Abstract
Distinction between grade II ependymomas and anaplastic ependymomas based on histopathological examination solely is problematic and, therefore, the management of intracranial ependymomas remains controversial. The aim of this study was to conduct a systematic review (SR) and meta-analysis (MA) of data published on immunohistochemical prognostic markers (IPM) in intracranial ependymomas (IE), and to establish an evidence-based perspective on their clinical value. Following the extensive search based on a strictly defined group of key words, 30 studies reporting results on IPM in IE were identified. Due to a pronounced inter-study heterogeneity, only 14 publications fulfilled the criteria for inclusion into SR. From the total of 67 immunohistochemical markers, 18 were found to correlate with prognosis. However, owing to inadequate data publishing, MA could be performed only with data on proliferation marker MIB-1 (Ki-67) from 5 publications, including 337 patients: The pooled hazard ratio for overall survival was 3.16 (95% confidence interval = 1.96–5.09; p < 0.001) implicating that patients suffering from tumors with higher immunohistochemical expression of MIB-1 had a significantly worse outcome. Marked inter-study heterogeneity and incomplete data publishing in primary studies significantly limited extent of the SR, and the possibility of performing MA. Although the prognostic impact of MIB-1 immunoexpression in IE could be confirmed, there remains lack of further reliable IPM that could be used in routine diagnosis. We encourage to search for new, useful markers, as well as to standardize lab-techniques and data interpretation algorithms across laboratories in order to increase data compatibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ependymomas are neuroectodermal tumors having their origin in the lining of the ventricular system of the central nervous system. Although they can occur at any age, they are of the greatest clinical importance in childhood. They represent the third most common brain tumors in this age group, following pilocytic astrocytomas and medulloblastomas, forming up to 12% of all pediatric intracranial malignancies [1]. The recent World Health Organization (WHO) grading system from 2007 [1] defines four categories of ependymal tumors: subependymomas and myxopapillary ependymomas (grade I tumors), ependymomas (grade II), and anaplastic ependymomas (grade III). Grade I ependymal tumors are clinically and histologically well-defined entities with a favorable outcome in the vast majority of patients [1]. On the other hand, the criteria for distinguishing between the grade II ependymomas and the anaplastic ependymomas according to the recent WHO grading system remained equivocal and thus the reproducibility of the prognostication based on histopathological examination remains problematic [2–5]. Grade III ependymoma is defined as an ependymal tumor with increased cellularity and brisk mitotic activity, often associated with microvascular proliferation and pseudopalisading necrosis [1]. None of these criteria has been properly quantified and their different weight in the diagnostic assessment has not been considered as well. Moreover, tumor necrosis was repeatedly questioned as an unfavorable prognostic factor [6–8]. Hence, the diagnosis of anaplastic ependymoma is neither easy nor reproducible. A number of publications concerning prognostication in ependymomas has been published. However, as these tumors are relatively rare, the studies on different tumor markers were performed mostly on series with a limited number of patients, contributing only a little to the classification system.
The aim of the present study was to perform a systematic review (SR) and meta-analysis (MA) of the available literature published on ependymomas focusing on the value of the immunohistochemical prognostic markers (IPM), as the systematic review and meta-analysis concerning these hasn’t been reported so far.
Materials and Methods
The systematic review was performed following the guidelines of NHS Centre for Reviews and Dissemination [9], using an approach reported previously [10–14]. Four sets of keywords were applied for literature search (Table 1). The keywords in EPENDYMOMA were used to identify the disease of interest; those in INTRACRANIAL TUMOR broaden the spectrum of papers being involved and thereby decreasing the risk of omission of relevant studies. TUMOR MARKER included specific keywords known to be potentially important a priori together with broader general terms (e.g. “marker”). The set CLINICAL AREA referred our search mainly to the area of diagnosis, prognosis and follow-up. A paper was included into further analysis in case at least one of the relevant terms from all of the above mentioned categories were present (conjunction of phrases from all categories) in either a title, abstract or within the keywords of the article. The online bibliographic databases Medline and Embase were chosen as a basis for identifying the relevant literature published from 1966 to October 2007. The search was restricted to English written literature. The potential relevance of the papers was determined independently by two reviewers reading titles and abstracts, mutual consent was required. The fulltexts of the selected reports were then used for the detailed analysis by all reviewers. The reference lists of these publications were searched for further relevant articles. There was no restriction regarding the age of the studied patient population. Review articles were not enrolled into our analysis because all the data had to be based on primary research only. All identified duplicates were eliminated. If multiple studies used the same or overlapping cohorts of patients while investigating the same prognostic marker, only the most complete or the most recent study was used. Next, only the papers providing quantitative results evaluating the prognostic value of immunohistochemical markers in ependymomas were included. As the association between the histological grade and the clinical outcome remains unclear [2–6], a correlation of IPM(s) with histological grade without proper clinical data represented another reason for exclusion. Studies on extracranial and grade I ependymomas were excluded as well, as their biological behavior is different [1, 15–17]. Studies comprising mixed tumors were rejected either. After this selection, the following detailed information on the studied cohort and immunohistochemistry were extracted: number of patients, age, localization of the tumors (supratentorial / infratentorial / spinal), histological grade (according to the WHO classification) and overall and/or progression free survival (OS, PFS) data. Hazard ratio (HR) and its confidence interval (CI) were directly collected from the tables or texts of the papers or, where possible, extracted from the published survival curves using methods described by Parmar et al [10]. Briefly, for each trial the time axis of Kaplan-Meier curve was split into non-overlapping intervals. The log hazard ratio was estimated for each interval and then combined with the others in a stratified way across intervals to obtain an overall log hazard ratio for each trial.
Detailed data on immunohistochemistry (antibody type and its dilution, pre-treatment methods, detection kit, cut-off values of each continuous variable and quantitative results of the test) were obtained as well. If the prognostic value of the immunohistochemical marker was studied in at least three papers, MA of this marker was performed. A DerSimonian-Laird random effects meta-analysis was performed to estimate the overall effect of a marker expression on prognosis [14, 18]. In brief, HRs and their variance obtained from the single studies were combined, so as the standard errors of the estimates were adjusted to reflect a measure of the extent of variation among the effects observed in different studies. It is the simplest and most conservative method of a random effects meta-analysis recommended for routine use while combining a small number of studies [18]. Heterogeneity among the studies was tested concurrently, using Cochrane statistics Q. Sensitivity analysis (inclusion of subgroups of studies into MA) was performed to test consistency of the pooled result. The software Comprehensive Meta Analysis, version 2.2.046 (Biostat Inc.) was used for statistical analysis.
Results
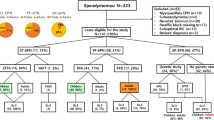
We identified 2,267 publications from the database search. Manual selection of the studies based on abstract analysis disclosed 94 relevant papers. Subsequently, a detailed study of the fulltexts showed that only 30 studies [6–8, 19–45] reported applicable results on immunohistochemical prognostic markers in ependymomas. Fourteen papers had to be excluded from a further analysis as it was impossible to clearly identify data on intracranial ependymomas of grade II and III due to a mixture with either spinal ependymomas [26–28, 30, 33, 38, 44, 45], grade I ependymomas (subependymomas or myxopapillary ependymomas) [23, 25, 33, 38, 39, 44], or other CNS tumors [24, 26–30, 33, 38, 39, 43–45]. Two studies [8, 35] were rejected as their results were included and updated in more recent papers [37, 40]. Cohort overlap had to be taken into account in 8 cases [6, 21, 22, 31, 32, 34, 36, 37]. In summary, 14 studies were eligible for SR and potentially for inclusion into MA (Table 2). A brief overview of immunohistochemical markers studied in these 14 papers and their impact on patients´ overall survival (OS) or progression free survival (PFS) are summarized in Tables 3 and 4. Hazard ratio (HR) and its confidence interval (CI) were specified in 6 studies only [6, 19, 22, 34, 36, 41]; extraction of HR and CI for some of the IPMs was possible from survival curves in further 5 studies [7, 20, 21, 31, 40]; in 3 studies sufficient data for extraction of HR and its CI were not provided [32, 37, 42].
MIB-1 (Ki-67)
MIB-1 was the most frequently studied antigen, investigated in total of 9 articles [6, 7, 20–22, 31, 32, 40, 41]. It represented the only marker that could be evaluated through MA. Due to cohort overlap only five papers [6, 7, 20, 21, 40] comprising 337 patients with the same primary endpoint (OS) were included into MA (Table 2).
The combined HR for all 5 eligible studies was 3.16 (95% CI = 1.96–5.09; p < 0.001) without statistical inter-study heterogeneity (Q = 5.56; p = 0.23), indicating that patients suffering from tumors with increased expressions of MIB-1 antigen in tumor cell nuclei had significantly worse survival (Fig. 1). The reported results of analysis of this marker using different primary endpoint (progression-free survival, PFS) in the individual studies included into SR were in concordance with this finding as well (Table 2).
Significant inter - study heterogeneity of staining techniques was apparent. Throughout the studies of MIB-1, the antibody dilution varied from 1:50 to 1:200, different antibody clones and pre-treatments were applied (Table 5). Moreover, the cut-off values of labeling index of this marker dichotomizing patients into two groups differed remarkably as well, varying from 1% to 25% (Fig. 1).
Other Nuclear Antigens
p53 was the second most common antigen studied; its higher expression correlated significantly with worse prognosis assessed both through PFS or OS [6, 20, 22, 36, 37]. There was cohort overlap in the studies by Korshunov et al. [36, 37] and by our group [6, 22]. The extent of immunohistochemical expression of Mdm2 (antigen murine double minute oncogene), negative regulator of p53, did not show any influence on patients´ prognosis in three papers [20, 36, 37].
Regarding the other proteins involved in the cell cycle regulation, cyclin D1 immunopositivity had only a marginally significant impact on PFS in a cohort of pediatric patients [6]. Decreased cyclin-dependent kinase inhibitor 2A (CDKN2A) immunopositivity investigated in 2 studies [36, 37] with cohort overlap, correlates with shorter PFS. p27 immunopositivity was shown to correlate significantly with worse PFS [37] as well. A higher expression of proliferating cell nuclear antigen (PCNA) was shown to correlate significantly with worse OS [20]. On the other hand, the expression of p21 had no impact on patients’ survival [37].
Topoisomerase II alpha (TopoIIalpha) immunopositivity correlates with an unfavorable outcome using both PFS and OS as a primary study endpoint [6, 21, 31, 36, 37]. Tabori et al [19] demonstrated a strong nuclear human telomerase reverse transcriptase (hTERT) immunopositivity in tumor cell nuclei in patients with a statistically significant worse overall survival. Among other immunohistochemical prognostic markers, significant relationship between OS and expression of survivin was found in one study [31]. Expression of hypoxia-related factor hypoxia-inducible factor alpha HIF-1A [32] did not show any prognostic relevance.
Cytoplasmatic Antigens
Regarding apoptosis-related markers, Bcl-2 correlated significantly with prognosis in one study on 31 pediatric patients [6] (using both PFS and OS as primary endpoints). However, its prognostic relevance was not proven in the study by Verstegen et al. investigating 51 patients of all ages and using OS [20]. Bax protein, investigated also in the study of Verstegen et al. [20], did not prove to be a relevant prognostic marker.
Among other cytoplasmatic antigens, Figarella-Branger et al. [42] documented a correlation between glial fibrillary acidic protein (GFAP) immunopositivity and better overall survival in 16 pediatric patients. Moreover, this group also showed that GFAP/vimentin ratio < 1 portends an increased risk of death [42]. In the study conducted by Zamecnik et al., however, expression of GFAP did not prove to be a significant predictor of prognosis [6, 22].
Korshunov et al. [37] confirmed a correlation between vascular endothelial growth factor (VEGF) immunopositivity and decreased PFS. The same group of authors showed in a study focused on chemoresistance-related proteins [34] that the risk of tumor recurrence decreases significantly in P-glycoprotein (P-GP), glutathione S-transferase pi (GST pi) and metallothioneins (MT) immunopositive tumors.
Membranous Antigens
Among membranous antigens, expression of epithelial membranous antigen (EMA) did not show any correlation with survival [6, 42]. On the other hand, Korshunov et al. [37] proved the correlation between epidermal growth factor receptor (EGFR) and decreased PFS. No correlation with overall survival was found in the case of ERBB1-4 expression [40]. Expression of hypoxia-related factor carbonic anhydrase 9 (CA9) [32] as well as of glycan HNK1 [42] did not show any prognostic relevance.
Extracellular Antigens
In the studies that fulfilled SR inclusion criteria the following extracellular matrix proteins were investigated: vitronectin, tenascin, laminin, collagen II, IV, VI were studied. The correlation of vitronectin and tenascin immunopositivity in the tumor invasion front, vasculature and intercellular spaces with decreased PFS was documented [22, 37]. The expression of the other antigens did not show any significant correlation with survival.
Discussion
The criteria for distinguishing between grade II ependymomas and anaplastic ependymomas according to the recent WHO grading system [1] based solely on histopathological evaluation remain elusive [2–6]. As a result, ependymomas represent one of the most controversial entities concerning the treatment strategy in human oncology which has a problematic impact on prognosis of these patients. Therefore, identification of tumor markers, which could be reproducible and easily used for differentiating between patients with less aggressive tumors, who might profit from a less intensive adjuvant therapy, and those with a high risk of tumor recurrence, is greatly desirable.
A systematic approach is currently the preferred format of reviewing the existing evidence regarding diagnosis, treatment, prognosis and overall effectiveness in medicine [46]. A few reviews on ependymomas have been published, among them the studies by Bouffet et al. [4] and Rickert et al. [47] are of importance. We performed the first systematic review of immunohistochemical prognostic markers in intracranial ependymomas through identification of the relevant literature indexed in the largest bibliographic databases on this topic and evaluated the relation of the individual markers to well-defined and reproducible criteria corresponding with prognosis of the patients.
Following the strict inclusion criteria for the published data, the MA could be performed only for MIB-1, as its impact on patients’ survival (OS) was properly documented in five publications. MIB-1 was studied in further four papers, which were not included into MA as their primary endpoint was different (PFS); MA for MIB-1 using PFS could not be performed as there was a cohort overlap among these studies. Interestingly, the HR and its variance in the study performed by Zamecnik et al. [6] differed from the other studies in MA. This could be due to a smaller cohort of patients, their age as well as the applied staining technique. We performed sensitivity analysis excluding this study (HR = 2.83, 95% CI =1.90–4.22) and it resulted in a statistically significant result that is in agreement with the MA from all the studies.
The other antigens including tumor cell structural proteins, extracellular matrix components and proteins involved in the cell cycle control and apoptosis were investigated regarding their prognostic relevance in the reports included into SR (Table 2). However, a limited number of these publications and inconsistent statistical reporting prevented quantitative pooling of their results by the means of MA.
While performing the systematic review, we experienced similar obstacles as reported previously [12, 46, 48]. The major problem was the inter - study heterogeneity; the inconsistency among studies was apparent in clinical factors (age, stage of disease, treatment modalities, length of follow-up), use of different study endpoints (PFS versus OS), as well as in different protocols for immunohistochemistry processing and evaluation. Above all, inadequate data publishing in primary studies limited quantitative synthesis of the results. Exclusion of non-English papers could cause also some bias in the SR. From the above mentioned reasons we admit that the review may not be fully comprehensive-primarily due to the strict inclusion criteria regarding quality of the studies.
Based on this study we may recommend that when conduction a study on prognostic markers, the endpoint outcome should be presented properly, e.g. as HR with some measure of precision. Coordination through cancer research groups may lead to creation of a prospective online database of trials which would help to prevent publication bias and may declassify individual patient data enabling subsequent analysis and evaluation [12, 48, 49]. In this review we confirmed a role of MIB-1 as a relevant prognostic marker; furthermore we gathered relevant literature and identified other possibly valuable immunohistochemical prognostic markers in intracranial ependymomas.
Abbreviations
- A:
-
adults
- C:
-
children
- CA9:
-
carbonic anhydrase
- CDKN2A:
-
cyclin-dependent kinase inhibitor 2A
- CI:
-
confidence interval
- CPP32:
-
putative cysteine protease
- EGFR:
-
epidermal growth factor receptor
- EMA:
-
epithelial membranous antigen
- ERBB:
-
epidermal growth factor receptor
- GFAP:
-
glial fibrillary acidic protein
- GST pi:
-
glutathione S-transferase pi
- HIF-1A:
-
hypoxia-inducible factor 1 alpha
- HNK1:
-
human natural killer
- HR:
-
hazard ratio
- hTERT:
-
human telomerase reverse transcriptase
- IHC:
-
immunohistochemistry
- infra:
-
infratentorial
- IPM:
-
immunohistochemical prognostic markers
- K-M:
-
Kaplan-Meier survival curve
- MA:
-
meta-analysis
- Mdm2:
-
murine double minute oncogene
- MT:
-
metallothioneins
- MVA:
-
multivariate analysis
- MW:
-
microwave pretreatment
- NA:
-
data not available
- NS:
-
non significant
- OS:
-
overall survival
- PCNA:
-
proliferating cell nuclear antigen
- PFS:
-
progression free survival
- P-GP:
-
P-glycoprotein
- SR:
-
systematic review
- SS:
-
statistically significant
- supra:
-
supratentorial
- TopoIIalpha:
-
topoisomerase II alpha
- UVA:
-
univariate analysis
- VEGF:
-
vascular endotelial growth factor
- WHO:
-
World Health Organization
References
McLendon RE, Wiestler OD, Kros JM, Korshunov A, Ng HK (2007) Ependymoma. In: Louis DN, Wiestler OD, Cavanee WK (eds) Classification of the Tumours of the Central Nervous System. Lyon, International Agency for Research on Cancer (IARC)
Foreman NK, Love S, Thorne R (1996) Intracranial ependymomas: analysis of prognostic factors in a population-based series. Pediatr Neurosurg 24:119–25
Pollack IF, Gerszten PC, Martinez AJ, Lo KH, Shultz B, Albright AL, Janosky J, Deutsch M (1995) Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery 37:655–66
Bouffet E, Perilongo G, Canete A, Massimino M (1998) Intracranial ependymomas in children: a critical review of prognostic factors and a plea for cooperation. Med Pediatr Oncol 30: 319–29; discussion 329–31
Robertson PL, Zeltzer PM, Boyett JM et al (1998) Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg 88:695–703
Zamecnik J, Snuderl M, Eckschlager T et al (2003) Pediatric intracranial ependymomas: prognostic relevance of histological, immunohistochemical, and flow cytometric factors. Mod Pathol 16:980–91
Kurt E, Zheng PP, Hop WC et al (2006) Identification of relevant prognostic histopathologic features in 69 intracranial ependymomas, excluding myxopapillary ependymomas and subependymomas. Cancer 106:388–95
Bennetto L, Foreman N, Harding B et al (1998) Ki-67 immunolabelling index is a prognostic indicator in childhood posterior fossa ependymomas. Neuropathol Appl Neurobiol 24:434–40
Khan KS, ter Riet G, Glanville J, Sowden AJ, Kleijnen J (2001) Undertaking Systematic Reviews of Research on Effectiveness: CRD Guidelines for Those Carrying Out or Commissioning Reviews. CRD Report 4, NHS Centre for Reviews and Dissemination, University of York, York
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–34
Machin D, Cheung YB, Parmar MK (2006) Survival Analysis. A Practical Approach, 2nd edn. John Wiley, Chichester
Riley RD, Abrams KR, Sutton AJ et al (2003) Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer 88:1191–8
Riley RD, Heney D, Jones DR et al (2004) A systematic review of molecular and biological tumor markers in neuroblastoma. Clin Cancer Res 10:4–12
Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F (2000) Methods for Meta-analysis in Medical Research. John Wiley and Sons, Ltd, Chichester
Rawlings CE, Giangaspero F, Burger PC, Bullard DE (1988) Ependymomas: a clinicopathologic study. Surg Neurol 29:271–81
Schroder R, Ploner C, Ernestus RI (1993) The growth potential of ependymomas with varying grades of malignancy measured by the Ki-67 labelling index and mitotic index. Neurosurg Rev 16:145–50
Takeuchi H, Kubota T, Sato K, Llena JF, Hirano A (2002) Epithelial differentiation and proliferative potential in spinal ependymomas. J Neurooncol 58:13–9
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–88
Tabori U, Ma J, Carter M et al (2006) Human telomere reverse transcriptase expression predicts progression and survival in pediatric intracranial ependymoma. J Clin Oncol 24:1522–8
Verstegen MJ, Leenstra DT, Ijlst-Keizers H, Bosch DA (2002) Proliferation- and apoptosis-related proteins in intracranial ependymomas: an immunohistochemical analysis. J Neurooncol 56:21–8
Wolfsberger S, Fischer I, Hoftberger R et al (2004) Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am J Surg Pathol 28:914–20
Zamecnik J, Chanova M, Tichy M, Kodet R (2004) Distribution of the extracellular matrix glycoproteins in ependymomas—an immunohistochemical study with follow-up analysis. Neoplasma 51:214–22
Shuangshoti S, Rushing EJ, Mena H, Olsen C, Sandberg GD (2005) Supratentorial extraventricular ependymal neoplasms: a clinicopathologic study of 32 patients. Cancer 103:2598–605
Schiffer D, Cavalla P, Migheli A, Giordana MT, Chiado-Piat L (1996) Bcl-2 distribution in neuroepithelial tumors: an immunohistochemical study. J Neurooncol 27:101–9
Schiffer D, Chio A, Giordana MT, Pezzulo T, Vigliani MC (1993) Proliferating cell nuclear antigen expression in brain tumors, and its prognostic role in ependymomas: an immunohistochemical study. Acta Neuropathol (Berl) 85:495–502
Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D, Epstein FJ (1996) Disseminated ependymomas of the central nervous system. J Neurosurg 85:618–24
Ritter AM, Hess KR, McLendon RE, Langford LA (1998) Ependymomas: MIB-1 proliferation index and survival. J Neurooncol 40:51–7
Roma AA, Prayson RA (2006) Expression of cyclo-oxygenase-2 in ependymal tumors. Neuropathology 26:422–8
Prayson RA (1998) Cyclin D1 and MIB-1 immunohistochemistry in ependymomas: a study of 41 cases. Am J Clin Pathol 110:629–34
Prayson RA (1999) Clinicopathologic study of 61 patients with ependymoma including MIB-1 immunohistochemistry. Ann Diagn Pathol 3:11–8
Preusser M, Wolfsberger S, Czech T, Slavc I, Budka H, Hainfellner JA (2005) Survivin expression in intracranial ependymomas and its correlation with tumor cell proliferation and patient outcome. Am J Clin Pathol 124:543–9
Preusser M, Wolfsberger S, Haberler C et al (2005) Vascularization and expression of hypoxia-related tissue factors in intracranial ependymoma and their impact on patient survival. Acta Neuropathol (Berl) 109:211–6
Nagashima T, Hoshino T, Cho KG, Edwards MS, Hudgins RJ, Davis RL (1988) The proliferative potential of human ependymomas measured by in situ bromodeoxyuridine labeling. Cancer 61:2433–8
Korshunov A, Sycheva R, Timirgaz V, Golanov A (1999) Prognostic value of immunoexpression of the chemoresistance-related proteins in ependymomas: an analysis of 76 cases. J Neurooncol 45:219–27
Korshunov A, Timirgaz V, Golanov A (1999) Prognostic value of aberrant p53 immunoexpression for the recurrence of ependymoma: An analysis of 76 cases. Neuropathology 19:380–385
Korshunov A, Golanov A, Timirgaz V (2001) p14ARF protein (FL-132) immunoreactivity in intracranial ependymomas and its prognostic significance: an analysis of 103 cases. Acta Neuropathol (Berl) 102:271–7
Korshunov A, Golanov A, Timirgaz V (2002) Immunohistochemical markers for prognosis of ependymal neoplasms. J Neurooncol 58:255–70
Ho DM, Hsu CY, Wong TT, Chiang H (2001) A clinicopathologic study of 81 patients with ependymomas and proposal of diagnostic criteria for anaplastic ependymoma. J Neurooncol 54:77–85
Guyotat J, Champier J, Jouvet A et al (2001) Differential expression of somatostatin receptors in ependymoma: implications for diagnosis. Int J Cancer 95:144–51
Gilbertson RJ, Bentley L, Hernan R et al (2002) ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res 8:3054–64
Figarella-Branger D, Civatte M, Bouvier-Labit C et al (2000) Prognostic factors in intracranial ependymomas in children. J Neurosurg 93:605–13
Figarella-Branger D, Gambarelli D, Dollo C et al. (1991) Infratentorial ependymomas of childhood. Correlation between histological features, immunohistological phenotype, silver nucleolar organizer region staining values and post-operative survival in 16 cases. Acta Neuropathol (Berl) 82: 208–16
Cruz-Sanchez FF, Garcia-Bachs M, Rossi ML et al (1992) Epithelial differentiation in gliomas, meningiomas and choroid plexus papillomas. Virchows Arch B Cell Pathol Incl Mol Pathol 62:25–34
Asai A, Hoshino T, Edwards MS, Davis RL (1992) Predicting the recurrence of ependymomas from the bromodeoxyuridine labeling index. Childs Nerv Syst 8:273–8
Athanasiou A, Perunovic B, Quilty RD, Gorgoulis VG, Kittas C, Love S (2003) Expression of mos in ependymal gliomas. Am J Clin Pathol 120:699–705
Egger M, Davey Smith G, Altman D (2001) Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ, London
Rickert CH, Paulus W (2005) Prognosis-related histomorphological and immunohistochemical markers in central nervous system tumors of childhood and adolescence. Acta Neuropathol (Berl) 109:69–92
Altman DG, Lyman GH (1998) Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat 52:289–303
Hutchon DJ (2001) Publishing raw data and real time statistical analysis on e-journals. Bmj 322(7285):530
Acknowledgments
The publication was supported in part by the grant VZ FNM 00064203 of the Ministry of Health of the Czech Republic and grant VZ MSM CR 0021620812 of the Ministry of Education. The authors state no financial conflicts of interest. We wish to thank Marie Hladikova for critical remarks on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Klara Kuncova and Ales Janda have contributed equally.
Rights and permissions
About this article
Cite this article
Kuncova, K., Janda, A., Kasal, P. et al. Immunohistochemical Prognostic Markers in Intracranial Ependymomas: Systematic Review and Meta-Analysis. Pathol. Oncol. Res. 15, 605–614 (2009). https://doi.org/10.1007/s12253-009-9160-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-009-9160-2