Abstract
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is overexpressed in advanced stage prostate adenocarcinomas. As a novel target for in vivo prognostic and therapeutic approaches, the distribution pattern of PSMA in primary and metastatic tumors is of significant interest. In this study we addressed the cellular distribution and heterogeneity of PSMA expression. Paraffin-embedded sections of 51 patients with primary prostate carcinoma and distant metastases were evaluated. Immunohistochemistry was used to determine the cellular localization, staining intensity and positive cell fraction which were related to tumor type and growth pattern. We demonstrated differences in the intracellular localization of the PSMA immunostaining which seem to be related to the tumor differentiation pattern. A significant number of the primary tumors (7/51) and metastases (6/51) presented with highly heterogeneous PSMA expression and in further 2 primary, and 8 metastatic tumors the staining was in the negative range (<10% positive tumor cells). A direct correlation between histological parameters and PSMA expression could not be demonstrated. Our findings clearly support the feasibility but also direct to potential failures of PSMA-targeted in vivo diagnostic and therapeutic approaches in prostate cancer patients with distant metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate-specific membrane antigen (PSMA) is a type II membrane protein with enzymatic functions, acting as a glutamate-preferring carboxypeptidase in human prostate tissue, which plays a role in folic acid utilization and metabolism [1, 2].
Although PSMA is also present in benign prostate epithelium it is highly overexpressed in the carcinomas of the prostate [3, 4], which is also reflected in elevated blood serum levels. An increase in expression was also reported in patients with hematogenous micrometastases of prostate carcinoma [5, 6].
In addition to the prostate, PSMA expression has been reported in several benign changes as well as in various kinds of other cancers, e.g. renal, colon and breast carcinomas [5, 7]. In many types of carcinomas a strong PSMA expression was also seen in the newly formed vessels resembling tumor related angiogenesis [1, 8].
The PSMA protein has intracellular, transmembrane and extracellular domains [1], while PSM’—a splice variant of the protein—remains intracellular. Both variants are recognized by commercially available highly specific monoclonal antibodies, e.g. 7E11 or 3E6 [1, 5] which are applicable for immunohistochemistry. The in vivo antibody binding of surface PSMA gives a promising opportunity for targeted imaging and therapeutic applications. The anti-PSMA clone 7E11 was recently introduced as a radiodiagnostic compound [9] and the further use of antibodies as immunotherapeutic compounds in prostate cancer is under investigation.
The aim of our study was to examine and compare the cellular localization and variability of PSMA expression as revealed by immunohistochemistry in primary prostate carcinomas and corresponding distant metastases from the same patient. Information on target heterogeneity and cellular distribution should help to identify factors that may interfere with the clinical utilization of PSMA based approaches.
Materials and Methods
Immunohistochemistry
During the time period from 1986 to 2007 a total of 51 cases were identified with both primary prostate adenocarcinomas and matched distant metastases in the archive of the Institute of Pathology, Medical University of Graz, Austria. Cases with only regional lymph node metastases were excluded. The mean age of the patients was 70 years (range 53–87 years). The primary prostate cancer samples included prostate core biopsies from 22 patients, the number of evaluated core biopsies ranging between 4 and 12 cylinders per patient. Transurethrally resected (TUR) material was evaluated from 29 patients.
Surgically removed distant metastases were obtained from the following sites: 31 from the skeletal system (17 spinal column, 13 femur, 1 humerus), 9 from the abdominal cavity (2 colon, 2 liver, 5 peritoneum), 3 from the central nervous system, 2 from the skin, 2 from the penis, 1 from the testis and 3 distant lymph node metastases were included.
All specimens were fixed in buffered 5% formaldehyde solution and embedded in paraffin according to standard operational procedures. Bone biopsy material was decalcified with EDTA. From the whole material 4 μm thick sections were routinely stained with hematoxylin and eosin and evaluated by two pathologists for the best representative material displaying areas rich in cancer cells. Simultaneously, each case was reclassified for Gleason score [10, 11] by the use of uniform criteria. Immunohistochemistry was performed according to our routine protocol. Briefly, representative sections were immunostained manually following antigen retrieval in 0.01 M Na-citrate buffer at pH 6.0 (95°C). The samples were incubated with the PSMA primary monoclonal antibody (clone 3E6, DAKO, Denmark, cat.no. M3620, dilution of 1:100). Specific binding was detected by the immunoperoxydase/DAB based detection kit EnVision (DAKO, cat. no. K5007). For control purposes slides were treated with isotype matched (IgG1) non-specific antibody under the same conditions.
All prostate carcinomas and/or the distant metastases which were negative for PSMA expression were additionally controlled by immunohistochemical staining for prostate specific antigen (PSA; DAKO, cat. no. A0562, working dilution 1:600).
Evaluation and Interpretation of IHC Staining
Microscopic evaluation included the semiquantitative determination of positive cell fraction, staining intensity, intratumoral variability (heterogeneity) and cellular localization. For this purpose large confluent areas were preferred, in case of core biopsies at least 1000 cells per section could be determined. All mentioned parameters were compared between the primary tumors and corresponding metastases as well as the Gleason score of the primary tumor.
Estimation of the PSMA-positive cell fraction was calculated by considering only the tumor cells of the sample and the analysis relied on the consensus of two independent histopathologists (S.M. and G. M.). PSMA positivity was classified according to the percentage of all positive tumor cells as negative (0–10%), low (positivity of 10–40%), moderate (positivity of 40–70%) and high (positivity of >70%).
The intensity of the IHC staining was estimated by using a 4 grade scoring system with intensity from 0 to 3, which reminds 0 (no staining), 1 (low), 2 (moderate), 3 (high) intensity.
Intracellular localization of the staining was determined and patterns of apical surface, cell membrane or cytoplasmic staining were documented.
For the evaluation and comparison of data groups standard statistical analysis was performed using the Fisher’s exact test for categorical data and Student’s T-test for continuous data [12]. The analysis was further supported with histograms showing the samples frequency of different categorical values and multivariable scatter plots revealing dependencies between particular stainings of PSMA expression. Tables 1 and 2 values represent arithmetical means and standard deviations.
Results
PSMA positivity was evident by immunohistochemistry in the great majority of the evaluated prostate carcinomas and matched metastatic tumors. The distribution of estimated positive fractions is presented in Fig. 1. A negative PSMA staining (less than 10% positive tumor cells) was observed in 2/51 primary tumors and in 8/51 metastases (Table 1). Only a single case was completely negative for PSMA in both the primary and metastatic tissue (1.9%) (Fig. 2a,b). The frequency of tumor cell positivity was found to be low in 7, moderate in 23 and high in 19 cases in primary tumors and 6, 16, and 21 in the metastases, respectively.
Scatter plot showing PSMA expression in percentage for 51 primary prostate cancers (x Axis) vs. distant metastases from the same patients (y Axis). Cut-off for PSMA positivity was determined at 10% and high expression at more than 80% of tumor cells. Values were highly variable and no statistical correlation between the two parameters could be stated
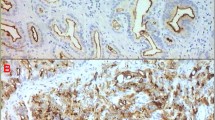
Microscopic appearance of PSMA immunohistochemistry in carcinomas of the prostate. a, c, e represent IHC stainings of the primary prostate carcinoma while b, d and f show metastases of the same patient. a and b were interpreted as PSMA negative. c and d show typical apical staining pattern. e and f are two examples for undifferentiated carcinomas with heterogeneous PSMA staining occurring predominantly in the cytoplasm of the tumor cells
There was no correlation between the rate of tumor cell positivity and the intensity of the IHC staining. As part of this heterogeneity larger cell groups completely lacking PSMA-related staining could be identified in the neighbourhood of the most intensively staining tumor cells. The strongest PSMA expression was seen at the luminal-apical surface of glandular structures in both primary and metastatic samples in tumors with glandular differentiation. The rest of the cell membrane usually showed a clear but moderate staining intensity. In several cases a cytoplasmic reaction dominated with similar intensity or covered the staining at the membrane surfaces by large. The overall intensity of the IHC staining was determined semi-quantitatively on a 4 grade scale (0/3+). The staining intensity was observed as low (1+) in 4 primary tumors and in 5 metastases, moderate (+2) in 12 and 13 and high (3+) in 33 and 28 in the PSMA positive cases, respectively.
Tumors with glandular/microglandular differentiation typically showed a partial membrane staining becoming confluent at the apical-luminal part of the glandular surface of membranes consistent with cell polarity (Fig. 2c,d). This pattern was observed in 27/51 primary tumors and in 25/51 (49.01%) metastases. Tumors with predominantly solid growth pattern and lacking glandular differentiation or cases with non-cohesive invasive morphology presented with intense diffuse cytoplasmic or mixed cytoplasmic and membranous staining (Fig. 2e,f). The cellular localization of the confirmed positive PSMA cases is denoted in Table 1.
PSMA related parameters were further evaluated in the context of the differentiation level by the use of the Gleason score of the primary tumors. A tendency but no statistical correlation of increased PSMA expression could be observed in regard to Gleason score in both primary (p > 0.1) and metastatic (p > 0.1) samples (Table 2).
Discussion
Overexpression of PSMA in the cell membrane of prostate cancer cells provides an optimal target for therapy and diagnosis. In vivo radioscintigraphic imaging using PSMA antibodies is a promising alternative for the demonstration of tumor spreading and metastatic activity [13, 14]. New PSMA-based therapeutic approaches have also been proposed [15]. However, the efficacy of these approaches highly depends on a homogenous and tumor cell-selective membrane expression of this molecule. Any variation (negativity, heterogeneity of expression, lack of membrane localization) may significantly limit the access of the therapeutic agent to the target cells resulting in therapy failure.
Our present study confirmed the frequent expression and the target potential of PSMA in advanced prostate carcinomas. In an earlier report by Ross et al. [16], about 50% of the evaluated primary tumors showed PSMA overexpression, which was associated with a mean Gleason score of 6.33 ± 1.21 indicating relatively well differentiated early stage prostate carcinomas [16]. This value was found to be much higher for the metastatic carcinomas evaluated here, which was anticipated from the selection of more advanced and also less differentiated cases (mean Gleason score 7.96 ± 1.17). In accordance with the advanced prostate cancer cohort 96.08% of the primary tumors and 84.32% of metastases showed expression of PSMA.
The major observation of our study was, that highly PSMA positive cases of any Gleason score also frequently contained larger areas with PSMA-negative cells, a feature independent of the metastatic phenotype. Cases with high amounts of PSMA negative tumor cell fractions made up the majority of the evaluated cohort. Negative or ambigious samples were all carefully revaluated and proved to be PSA positive by immunohistochemistry providing further evidence for their prostate origin. The fact, that primary tumor and metastasis were uniformly negative in only a single case suggests that completely PSMA negative prostate carcinomas are rare. Local biological factors and the tumor cell microenvironment, on the other hand, may significantly contribute to the up- and downregulation of PSMA resulting in tumor heterogeneity. Finally, a virtual negativity in highly heterogenous tumors may eventually occur by the sampling of a region with repressed PSMA (e.g. in prostate core biopsies).
Clinical anti-PSMA approaches rely on the specific binding of the antibody to the target located to the cell surface [17], therefore, the cellular distribution of positivity is of major impact. Cell surface positivity was observed in the majority of the evaluated samples, most of them presenting with predominant luminal positivity if glandular differentiation was present. However, cytoplasmic staining without evident membrane positivity was also observed in a relevant part of the evaluated tumor samples. Previous studies described cytoplasmic PSMA as a splice variant (PSM`) which lost its ability to be integrated in the lipid bilayer as a transmembrane protein [17]. The biological relevance of this variant is not yet known. We interpret, that cytoplasmic PSMA positivity presented in our cases represent the overexpression of the PSM’ splice variant in prostate cancer. This kind of overexpression may have a clinical impact as this PSMA splice variant will not be accessible for antibodies in vivo despite the immunohistochemical positivity. Therefore, cytoplasmic PSMA positivity should be considered equal to PSMA negativity in future IHC based studies.
In summary, total or partial PSMA negativity as well as cytoplasmic positivity could be demonstrated in both primary tumors and distant metastases, without correlations to further histological or anatomical features (Gleason score, histological subtype, localization of metastases). Similar to the demonstration of target expression in several well established therapeutic approaches (e.g. the anti-HER-2 therapy) the determination of PSMA by immunohistochemistry in individual prostate carcinomas can provide information regarding indication and pitfalls of PSMA-based anticancer treatment, i.e. recombinant anti-PSMA antibody therapy [18].
Abbreviations
- A:
-
Apical
- AB:
-
Antibody
- C:
-
Cytoplasmic
- GM:
-
Gabor Mehes
- IHC:
-
Immunohistochemistry
- M:
-
Membrane
- PSMA:
-
Prostate-Specific Membrane Antigen
- PSA:
-
Prostate Specific Antigen
- SD:
-
Standard Deviation
- SM:
-
Sebastian Mannweiler
- TUR:
-
Transurethrally Resected
References
Chang SS (2004) Overview of prostate-specific membrane antigen. Rev Urol 6:S13–S16
Pinto JT, Suffoletto BP, Berzin TM et al (1996) Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res 2:1445–1451
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP (1998) Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 82:2256–2261
Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, Hautmann RE, Gschwendt JE, Kuefer R, Rubin MA (2007) Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol 38:696–701
Horoszewicz JS, Kawinski E, Murphy GP (1987) Monoclonal antibodies to a new antigenic marker in epithelial cells and serum of prostatic cancer patients. Anticancer Res 7:927–936
Moreno JG, Croce CM, Fischer R et al (1992) Detection of hematogenous micrometastases in patients with prostate cancer. Cancer Res 52:6110–6112
Silver DA, Pellicer I, Fair WL et al (1997) Prostate-specific membrane antigen expression in normal and malignant human tissue. Clin Cancer Res 3:81–85
Chang S, O’Keefe DS, Bacich DJ, Reuter VE, Heston WD Gaudin PB (1999) Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res 5:2674–2681
Wright GL, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF, Moriarty R (1996) Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 48:326–334
Gleason DF (1977) Histologic grading and clinical staging of prostatic carcinoma. In: Tannebaum M (ed) Urologic pathology: the prostate. Lea & Febiger, Philadelphia, pp 171–197
Lopez-Beltran A, Mikuz G, Luque RJ, Mazzucchelli R, Montironi R (2006) Current practice of Gleason grading of prostate carcinoma. Virchows Arch 448(2):111–118
Douglas G. Altman: Practical Statistics for Medical Research Chapman & Hall/CRC 1 edition (November 22, 1990)
Bander NH (2006) Technology insight: monoclonal antibody imaging of prostate cancer. Nat Clin Pract Urol 3(4):216–225
Sodee DB, Sodee AE, Bakale G (2007) Synergistic value of single-photon emission computed tomography/computed tomography fusion to radioimmunoscintigraphic imaging of prostate cancer. Semin Nucl Med 37(1):17–28
Elsässer-Beile U, Wolf P, Gierschner D, Bühler P, Schultze-Seemann W, Wetterauer U (2006) A new generation of monoclonal and recombinant antibodies against cell-adherent prostate specific membrane antigen for diagnostic and therapeutic targeting of prostate cancer. Prostate 66(13):1359–1370
Ross JS, Sheehan CHE, Fisher HAG, Kaufman RP, Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BVS (2003) Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 9:6357–6362
Zhigang Z, Wenly S (2004) Prostate stem cell antigen (PSCA) expression in human prostate cancer tissue and its potential role in prostate carcinogenesis and progression of prostate cancer. W J Surg Oncol 2:1–13
Bühler P, Wolf P, Gierschner D, Schabel I, Katzenwadel A, Schultze-Seemann W, Wetterauer U, Tacke M, Swamy M, Schamel WW, Elsässer-Beile U (2008) A bispecific diabody directed against prostate-specific membrane antigen and CD33 induces T-cell mediated lysis of prostate cancer cells. Can Immunol Immunother 57(1):43–52
Acknowledgments
The authors thank Elisabeth Patz and Anja Hausleitner for their excellent technical assistance and Harald Zöbl for the graphical layout and design.
Legal declaration
IRB approval formally obtained according to the votum 12-159 ex 01/02 at Medical University Graz, Austria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mannweiler, S., Amersdorfer, P., Trajanoski, S. et al. Heterogeneity of Prostate-Specific Membrane Antigen (PSMA) Expression in Prostate Carcinoma with Distant Metastasis. Pathol. Oncol. Res. 15, 167–172 (2009). https://doi.org/10.1007/s12253-008-9104-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-008-9104-2