Abstract
Extracellular matrix metalloproteinase inducer (EMMPRIN, also named as CD147) is a multifunctional membrane glycoprotein over-expressed in many kinds of human solid tumors. It has been demonstrated to be involved in tumor invasion and angiogenesis. The aim of this study was to analyze the clinicopathological characteristics of the expression of CD147 in human prostate cancer (PCa), and to evaluate its clinical significance in the histologic classification and prognosis of PCa. CD147 protein expression in paraffin-embedded specimens gathered from 62 cases of PCa and 30 cases of benign prostatic hyperplasia (BPH) were detected by the method of immunohistochemistry. The association of CD147 protein expression with the clinicopathological characteristics and with the prognosis of PCa was subsequently assessed. CD147 expression were positively expressed in 51/62 (82.3%) of PCa and 4/30 (13.3%) of BPH cases, respectively. The positive expression rate of CD147 in PCa tissues was significantly higher than that in BPH. The positive expression of CD147 was dramatically associated with TNM grade (p < 0.001), the depth of the prostatic wall invasion (p = 0.008), GLEASON Score (p = 0.001) and Histologic grade (p = 0.001). The patients with CD147 expression were associated with a poor prognosis of PCa (p = 0.01) and the survival rate of the patients with a strong positive expression of CD147 was the lowest (p = 0.01). The results suggest that the expression of CD147 may be an important feature of PCa and the detection of its expression may benefit us in the prediction of the prognosis of PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most frequent cause of cancer-related death for Western men. Its incidence is continuously rising, with over 200,000 new cancers and 35,000 ~ 40,000 deaths per year [1]. Carcinogenesis and the mechanisms influencing the progression and prognosis of PCa is a multistep process, involving both genetic insults to epithelial cells and changes in epithelial–stromal interactions [2]. Despite current therapeutic methods, many patients develop metastases. Its prognosis rate varies greatly for patients with different clinical stages and pathological grades. As we know, a prostate-specific antigen (PSA) test measures the amount of PSA in the blood. PSA is released into a man’s blood by his prostate gland. Healthy men have low amounts of PSA in the blood, but the amount of PSA in the blood normally increases as a man’s prostate enlarges with age. PSA may increase as a result of an injury, a digital rectal exam, sexual activity, inflammation of the prostate gland or PCa [3, 4]. Since the use of PSA in the diagnosis of PCa has a lower specificity under some conditions, it may not reflect the biological properties of PCa exactly [5], it is, therefore, of great significance to search for a more sensitive, more specific PCa marker that can provide valuable information for diagnosis and treatment of the disease.
The degradation of the extracellular matrix (ECM) surrounding primary tumors and metastases is critical for the invasion and metastasis of epithelial tumor cells [6]. Matrix metalloproteinases (MMPs) expression by stromal [7] and endothelial cells [8] can be regulated by various soluble or cell-bound factors, such as extracellular Matrix Metalloproteinase Inducer (EMMPRIN, also known as CD147). CD147 has an abundant expression in malignant tumor tissues compared to normal tissues and may facilitate tumor metastasis by activating MMP production, modulating cell-substrate and adhesion processes [9–12]. Given the important function of CD147 in the progress of tumor tissues, some reports have demonstrated that it has some association with the outcome of breast cancer [13]. However, there are few reports that simultaneously investigate the clinicopathological features of the expression of CD147 in PCa. In the present study, we analyzed the association between CD147 and TNM grade, the depth of prostatic wall invasion, GLEASON Score, as well as histologic grade, so as to evaluate the clinical significance of this marker in the progression and the prognosis assessment of PCa.
Materials and Methods
Patients and Tissue Samples
Sixty-two fresh PCa tissues (aging 58 ~ 86 years, mean ± SD = 73.9 ± 12.1 years, TNM staging from I to III) and paired 30 BPH tissues were kindly provided by the Biological Research Center, Fourth Military Medical University. All of the tissues were obtained immediately during the operation of transurethral resection prostate and suprapubic prostatectomy.
None of the patients recruited in this study had chemotherapy or radiotherapy before the surgery. All of them received resection of PCa during March 6th 2002 to June 18th 2005 in the Department of Urinary Surgery, Xijing Hospital, Fourth Military Medical University, China. The pathological diagnosis was performed preoperatively and confirmed postoperatively. All patients were reviewed and all specimens were re-examined in March, 2007. TNM grade, the depth of prostatic wall invasion, GLEASON Score and histological grade were examined by the same group of two senior pathologists experienced in PCa diagnosis. All patients were given a 5 year follow-up. For the analysis of survival and follow-up, the date of prostatectomy was used to represent the beginning of the follow-up period. All of the patients whose cause of death was not related with PCa were excluded when we collected investigative cases. The study was approved by the Research Ethics Committee of the Fourth Military Medical University, China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Reagents
Mouse anti-CD147 monoclonal antibody, which recognizes the 50–60 kDa CD147 antigen, and its isotype-matched control monoclonal antibody (MT8) were both collected by the Biological Research Center, Fourth Military Medical University. CD147 antibodies reacted specifically with human CD147, and reactivity has been further confirmed with human breast BT-20 and colon cancer SW480 cell lines as specified by the manufacturers. UltraSensitive™ SP kit used for immunostaining was offered by Fuzhou Maixin Biotechnology Inc. Ltd.
Immunohistochemistry Analysis
The specimens were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. The paraffin-embedded tissues were cut at 3 μm and then deparaffinized with xylene and rehydrated for further H&E or peroxidase (DAB) immunohistochemistry staining employing DAKO EnVision System (Dako Diagnostics, Zug, Switzerland). Following a simple proteolytic digestion and a peroxidase blocking, the tissue slides were incubated with the primary antibody against respective target protein at a dilution of 1:1,000 overnight at 4°C. After washing, peroxidase labeled polymer and substrate-chromogen were then employed in order to visualize the staining of the interested proteins.
Following a hematoxylin counterstaining, the immunostaining was scored by two independent experienced pathologists, who were blinded to the clinicopathological data and clinical outcomes of the patients. The scores of the two pathologists were compared and any discrepant scores were trained by re-examining the staining by both pathologists to achieve a consensus score. The number of positive-staining tumor cells showing immunoreactivity on the cell membranes and cytoplasm in ten representative microscopic fields was counted and the percentage of positive cells was calculated. The immunostaining was assessed for staining intensity (grades 0 ∼ 3) using light microscopy. The criteria used for assessment were as previously reported [11], where: 0 (negative, <5%); 1 (weak, 6–25%); 2 (moderate, 26–50%); 3 (strong, >51%) of the tumor cells stained.
Statistical Analysis
The software of SPSS version13.0 for Windows (SPSS Inc, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) was used for statistical analysis. Continuous variables were expressed as \(\overline X \pm s\). Statistical analysis was performed with Fisher’s exact test for any 2×2 tables, Pearson χ2 test for non—2×2 tables, chi-square trend test (the Log Rank, Mantel–Cox) for ordinal datum, Kaplan–Meier and Cox Regression methods for the question of survival analysis. Differences were considered statistically significant when p was less than 0.05.
Results
Expression and Location of CD147 in PCa Tissues
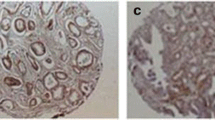
Heterogeneous areas of CD147 immunostaining were found in the 62 specimens of PCa and 30 specimens of BPH as shown in respecitive image (Fig. 1). CD147 was strongly expressed on the membrane and partly in the cytoplasm of PCa cells. It was positively, although, weakly stained on a few stromal cells. Scattered areas of weak heterogeneous epithelial cell membrane CD147 immunostaining were observed in 4/30 (13.3%) BPH specimens (Fig. 1b) whilst 51/62 (82.3%) PCa tissues were positive for CD147(score 1 ∼ 3). For CD147 positive primary PCa sections, heterogeneous weak and moderate (score 1 ∼ 2) and strong staining (score 3) were found in 32/62 (51.6%) and 19/62 (30.7%), respectively (Table 1). No regional heterogeneity has been observed within and between individual glands, and between central and infiltrative zones of the tumor.
Immunohistochemical staining for CD147 in PCa (Original magnification×400). a~b, CD147 negative staining in normal prostate tissue and BPH tissue; c, isotype controls of CD147 negative staining in PCa tissues; d~f, CD147 low, intermediate, high positive expression in PCa tissues (GLEASON scores < 7, < 7 and ≥7, respectively) were found in cell membrane and cytoplasm at various levels in PCa tissues respectively. The arrow indicates the positive area in the tumor tissues
Association Between CD147 Expression and the Clinicopathological Characteristics of PCa
The association between CD147 expression and the clinicopathological features of PCa patients is shown in Table 2. A positive expression of CD147 tended to be associated with the depth of prostatic wall invasion (p = 0.008), GLEASON Score (p = 0.001), and histologic grade (p < 0.001). The incidence of CD147 positive expression was significantly higher in stages IIb ∼ III than in stages I ∼ IIa (p < 0.001).
Prognostic Implications of CD147 Expression in PCa
The association between the 5 year survival rate of PCa patients and the expression of CD147 was analyzed using Kaplan–Meier method. According to the expression of CD147, the patients were categorized into three groups: 0, 1 ~ 2 and 3. The Chi-square value by Log Rank (Mantel–Cox) indicated a significant difference among different groups with regard to the expression status of CD147 (Table 3) (p < 0.01). The results by pairwise comparisons showed that there is a significant difference in survival rates between patients with CD147 stronger positive expression (grade 3) and any of the two other groups (grades 1 ~ 2) (p < 0.01). In all three groups, patients with CD147 stronger positive expression had the poorest prognosis. Using Cox regression analysis on the 62 patients, the expression of CD147 seemed to be an independent prognostic indicator (Fig. 2).
Kaplan–Meier survival curves for CD147 expression in PCa. a, categorized by negative CD147 expression (11 cases); b, categorized by weak~moderate positive CD147 expression (23 cases); c, categorized by stronger positive CD147 expression (28 cases). Survival was significantly poor for patients with stronger positive CD147 expression than those with negative expression (p < 0.01)
Discussion
PCa is the most commonly occurring cancer in men and advanced metastatic PCa is currently incurable. It has been found that 5 year survival rates for PCa patients were 58.6% on average and the 10 year survival rates for I–III grade patients with PCa were 77.4%, 75.3%, 26.5%, respectively [14]. Because of the limitations in efficient detection techniques, clinicians cannot determine which patients should be treated immediately. Given these reasons, to find the molecular markers associated with the progression and prognosis of PCa is a great challenge to clinicians and basic scientists.
To our knowledge, PSA is the most common indicator used for PCa diagnosis, but its specificity to PCa tissues is not very high. Some benign lesion, such as prostatitis, BPH also can lead to elevated PSA. In addition, PSA is not capable of identifying metastasis and prognosis. Fortunately, previous studies have demonstrated that CD147 can potentially serve as a new target for anti-tumor therapy [15]. They showed that the CD147 expression could frequently be detected in the vast majority of human malignancies as well as in a subset of benign tumors. Nevertheless, there are significant differences both in the intensity and distribution of CD147 staining among different malignant tumors as well as benign lesions [16–18]. CD147 expressed on the tumor cell surface and stimulates nearby fibroblasts and endothelial cells [19–21]. CD147 has been indicated to have characteristics of an adhesive molecule and be able to mediate cell-to-cell or cell-to-matrix adhesion [22–25]. Some other studies [26–29] showed that the molecule facilitates the production of MMPs from fibroblasts and tumor matrix cells, the latter can almost degrade all components in basal membranes and extracellular matrixes. MMPs may also participate in late events during cancer metastatic spread, when cancer cells enter, survive and exit blood vessels or lymphatics. CD147 also can affect expressions of vascular endothelial growth factor (VEGF) at RNA and protein level, as well as promote the growth of the tumor vascular system [30, 31]. Moreover, CD147 is a multifunctional protein whose functional diversity may relate to distinct post-translational modifications. Differential modification through glycosylation may be cell-type specific or associated with malignancy [32].
Since CD147 expression and biological role in primary PCa and in the metastatic microenvironment has not been fully investigated, in the present study, the clinicopathological significance of CD147 in PCa was evaluated. Our data indicated that the positive expression rate of CD147 in PCa tissue (82.3%) was significantly higher (p < 0.05) than that in BPH tissue, only 13.3% of which showed as a weak positive. In tumor clinics and pathology analysis, patients with outer cyst invasion and high GLEASON score (>7 points) have higher rates of CD147 positive expression than those with inner cyst tumor and low GLEASON score (<7 points). With the increment of TNM stage and histological grade, positive rate, especially stronger positive expression rate of CD147 in patients also increased, which indicated that CD147 expression is positively associated with PCa invasion, metastasis and the level of malignance. CD147 detection can improve the determination of the biological characteristics of PCa tissues and indirectly reflect the prognosis of patients. The prognosis of CD147-positive patients is found to be significantly worse than that of CD147-negative patients in this study. The positive association between CD147 expression and the invasive depth, TNM stage, GLEASON score and histological grading of tumor which were found in this study, may explain the poor prognosis role of CD147. Therefore, CD147 could overcome the weakness of inadequate specificity of PSA, so as to enhance the diagnosis efficiency, characterize the clinical progression and biological properties for clinicians, and improve the efficiency to predict the prognosis of PCa.
In conclusion, CD147 expression may play an important role in the progression of PCa. A detection of it may benefit us in the prediction of the prognosis of PCa. In the near future, we may extend the follow up period in order to determine the prognosis effect of PCa more exactly.
References
Schrgder FH, Wildhagen MF, ERSPC (2001) Screening for prostate cancer: evidence and perspectives. BJU Int 88:811–817
Holmberg L, Bill-Axelson A, Helgesen F (2002) A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Eng J Med 347:781–789
Hochreiter WW (2008) The issue of prostate cancer evaluation in men with elevated prostate-specific antigen and chronic prostatitis. Andrologia 40:130–133
Loeb S, Catalona WJ (2008) What to do with an abnormal PSA test. Oncologist 13:299–305
Kehinde EO, Maghrebi MA, Anim JT (2008) The importance of determining the aggressiveness of prostate cancer using serum and tissue molecular markers. Can J Urol 15:3967–3974
Wang L, Wu G, Yu L, Yuan J, Fang F, Zhai Z et al (2006) Inhibition of CD147 expression reduces tumor cell invasion in human prostate cancer cell line via rna interference. Cancer Biology & Therapy 5:608–614
Gabison EE, Mourah S, Steinfels E et al (2005) Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas. Am. J. Pathol 166:209–219
Li Y, Shang P, Qian A et al (2003) Inhibitory effects of antisense RNA of HAb18G/CD147 on invasion of hepatocellular carcinoma cells in vitro. World J Gastroente 9:2174–2177
Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C (2004) The microvesicle as a vehicle for EMMPRIN in tumorstromal interactions. Oncogene 23:956–963
Millimaggi D, Mari M, D’Ascenzo S, Carosa E, Jannini EA, Zucker S et al (2007) Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 9:349–357
Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M (2006) Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 56:359–367
London CA, Sekhon HS, Arora V, Stein DA, Iversen PL, Devi GR (2003) A novel antisense inhibitor of MMP-9 attenuates angiogenesis, human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther 10:823–832
Mi Z, Oliver T, Guo H, Gao C, Kuo PC (2007) Thrombin-cleaved COOH (-) terminal osteopontin peptide binds with cyclophilin C to CD147 in murine breast cancer cells. Cancer Res 67:4088–4097
Kakehi Y (2003) Watchful waiting as a treatment option for localized prostate cancer in the PSA era. Jpn J Clin oncol. 33:1–5
Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM et al (2007) Up-regulation of CD147 and matrix metalloproteinase-2, -9 induced by P-glycoprotein substrates in multidrug resistant breast cancer cells. Cancer Sci 98:1767–1774
Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G et al (2006) High incidence of EMMPRIN expression in human tumors. Int J Cancer 119:1800–1810
Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H et al (1995) The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 55:434–439
Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S et al (2002) EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis 19:697–702
Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P et al (2005) Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 65:3193–3199
Biswas C (1982) Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun 109:1026–1034
Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ et al (2005) MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis 22:565–573
Sun J, Hemler ME (2001) Regulation of MMP-1 and MMP-2production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer research 61:2276–2281
Marieb EA, Zoltan-Jones A, Li R (2004) Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Research. 64:1229–1232
Li HG, Xie DR, Shen XM (2005) Clinicopathological significance of expression of paxillin, syndecan-l and EMMPRIN in hepatocellular carcinoma. World J Gastroenterol 11:1445–1451
Lanyi M, Antonescu CR (2002) The precrystalline cytoplasmic granules of alveolar soft part sarcoma contain monocarboxylate transporter 1 and CD147. Am J Pathol 160:1215–1221
Seitz C, Djavan B (2006) Biological markers of prostate cancer. Ann Urol (Paris). 40:329–335
Zucker S, Hymowitz M, Rollo EE et al (2001) Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol 158:1921–1928
Tang Y, Kesavan P, Marian T (2004) Tumor-stroma interaction:positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expressio and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res 2:73–80
Kanekura T, Chen X, Kanzaki T (2002) Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer 99:520–528
Jia L, Wang H, Qu S, Miao X, Zhang J (2008) CD147 regulates vascular endothelial growth factor-expression, tumorigenicity, and chemosensitivity to curcumin in hepatocellular carcinoma. IUBMB Life 60:57–63
Tang Y, Nakada MT, Rafferty P, McCabe FL, Millar H, Cunningham M et al (2006) Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res 4:371–377
Madigan MC, Kingsley EA, Cozzi PJ, Delprado WJ, Russell PJ, Li Y (2008) The role of extracellular matrix metalloproteinase inducer protein in prostate cancer progression. Cancer Immunol Immunother 57(9):1367–1379 doi:10.1007/s00262-008-0473-x
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Guangdong Province (No.04003650) and the Key Programs of Science and Technology of Guangzhou city (No. 200323 – E4053) and National High Technology Research and Development Project of China (No.2006AA02A245).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhao-dong Han, Xue-cheng Bi, and Wei-jun Qin contributed equally to this article.
Rights and permissions
About this article
Cite this article
Han, Zd., Bi, Xc., Qin, Wj. et al. CD147 Expression Indicates Unfavourable Prognosis in Prostate Cancer. Pathol. Oncol. Res. 15, 369–374 (2009). https://doi.org/10.1007/s12253-008-9131-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-008-9131-z