Abstract
Caveolin-1 is the major structural protein in caveolae, implicated in oncogenesis and angiogenesis. The connections between caveolin-1 and progression and angiogenesis of hepatocellular carcinoma (HCC) is still not clear. Thus we investigated the relationship of caveolin-1 expression, vascular endothelial growth factor (VEGF) expression, microvessel density (MVD), and unpaired artery (UA) with the clinicopathologic features of patients with HCC. Formalin-fixed, paraffin-embedded tissue sections of HCC from 75 patients who had undergone an initial hepatectomy were stained immunohistochemically with specific antibodies against caveolin-1, VEGF, CD34 and α-SMA. The levels of caveolin-1, VEGF, MVD and UA were correlated with the clinicopathologic variables, and tissue sections were also analyzed by dual-label immunofluorescence. We found that increased expression of caveolin-1 was associated with metastasis and with a worse prognosis of HCC. Caveolin-1 expression correlates positively with VEGF, MVD and UA. These results suggest that caveolin-1 may play an important role in the progression of HCC and angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caveolin-1, a 21–24 kDa membrane protein, is a principal structural component of the plasma membrane invaginations known as caveolae. Caveolin-1 interact directly with many key signaling molecules, including Src family tyrosine kinases, endothelial nitric oxide synthase and G proteins [1]. It has been demonstrated that caveolin-1 is involved in the pathogenesis of several important human diseases, such as cancer, atherosclerosis, vasculoproliferative diseases, and diabetes. Recent studies reveal that caveolin-1 is down-regulated in pulmonary adenocarcinoma and sarcoma [2, 3]. However, elevated expression of caveolin-1 has been associated with the metastasis of esophageal squamous cell carcinoma and pancreatic ductal adenocarcinoma, and the overexpression of caveolin-1 is negatively correlated with patient survival [4, 5]. These findings indicate that, depending on the tumor cell type, caveolin-1 might function both as a tumor suppressor and as a tumor-promoting gene.
Caveolin-1 may also contribute to the progress of angiogenesis, which is a very important mechanism in tumor progression and metastasis. It has been reported that overexpression of endothelial-specific caveolin-1 reduces vascular endothelial growth factor (VEGF)-stimulated vascular permeability and decreases VEGF-mediated angiogenesis [6], whereas a decrease in caveolin-1 abundance promotes angiogenesis and prevents its termination by mural cell recruitment [7]. However, it appears that caveolin-1 may have dual roles in angiogenesis, as another study reports that increased expression of caveolin-1 and microvessel density (MVD) correlated with metastasis and poor prognosis in renal cell carcinoma [8].
Hepatocellular carcinoma (HCC) is one of the most common solid tumors throughout the world, and it is characterized by hypervascularization, high recurrence, metastasis and dismal prognosis. Therefore, it is important to identify any biologic markers that seem to track with metastasis and prognosis of HCC. Several in vitro and in vivo investigations indicate that caveolin-1 may play a direct role in the progression and angiogenesis of HCC [9–12]. However, the connections between caveolin-1 and these two steps of HCC development are still not clear. Thus, in the present study, we investigated the expressions levels of caveolin-1 and VEGF, MVD (labeled by CD34) and unpaired artery (UA) formation (labeled by alpha smooth muscle actin (α-SMA)) in HCC and analyzed them with respect to clinicopathologic features and patient prognosis. The relationships between immunohistochemical markers were also assessed.
Materials and Methods
Patients and Specimens
Formalin-fixed, paraffin-embedded, archival surgical specimens from 75 patients (68 men and seven women, mean age of 45.6 years, range 25–75) diagnosed with HCC were assessed. All patients had undergone an initial hepatectomy at our institution between May 2006 and March 2007 consecutively with curative intention. Tumors were staged according to the 2002 revised TNM system by National Comprehensive Cancer Network (NCCN). This study was approved by the ethical committee of Southwest Hospital, Third Military Medical University, and written informed consent was obtained from all patients.
Immunohistochemical Analysis
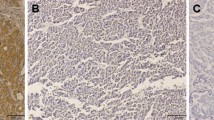
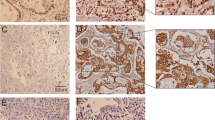
Paraffin-embedded blocks were sectioned at 4 μm, deparaffinized and rehydrated. After microwave pretreatment in citrate buffer (pH 6.0) for antigen retrieval, slides were immersed in 3% hydrogen peroxide for 10 min to block the endogenous peroxidase activity. The sections were blocked with blocking reagent in normal goat serum (ZLI-9020, ZSGB-BIO, Beijing, China) for 20 min. Sections were then incubated with a rabbit polyclonal antibody to caveolin-1 (diluted 1:500; Santa Cruz Biotechnology, Inc., USA), a mouse monoclonal antibody to VEGF (dilution 1:500; Santa Cruz Biotechnology, Inc., USA), a mouse monoclonal antibody to CD34 (clone QBEnd/10, diluted 1:100; NeoMarkers for Lab Vision Corporation, San Francisco, CA, USA) or a mouse monoclonal antibody to α-SMA (clone 1A4, diluted 1:100; Dako North America, Inc., California, USA) for 2 h at 37°C in a humidified chamber. After a second incubation with two-step IHC detection reagent (PV-6001/6002, ZSGB-BIO, Beijing, China), reaction products were visualized by immersing slides in 3,3′diaminobenzidine tetrachloride with substrate from a kit (ZLI-9032, ZSGB-BIO, Beijing, China). Finally, The slides were counterstained with Mayer’s haematoxylin. Positive staining of smooth muscle cells or endothelium, known to be abundant in caveolin-1 expression, provided an internal positive control for caveolin-1 immunostaining (Fig. 1a). Negative controls were obtained by using phosphate buffer saline (PBS) as the primary antibody.
Immunohistochemical staining for caveolin-1, VEGF, CD34 and α-SMA. a, Endothelial cells are stained as an internal control, but cancer cells are not stained (caveolin-1 intensity 0) (×400). b, The cytoplasm of almost all cancer cells is strongly stained for caveolin-1 (caveolin-1 intensity 3) (×400). c, Most of the cancer cell cytoplasm is strongly stained for VEGF (VEGF intensity 3)(×400). d, Intratumoral SECs are labeled by CD34 (×400). e, Intratumoral smooth muscle cells of UA are labeled by α-SMA (×400). f, Caveolin-1 expression in SECs (green arrow) and smooth muscle cells of UA (red arrow)(×400)
Evaluation of Immunohistochemical Staining
Immunostaining was independently evaluated by three pathologists unaware of the sample origin. There was disagreement regarding the conclusions of the immunostaining in <10% of the slides examined, and a consensus was reached on further review.
Caveolin-1 and VEGF expression were based on the presence of cytoplasmic and/or membranous staining. Caveolin-1 and VEGF immunostaining were semi-quantitatively estimated based on intensity and proportion (percentage of positive cells). The caveolin-1 or VEGF intensity was subdivided into four categories: 0 (negative), 1 (weak), 2 (moderate, same intensity of smooth muscle cells) and 3 (strong, Fig. 1b, c). The proportion of caveolin-1 or VEGF staining (percentage of positive cells) was also classified using five categories: 0 (≤10%), 1 (11–25%), 2 (26–50%), 3 (51–75%) and 4 (>75%). A caveolin-1 or VEGF score for each case was generated by multiplying the values for the two variables. For further analysis, a mean score of 6 was used as a cut-off value for caveolin-1 expression to divide the samples into a high-expression group (≥6) and a low-expression group (<6). This cut-off value was adopted from a previous report [13].
MVD was determined using CD34-stained slides. Individual microvessels were counted in the area of highest vascularity at ×200 in five selected microscopic fields. Any brown-staining endothelial cell or cluster that was separated from other nearby microvessels was counted. The presence of a vascular lumen was not necessary to identify a microvessel. Large anastomosing sinusoidal vessels were counted as a single vessel. Large vessels with thick muscular walls were excluded from the count. A modified method using the procedure of Tanigawa et al. [14], which considered the length of a microvessel to be about 40 mm (an estimate that was calculated as one point for counting under 200 magnification), was applied in those cases. The microvessel count was expressed as the mean number of vessels in the selected area (Fig. 1d). The mean value was used as a cut-off point for survival analysis.
UA formation was defined as the presence of α-SMA-positive vessels that are located in the tumor parenchyma and are not accompanied by obvious fibrous/portal vein or bile duct. Arterioles were distinguished from veins by the ratio of minimum external diameter, which exceeds 1/10. Slides were examined in a ×100 field, and ten visual fields were randomly selected from each section. The arterioles were counted, and the total arteriole area was calculated (Fig. 1e). The mean value was also used as a cut-off point for survival analysis.
The expression of caveolin-1 in sinusoidal endothelial cells (SECs) and in smooth muscle cells of UA was quantitatively estimated as the method of MVD (labeled by CD34) and UA (labeled by α-SMA) count (Fig. 1f).
Immunofluorescence Analysis
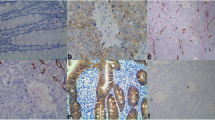
To determine whether the location of caveolin-1 expression in neovascularization is coincident with the SECs labeled by CD34 or the smooth muscle cells of UA labeled by α-SMA, dual-label immunofluorescence was performed. The experimental procedure is the same as that used for previous immunohistochemical analyses prior to incubation with primary antibodies. These sections were incubated with primary antibodies for two hours at 37°C in a humidified chamber. The primary antibodies (as immunohistochemisty) were used at the following dilutions: caveolin-1 pAb (1:300), CD34 mAb (1:50) and α-SMA mAb (1:100). Hoechst dye (Sigma-Aldrich Biotechnology, USA) was used at a concentration of 10 μg/mL. Afterward, the sections were washed with PBST (PBS+Tween) and incubated with secondary antibodies for one hour at 37°C in a humidified chamber. Rabbit and mouse rhodamine (TRITC)- and fluorescein (FITC)-conjugated secondary antibodies (Pierce Biotechnology, USA) were used at a dilution of 1:50. The sections were then mounted with VECTASHIELD H-1,000 reagent (Vector Laboratories, Inc., USA). In the end, the sections were evaluated and captured using a time-lapse imaging system (Leica, Germany) (Fig. 2).
Dual-label immunofluorescent analysis shows the colocalization of caveolin-1 (a, red) and CD34 (b, green) in the SECs (c, yellow). Additionally, caveolin-1 (d, red) colocalizes with α-SMA (e, green) in the smooth muscle cells of UA (f, yellow). Blue-staining in panels c and f represents the cellular nuclei that have taken up the Hoechst dye. All images were taken at the same magnification of ×200
Statistical Analysis
An independent sample t-test was used to analyze the correlation between caveolin-1 intensity, proportion or score and clinicopathological variables, including tumor size (the longest diameter of the tumor), tumor capsule, TNM stage, portal vein tumor thrombus (PVTT) and metastasis. Either a chi-square test or a Mann–Whitney U-test was used to analyze the association between caveolin-1 expression, VEGF expression, UA or MVD and clinicopathological variables. Spearman’s correlation test was applied for correlations among caveolin-1 expression, VEGF expression, UA and MVD. The survival calculations were illustrated with Kaplan-Meier curves and univariate and multivariate analyses using the log-rank test or the Cox proportional hazards regression model. In all analyses, a difference of P < 0.05 was used as an indication of statistical significance. Statistical analysis was performed using the SPSS version 13.0 software package (SPSS Inc., Chicago, USA).
Results
Clinicopathologic Features of the Patients
In this series, the duration of symptoms varied from one week to two years, with a median duration of two months. The primary tumor sites were at Couinaud V, VI, VII and VIII segments in 39 patients, Couinaud II, III and IV segments in 33 patients and Couinaud I segment in three patients. Seventy patients displayed clinical symptoms, such as right upper quadrant pain and abdominal distention. All resection margins were histologically clear of tumors. Thirty tumors were TNM stage I or II, and 45 tumors were TNM stage III or IV. Tumor sizes ranged from 1.0 cm to 19.0 cm, with a median size of 7.5 cm. All were completed outpatient follow-ups after surgery. Thirty-seven patients were disease free at a median (range) follow-up of 54 (32–75) weeks; 4 patients died from some other factors but tumor; the other 34 patients had intrahepatic tumor recurrence or/and distant metastases at a median (range) of 18 (4–72) weeks after the hepatectomy. Twenty-nine patients died during follow-up, 25 from intrahepatic tumor recurrence or/and distant metastases, three from hepaticfailure due to final stage of cirrhosis, and one from massive hemorrhage of the upper alimentary tract.
Immunohistochemical Findings and Clinicopathologic Variables
The levels of caveolin-1 expression in samples from 75 patients are summarized in Table 1. Caveolin-1 intensity (P = 0.001), proportion (P = 0.002) and score (P = 0.001) were significantly correlated with metastasis. Caveolin-1 score also correlated with tumor size (P = 0.034) (Table 2). Caveolin-1 expression (P < 0.001), VEGF expression (P < 0.001), MVD (P = 0.005) and UA count (P < 0.001) were significantly linked with metastasis, while VEGF expression (P = 0.004) and UA count (P = 0.011) were also significantly associated with tumor size (Table 3).
Correlation Between Caveolin-1 Expression, VEGF Expression, MVD and UA
Spearman correlation analysis revealed that Caveolin-1 expression was correlated positively with VEGF (r = 0.293, P = 0.011), MVD (r = 0.361, P = 0.001) and UA (r = 0.388, P = 0.001). MVD (r = 0.497, P < 0.001) and UA (r = 0.303, P = 0.008) were positively associated with VEGF expression. MVD was significantly higher in the tumors with higher caveolin-1 expression (P = 0.002) or VEGF expression (P < 0.001) compared to those without. The expression of caveolin-1 in SECs and smooth muscle cells of UA was significantly correlated with MVD (labeled by CD34, r = 0.967, P < 0.001) and UA count (labeled by α-SMA, r = 0.956, P < 0.001).
Dual-label immunofluorescence analysis showed the colocalization of caveolin-1 and CD34 in the SECs. Additionally, caveolin-1 colocalized with α-SMA in the smooth muscle cells of UA.
Survival Analysis
A Kaplan-Meier survival curve showed that the patients with higher caveolin-1 expression had a significantly worse survival rate than patients with lower caveolin-1 expression (P = 0.014, Fig. 3a). The survival rate of patients with higher VEGF (P = 0.003, Fig. 3b), MVD (P = 0.012, Fig. 3c) or UA (P = 0.025, Fig. 3d) was significantly worse than that of patients with lower VEGF expression, MVD or UA.
Kaplan-Meier cancer-specific survival curves according to a, caveolin-1 expression (<6, blue; ≥6, green), where the survival rate of patients with higher caveolin-1 expression was significantly worse than that of patients with lower expression (P = 0.014); b, VEGF expression (<6, green; ≥6, blue), where the survival rate of patients with higher VEGF expression was significantly worse than that of patients with lower expression (P = 0.003); c, UA (low, blue; high, green), where the survival rate of patients with higher UA formation was significantly worse than that of patients with lower UA formation (P = 0.025); d, MVD (low, blue; high, green), where the survival rate of patients with a higher MVD was significantly worse than that of patients with a lower MVD (P = 0.012)
Univariate analyses identified tumor size, PVTT, metastasis, caveolin-1 intensity, caveolin-1 score, caveolin-1 expression, VEGF expression, MVD and UA as significant prognostic factors for cancer-specific survival. Multivariate analyses indicated that only tumor size and VEGF expression were an independent prognostic factor. Caveolin-1 expression was not an independent factor.
Discussion
The role of caveolin-1 expression in tumors is still controversial. Studies of Rous sarcoma virus-transformed chicken embryonic fibroblasts helped to initially identify caveolin-1 as a target for inactivation during oncogenesis [15]. Williams et al. [16] showed that caveolin-1 deficient mice promoted mammary tumorigenesis and dramatically enhanced lung metastasis in vivo. In contrast, several more recent studies have found that caveolin-1 expression was elevated in some cancers, including prostate cancers [17], esophageal carcinomas [4], and meningiomas [18]. In these neoplasias, caveolin-1 overexpression correlates with a high histological grade, a high tumor stage, metastasis and poor prognosis. Recently, Bailey et al. [19] showed that caveolin-1 expression could be up-regulated during epithelial-to-mesenchymal transition, and furthermore, once expressed, caveolin-1 could greatly influence cancer cell adhesion. Barresi at al. [20] showed that the rate of caveolin-1 expression significantly correlated with MVD counts in meningiomas, suggesting that caveolin-1 may mediate the progression of these tumors by stimulating the angiogenic process. Therefore, although many studies have explained the correlation of caveolin-1 with cancers, the exact role of caveolin-1 in cancers is still paradoxic.
For HCC, Yerian et al. [21] reported that caveolin expression levels were a continuum, increasing during the progression from normal to cirrhotic liver, further increasing in macroregenerative and dysplastic nodules (MDN) and then decreasing with the development of HCC. Moreover, they demonstrated that MDN SECs showed an increased intensity of caveolin immunostaining relative to adjacent cirrhotic liver. However, Zhou et al. [11] showed that caveolin-1 has dual functions in mouse hepatocarcinoma cells with different invasive ability, both as a tumor suppressor protein and as a lymphatic metastasis promoter protein. Furthermore, down-regulation of caveolin-1 reduces lymphangiogenic factor VEGFA expression. Chio et al. [12] reported that the expression of caveolin-1 in the smooth muscle cells of unpaired arteries was significantly correlated with the number of unpaired arteries, which has been used to screen neovascularization and arterial blood supply in HCC and might have predictive utility for patient outcome. Otherwise, previous studies reported that VEGF [22], MVD [14] and UA [23] were associated with the progression of HCC and angiogenesis. Therefore, it is necessary to further examine the relationship between caveolin-1 and VEGF, UA or MVD in HCC.
In the present study, only HCC was studied to exclude the influence of histological type, and caveolin-1 or VEGF immunostaining was semi-quantitatively estimated based on intensity and proportion. The caveolin-1 or VEGF score for each case was generated by multiplying the values for the two variables. The caveolin-1 intensity, proportion and score were significantly correlated with metastasis. The caveolin-1 score was also significantly associated with tumor size. The survival rate of patients with higher caveolin-1 expression was significantly worse than that of patients with lower caveolin-1 expression. These results suggest that caveolin-1 expression is correlated with metastasis and survival in HCC. As multivariate analyses indicated that caveolin-1 expression was not an independent prognostic factor for cancer-specific survival.
In many studies, caveolin-1 has already been suggested to be involved in angiogenesis. Specifically, this research has shown that caveolin-1 can regulate endothelial cell growth and tubule formation [24, 25]. A study on multiple myeloma has shown that VEGF secreted by neoplastic cells is able to induce Src-mediated caveolin-1 phosphorylation within the caveolae of the same cells [26]. Because HCC is a highly vascularized solid tumor and angiogenesis is correlated with prognosis in HCC, we assumed that caveolin-1 might play a direct role in the progression of HCC and angiogenesis. In this study, caveolin-1 expression was significantly correlated with metastasis and positively associated with VEGF, intratumoral MVD and UA, which are known to increase with the biological aggressiveness of HCC. These associations suggest that angiogenesis may be affected by caveolin-1 during the progression of HCC. Besides, the number of SECs and smooth muscle cells of UA labeled by caveolin-1 was nearly the same as that of SECs labeled by CD34 and smooth muscle cells of UA labeled by α-SMA, respectively. Thus, we speculate that caveolin-1 may not only stimulate tumor neo-angiogenesis through inducing the production of angiogenic factors, such as VEGF, but it may also be directly involved in endothelial cell growth and tubule formation.
In summary, increased expression of caveolin-1, VEGF, MVD and UA is associated with metastasis and a negative prognosis in HCC. Caveolin-1 expression is correlated with VEGF, MVD and UA. In addition, the number of caveolin-1 expression in the SECs and the smooth muscle cells of UA was nearly coincident with that of SECs labeled by CD34 and smooth muscle cells of UA labeled by α-SMA. Our findings suggest that caveolin-1 may favor the progression of HCC by mediating neoangiogenesis. Immunohistochemistry using a caveolin-1 antibody would provide a simply and significantly biological method useful for screening tumor cells and neovascularization in HCC, and this antibody IHC may have predictive utility for patients’ outcome. In view of these data, we think that further studies investigating the role of caveolin-1 in HCC may open new insights about the possibility of novel therapeutic strategies able to block the tumor’s blood supply and to decrease cellular proliferation in HCC.
Abbreviations
- HCC:
-
hepatocellular carcinoma
- MVD:
-
microvessel density
- UA:
-
unpaired artery
- VEGF:
-
vascular endothelial growth factor
- α-SMA:
-
alpha smooth muscle actin
- SECs:
-
sinusoidal endothelial cells
- MDN:
-
macroregenerative and dysplastic nodules
References
Okamoto T, Schlegel A, Scherer PE, Lisanti MP (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422
Wikman H, Kettunen E, Seppänen JK, Karjalainen A, Hollmén J, Anttila S, Knuutila S (2002) Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene 21:5804–5813
Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U (2001) Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol 158:833–839
Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H (2002) Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer 94:929–933
Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T, Kondo S, Katoh H (2002) Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer 87:1140–1144
Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK (2001) Caveolin-1 expression is associated with high-grade bladder cancer. Urology 58:811–814
Dewever J, Frérart F, Bouzin C, Baudelet C, Ansiaux R, Sonveaux P, Gallez B, Dessy C, Feron O (2007) Caveolin-1 is critical for the maturation of tumor blood vessels through the regulation of both endothelial tube formation and mural cell recruitment. Am J Pathol 171:1619–1628
Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ (2004) Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int 93:291–296
Anders RA, Yerian LM, Tretiakova M, Davison JM, Quigg RJ, Domer PH, Hoberg J, Hart J (2003) cDNA microarray analysis of macroregenerative and dysplastic nodules in end-stage hepatitis C virus-induced cirrhosis. Am J Pathol 162:991–1000
Hirasawa Y, Arai M, Imazeki F, Tada M, Mikata R, Fukai K, Miyazaki M, Ochiai T, Saisho H, Yokosuka O (2006) Methylation status of genes upregulated by demethylating agent 5-aza-2′-deoxycytidine in hepatocellular carcinoma. Oncology 71:77–85
Zhou H, Jia L, Wang S, Wang H, Chu H, Hu Y, Cao J, Zhang J (2006) Divergent expression and roles for caveolin-1 in mouse hepatocarcinoma cell lines with varying invasive ability. Biochem Biophys Res Commun 345:486–494
Choi HN, Kim KR, Park HS, Jang KY, Kang MJ, Lee DG, Kim YK, Cho BH, Cha EJ, Moon WS (2007) Expression of caveolin in hepatocellular carcinoma: association with unpaired artery formation and radiologic findings. Korean J Hepatol 13:396–408
Park SS, Kim JE, Kim YA, Kim YC, Kim SW (2005) Caveolin-1 is down-regulated and inversely correlated with HER2 and EGFR expression status in invasive ductal carcinoma of the breast. Histopathology 47:625–630
Tanigawa N, Lu C, Mitsui T, Miura S (1997) Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology 26:1216–1223
Glenney JR Jr (1989) Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem 264:20163–20166
Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP (2004) Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. J Biol Chem 279:51630–51646
Yang G, Addai J, Wheeler TM, Frolov A, Miles BJ, Kadmon D, Thompson TC (2007) Correlative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesis. Hum Pathol 38:1688–1695
Barresi V, Cerasoli S, Paioli G, Vitarelli E, Giuffrè G, Guiducci G, Tuccari G, Barresi G (2006) Caveolin-1 in meningiomas: expression and clinico-pathological correlations. Acta Neuropathol 112:617–626
Bailey KM, Liu J (2008) Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase. J Biol Chem 283:13914–13724
Barresi V, Cerasoli S, Tuccari G (2008) Correlative evidence that tumor cell-derived caveolin-1 mediates angiogenesis in meningiomas. Neuropathology 28:472–478
Yerian LM, Anders RA, Tretiakova M, Hart J (2004) Caveolin and thrombospondin expression during hepatocellular carcinogenesis. Am J Surg Pathol 28:357–364
Mitsuhashi N, Shimizu H, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Yoshidome H, Kato A, Nukui Y, Miyazaki M (2003) Angiopoietines and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology 37:1105–1113
Morinaga S, Imada T, Shimizu A, Akaike M, Sugimasa Y, Takemiya S, Takanashi Y (2001) Angiogenesis in hepatocellular carcinoma as evaluated by alpha smooth muscle actin immunohistochemistry. Hepatogastroenterology 48:224–228
Liu J, Wang XB, Park DS, Lisanti MP (2002) Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem 277:10661–10668
Griffoni C, Spisni E, Santi S, Riccio M, Guarnieri T, Tomasi V (2000) Knockdown of caveolin-1 by antisense oligonucleotides impairs angiogenesis in vitro and in vivo. Biochem Biophys Res Commun 276:756–761
Podar K, Shringarpure R, Tai YT, Simoncini M, Sattler M, Ishitsuka K, Richardson PG, Hideshima T, Chauhan D, Anderson KC (2004) Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res 64:7500–7506
Acknowledgements
Special thanks to Dr. Lin-Ying Chen for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, ZB., Cai, L., Zheng, SG. et al. Overexpression of Caveolin-1 in Hepatocellular Carcinoma with Metastasis and Worse Prognosis: Correlation with Vascular Endothelial Growth Factor, Microvessel Density and Unpaired Artery. Pathol. Oncol. Res. 15, 495–502 (2009). https://doi.org/10.1007/s12253-008-9144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-008-9144-7