of CD4+CD25−FOXP3− cells at the periphery. Regulatory T cells are crucial in preventing autoimmune responses, and are implicated in a broad spectrum of autoimmune diseases, as well as in allograft rejection, graft-versus-host disease and allergy.
The role of Treg cells in cancer has recently been the focus of intense investigation. These cells have been found in increased number in the peripheral blood of cancer patients compared to healthy controls, and their accumulation in the tumor tissue has been demonstrated in the case of many cancer types (reviewed in ref. [4]).
Although a high number of functional regulatory T cells in the tumor microenvironment may be indicative of a local suppression of T cell immunity, the pathophysiological importance of Treg accumulation in human malignancies has remained questionable. Reports on the prognostic role of tumor-infiltrating Tregs are inconclusive; while in some cancer types their increased level has been correlated with poor outcome, as in the case of ovarian, breast, pancreatic and hepatocellular carcinomas [5–9], in others including prostate and renal cancers no significant associations were found [10, 11], and in the case of head and neck carcinomas, colorectal cancer, as well as in several lymphoma types a marked infiltration by Tregs showed correlation with improved survival [12–17].
Few studies have addressed the presence of regulatory T lymphocytes and its potential clinical correlates in melanoma, most of them comprising data on a limited number of patients and yielding controversial results. Mourmouras et al characterized cutaneous melanocytic lesions including melanomas with respect to the prevalence of Tregs, and found a lower percentage of Tregs in vertical than in radial growth phase melanomas [18]. Contrary to this finding, another small-scale study described higher prevalence of these cells in VGP compared to RGP, and also in stage III–IV tumors compared to stage I ones [19]. Miracco et al, in a study performed on vertical growth phase melanomas, reported that high intra- and peritumoral percentage of Tregs predicted local recurrence [20]. On the other hand, no correlation of the number of FOXP3+ lymphocytes with Breslow index or patient survival was found by Hillen et al [21]. Thus far, no systematic studies were performed correlating the extent of Treg infiltration of primary cutaneous melanoma with several clinicopathologic factors in large patient cohorts, providing conclusive data on the clinical relevance of these cells.
In a previous study examining the prevalence of activated T cells in cutaneous melanomas, we found a decreased peritumoral infiltration by T lymphocytes expressing CD25 or OX40 in tumors developing distant metastases during a follow-up period of 5 years, compared to nonmetastatic or lymph node metastatic ones. Accordingly, high peritumoral densities of CD25+ or OX40+ lymphocytes were associated with longer survival of the patients [22]. Furthermore, we also identified the density of DC-LAMP+ mature DCs, as well as combinations of high peritumoral CD1a+ or DC-LAMP+ cell densities with high numbers of CD25+ or OX40+ lymphocytes as prognostic factors in melanoma patients [23]. This suggests that the presence of activated T cells and antigen presenting DCs at the primary site could be a marker of a functional immune response against melanoma progression and influence the outcome of the disease. In the present study we examined the role of regulatory T cells, another subset of T lymphocytes with a presumed opposing function, in these processes. We investigated the prevalence of lymphocytes expressing the FOXP3 marker by immunohistochemistry in primary tumor samples from 97 patients with cutaneous melanoma, and evaluated with regard to its association with tumor thickness, the development of metastases, patients’ survival, and other clinicopathologic parameters.
Materials and Methods
Tumor Samples
Archival tissue samples were obtained from 97 patients with primary cutaneous melanoma who underwent surgery between 1980 and 2001 at the Institute of Dermato-Venerology, Semmelweis University, and at the National Institute of Oncology, Budapest. Patients were selected in order to obtain a study group involving a higher number of intermediate-thickness or thick (>1.0 mm) melanoma samples than their normal ratio, which have a more uncertain prognosis than thin tumors. The study was approved by the ethics committees of both institutions. Patients did not receive any anticancer treatment prior to surgery. Clinical and pathological characteristics are summarized in Table 1. The tumors were grouped into four thickness categories based on the AJCC staging system [24] (≤1.0, 1.01–2.0, 2.01–4.0, >4.0 mm), and into three categories according to disease progression (nonmetastatic, lymph node metastatic, visceral metastatic). Distribution according to stages [24] was: st. IA, 10; IB, 11; IIA, 17; IIB, 28; IIC, 14; IIIA, 14; IIIB, 3. Surviving patients had follow-up data for at least 5 years; none of the patients died of melanoma-unrelated causes within 5 years. Forty-five patients had no metastases developed during the follow-up period, while eight had metastases confined to regional lymph nodes, which were excised. Fourty-four patients developed distant visceral metastases. Five-year survival of patients in both the nonmetastatic and the lymph node metastatic groups was 100%, while only two patients developing distant visceral metastases survived for more than 5 years (62 and 72 months). Tumors with clinical regression and/or histological signs of extensive regression were not included in the study.
Immunohistochemical Detection of Infiltrating Cells
Three-μm sections cut from formalin-fixed, paraffin-embedded cutaneous melanoma samples were used. Immunohistochemistry was performed as described earlier [22], using monoclonal anti-FOXP3 (236A/E7; Abcam Inc., Cambridge, MA) primary antibody, followed by biotin/streptavidin-peroxidase method (LSAB2 System, HRP; Dako, Glostrup, Denmark) and visualization with 3-amino-9-ethylcarbazole (Vector Laboratories, Inc., Burlingame, CA).
Evaluation of the Immune Reactions
Slides were examined using a graticule of 10 × 10 squares, calibrated as 0.25 mm2 at 200× magnification. Because the distribution of stained cells was heterogeneous, the entire tumor area was analyzed in every case, and density of positive cells/mm2 is given. Although a few larger cells with morphological features of melanoma cells also showed nuclear FOXP3 staining in some instances, the majority of labeled cells had lymphocytic morphology, and only these cells were counted. The number of FOXP3+ lymphocytes was registered separately in intratumoral (infiltrating melanoma cell nests) and peritumoral areas (distributed in the infiltrate along the margin and the base of melanomas). In 3 areas of the highest density of positive cells the ratio of FOXP3+ lymphocytes was also determined as a percentage of the total cell number in the given field, using high magnification (400×). The proportion of patients with significant density of FOXP3+ cells was calculated using cutoff values set up separately for intra- and peritumoral localization as well as for the ratio of positive cells (30 and 220 cells/mm2, and 11%, respectively), based on the median of the given variable in the whole patient group.
Statistical Analysis
Comparisons between cell densities in different tumor groups was made using the Mann-Whitney U test and Kruskal-Wallis test, while χ2 test was used for comparing the proportions of samples with high cell densities. Association between tumor thickness and cell density values was evaluated by the Pearson test. Univariate analysis of survival was performed by the Kaplan-Meier method, and the statistical analysis was carried out by the Mantel-Cox test. All statistics were calculated using the BMDP Statistical Software Pack.
Results
Detection of FOXP3+ Lymphocytes in Melanoma Samples
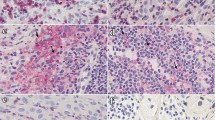
In the melanoma samples, nuclear staining for FOXP3 was detected predominantly in small lymphocytes (Fig. 1). Occasionally, larger cells with morphological features of melanoma cells with nuclear FOXP3 staining were noted. Few positive cells were found in the uninvolved skin or dermal areas.
The density of FOXP3+ lymphocytes was determined separately in intratumoral (inside melanoma cell nests) and peritumoral areas (surrounding the tumor deposits). Lymphocytes stained for FOXP3 were more abundant in the tumor stroma, with a six-fold difference in average compared to their intralesional density (mean±SD, 292.2 ± 211.5 vs. 48.8 ± 63.2 cells/mm2). In three areas of the highest density of positive cells the ratio of FOXP3+ lymphocytes was also calculated as a percentage of the total cell number in the given field, and was estimated as 11.9 ± 5.8% for the whole patient group.
Correlations Between FOXP3+ Cell Density and Clinicopathologic Parameters
The degree of either intra- or peritumoral infiltration by FOXP3+ lymphocytes, or the ratio of positive cells did not show significant correlation with Breslow index (r = −0.1285, r = −0.1193 and r = −0.0048, respectively; p > 0.05). Although intermediate thickness tumors (between 1 and 4 mm) displayed increased intratumoral density (p = 0.0039 by Kruskal-Wallis test), no such differences were found in the case of either peritumoral density or the ratio of positivity (Fig. 2a). Moreover, no difference was found in FOXP3+ cell number between primary tumors according to their metastasis formation during a follow-up period of 5 years (p = 0.8292, p = 0.2337 and p = 0.1803 for intra-, peritumoral density and positivity ratio, respectively; Fig. 2b).
For intra- and peritumoral densities, as well as for the ratio of FOXP3+ cells, we calculated the proportions of samples with values higher than the median of the given variable in the whole patient group (30 and 220 cells/mm2, and 11%, respectively). Analyzing the relationship between the ratio of samples with high FOXP3+ cell numbers and tumor thickness or the metastatic pattern of the tumor, no significant differences were observed, with the exception of a higher frequency of samples with elevated intratumoral density of labeled cells in the groups of intermediate thickness tumors (Table 2). The distribution of melanomas with high FOXP3+ cell densities was also analyzed according to other clinicopathologic factors as patient age and gender, tumor location and the presence or absence of ulceration, with no associations found in the case of any of these parameters (Table 2). Concerning histological type, SSM and NM cases were compared only in the >2.0 mm thickness categories, since only 2 nodular melanomas were included in the thinner tumor groups; in these cases no difference in FOXP3+ cell content was found.
Survival Analysis According to FOXP3+ Cell Density
To evaluate the prognostic impact of the infiltration by FOXP3+ lymphocytes, we performed Kaplan-Meier analysis using the medians as cutoff levels as described above. Neither intra- or peritumoral densities, nor the ratio of positive cells showed any significant association with the survival of the patients (p = 0.4890, p = 0.6245 and p = 0.2711, respectively). The percentage of patients with more than 5 years survival was similar irrespective of the amount of FOXP3+ cells (60% vs. 53%, p = 0.5327, 59% vs. 54%, p = 0.6181 and 51% vs. 62%, p = 0.2773, for high or low intra- and peritumoral cell densities, and high or low positive cell ratios, respectively).
Discussion
In the present report, we determined the density of FOXP3+ lymphocytes in primary tumors of patients with malignant melanoma, and analyzed in relation to clinicopathologic parameters.
FOXP3+ cells were more prevalent in the stromal, lymphocyte-rich areas than in tumor cell nests, similarly to observations on other tumor types [6, 12]. Occasional positive staining of larger cells with morphological features of melanoma cells could also be found, in accordance with observations by Ebert et al [25]. Nevertheless, the overwhelming majority of FOXP3 staining was detected in small lymphocytes. We did not make attempts to characterize the labeled cell type(s) more precisely by double immunohistochemical staining. Although the expression of FOXP3 could be induced in vitro in CD4+CD25− and CD8+ lymphocytes as well [26–29], in previous immunohistochemical studies on melanoma all FOXP3+ cells expressed CD3 and almost all of them CD4 and CD25 as well [18, 19], suggesting that the vast majority of tumor-infiltrating FOXP3+ cells are CD4+CD25+ T lymphocytes.
The use of archival samples in our study did not allow the examination of the functional characteristics of the labeled cells. Since CD25 is expressed on activated T lymphocytes beside Tregs, FOXP3 has been considered the most reliable marker for immunohistochemical determination of the latter cells. It is a transcription factor critical for the development and function of regulatory T cells [30–33], and its expression is generally considered to define Tregs with suppressive capability [31–34]. On the other hand, in recent studies it became clear that the role of human FOXP3 is different from that of its murine counterpart, being a consequence of activation status, and responsible for an anergic phenotype, but not necessarily for a regulatory function of cells that express it [4, 26, 28, 34–36]. Nevertheless, a suppressive capacity of CD4+CD25highFOXP3+ cells isolated from human tumors was demonstrated by Kryczek et al. [37].
In any case, FOXP3-positive lymphocytes infiltrating primary human melanomas do not appear to exert a suppressor activity that would be reflected in the later course of the disease. Although their presence seemed to make a difference in terms of local recurrence of VGP melanomas [20], it did not influence either the development of metastases or survival of melanoma patients according to our study. With regard to the lack of effect on survival, our results corroborate those of an earlier report [21]. To the best of our knowledge, however, ours is the first study that systematically analyzed the associations of Treg infiltration with several clinicopathologic factors in a large patient cohort, in an attempt to provide conclusive data on the clinical relevance of these cells in melanoma. Since the amount of FOXP3+ cells did not show association with any of the studied parameters: patient age or gender, tumor thickness, location, histological type, ulceration and, most importantly, the outcome of the disease, as mentioned above, FOXP3+ cell infiltration in the primary tumor does not seem to have a significant impact on the course of the disease in cutaneous melanoma. Previous studies aiming at the clinical relevance of tumor-infiltrating Tregs have given rise to contradictory results, showing correlation with poor outcome in some cancer types [5–9] but no association or even correlation with improved survival in others [10–17, 21]. It could be suggested that each tumor type behaves differently in this context [4], depending perhaps on other players on the scene of antitumor immune reactions and their complex functional interrelationships with Tregs as well as with tumor cells.
In conclusion, we have shown that the density of FOXP3+ lymphocytes in the primary tumor is not significantly associated with clinicopathologic parameters and has no prognostic value in patients with cutaneous melanoma.
References
Paschen A, Eichmüller S, Schadendorf D (2004) Identification of tumor antigens and T-cell epitopes, and its clinical application. Cancer Immunol Immunother 53:196–203
Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S (2000) Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 74:181–273
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5:263–274
Knutson KL, Disis ML, Salazar LG (2007) CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother 56:271–285
Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Hiraoka N, Onozato K, Kosuge T, Hirohashi S (2006) Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12:5423–5434
Bates GJ, Fox SB, Han C et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Kobayashi N, Hiraoka N, Yamagami W et al (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13:902–911
Gao Q, Qiu S-J, Fan J et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593
Fox SB, Launchbury R, Bates GJ et al (2007) The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2α but not HIF-1α. Prostate 67:623–629
Siddiqui SA, Frigola X, Bonne-Annee S et al (2007) Tumor-infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res 13:2075–2081
Badoual C, Hans S, Rodriguez J et al (2006) Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancer. Clin Cancer Res 12:465–472
Salama P, Phillips M, Grieu F et al (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Álvaro T, Lejeune M, Salvadó MT et al (2005) Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11:1467–1473
Carreras J, Lopez-Guillermo A, Fox BC et al (2006) High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108:2957–2964
Gjerdrum LM, Woetmann A, Odum N et al (2007) FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia 21:2512–2518
Lee NR, Song EK, Jang KY et al (2008) Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma 49:247–256
Mourmouras V, Fimiani M, Rubegni P et al (2007) Evaluation of tumour-infiltrating CD4+CD25+FOXP3+ regulatory T cells in human cutaneous benign and atypical naevi, melanomas and melanoma metastases. Br J Dermatopathol 157:531–539
De Panfilis G, Campanini N, Santini M et al (2008) Phase- and stage-related proportions of T cells bearing the transcription factor FOXP3 infiltrate primary melanoma. J Invest Dermatol 128:676–684
Miracco C, Mourmouras V, Biagioli M et al (2007) Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep 18:1115–1122
Hillen F, Baeten CIM, van de Winkel A et al (2008) Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother 57:97–106
Ladányi A, Somlai B, Gilde K et al (2004) T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res 10:521–530
Ladányi A, Kiss J, Somlai B et al (2007) Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother 56:1459–1469
Balch CM, Buzaid AC, Soong S-J et al (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19:3635–3648
Ebert LM, Tan BS, Browning J et al (2008) The regulatory T cell-associated transcription factor FOXP3 is expressed by tumor cells. Cancer Res 68:3001–3009
Morgan ME, van Bilsen JH, Bakker AM et al (2005) Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 66:13–20
Roncador G, Brown PJ, Maestre L et al (2005) Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol 35:1681–1691
Wang J, Ioan-Facsinay A, van der Voort EI et al (2007) Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 37:129–138
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336
Fontenot JD, Rasmussen JP, Williams LM et al (2005) Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22:329–341
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Yagi H, Nomura T, Nakamura K et al (2004) Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol 16:1643–1656
Walker MR, Kasprowicz DJ, Gersuk VH et al (2003) Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest 112:1437–1443
Allan SE, Passerini L, Bacchetta R et al (2005) The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 115:3276–3284
Allan SE, Crome SQ, Crellin NK et al (2007) Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19:345–354
Ziegler SF (2006) FOXP3: of mice and men. Annu Rev Immunol 24:209–226
Kryczek I, Liu R, Wang G et al (2009) FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 69:3995–4000
Acknowledgements
We thank Katalin Derecskei, Ibolya Sinka and Miklós Kónya (National Institute of Oncology) for their excellent technical assistance. The study was supported by Hungarian Ministry of Health grant ETT 308/2003 (AL), Hungarian Scientific Research Fund grant OTKA K 72836 (AL), and NKFP1a-0024-05 (JT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ladányi, A., Mohos, A., Somlai, B. et al. FOXP3+ Cell Density in Primary Tumor Has No Prognostic Impact in Patients with Cutaneous Malignant Melanoma. Pathol. Oncol. Res. 16, 303–309 (2010). https://doi.org/10.1007/s12253-010-9254-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-010-9254-x