Abstract
Hantavirus infection is a global health challenge, causing widespread public concern. In recent years, cases of hantavirus infection in pregnant women have been reported in many countries. The infected pregnant women and their fetuses appear to have more severe clinical symptoms and worse clinical outcomes. Hence, to study the prevalence of hantavirus infection in pregnant women, this study will focus on the epidemiological distribution of the virus, different virus species penetrating the placental barrier, and factors affecting the incidence and clinical outcome of the infection in pregnant women and their fetuses. In addition, this review will also discuss the diagnostic tools and treatments for pregnant patients and provide an overview of the relevant future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hantaviruses are negative-sense, single-stranded, enveloped RNA viruses belonging to the family Hantaviridae. Since the first hantavirus was isolated in 1978, many countries and regions have reported a series of hantavirus infections continuously (Lee et al. 1978). Hantaviruses cause two clinical syndromes in humans; designated hemorrhagic fever with renal syndrome (HFRS), and hantavirus cardiopulmonary syndrome (HPS). HFRS mostly occur in Eurasian countries, caused by Hantaan virus (HTNV), Seoul virus (SEOV), Puumala virus (PUUV) and Dobrava virus (DOBV) (Avsic-Zupanc et al. 2019). The clinical features of HFRS are severe systemic manifestations, including fever, hemorrhage and acute renal failure. HPS is mainly a group of syndromes with respiratory system involvement, caused by Sin Nombre virus (SNV), Andes virus, and other viruses. The leading cause of death in HPS is acute progressive non-cardiogenic pulmonary edema and respiratory failure (Peters et al. 1999).
Hantaviruses are zoonotic viruses that can be carried by small rodents. Approximately 20,000 cases of hantavirus-related diseases occur worldwide each year (Jiang et al. 2017). Furthermore, there is a clear indication that the incidence rate increases every year (Jiang et al. 2017; Watson et al. 2014). The mortality rates reported are 12% for HFRS and 60% for HPS (Zhang et al. 2010; Jonsson et al. 2010). Pregnant women are afflicted by inhalation of host secretions and excretions carrying the virus, or by exposure to rodent carriers (Pedrosa and Cardoso 2011). However, the cases of women infected with hantavirus during pregnancy are rarely reported even in areas where hantaviruses are concentrated, which poses certain difficulties for subsequent research and clinical management. Here, in order to contribute to the diagnosis, treatment, and prognosis prediction, we reviewed past cases of hantavirus infections in pregnant women.
Hantavirus Infection during Pregnancy
Global Distribution of Hantavirus Infection during Pregnancy
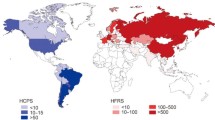
As reported in the existing literature, pregnant women with hantavirus infection mainly live in areas where hantaviruses are prevalent. Almost every pregnant woman infected with hantaviruses during pregnancy has a clear history of exposure to the epidemic area (Fig. 1). Therefore, the epidemiological distribution primarily relies on the rodent host distribution, and it is influenced by factors such as the climate, environment, and food availability, which can make up a unique rodent host-hantavirus system. The rodent hosts of principal disease-causing hantaviruses are Apodemus agrarius (HTNV) in Asia, Myodes glareolus (PUUV) in Europe, and Peromyscus maniculatus (SNV) in the Americas (Watson et al. 2014; Kariwa et al. 2007; Vaheri et al. 2013).
Incidence and Clinical Outcomes of Different Hantavirus Species during Pregnancy
Cases of pregnant women infected by HTNV and SEOV hantaviruses are more than that infected by other hantavirus species (Fig. 1, Table 1). This is related to the high incidence rate and large population in the areas where these two virus species are primarily distributed. China and South Korea are the top two hantavirus infection areas (Kariwa 2017), and this is consistent with the distribution of their rodent hosts, which is also in line with the final findings of this review.
Hantavirus infection with different species during pregnancy also has different results for pregnant women. As observed from our statistical review, the mortality rate of pregnant women infected with HTNV/SEOV was 10.5% (27/256) (Table 1), and that of those infected with SNV was 25.0% (2/8) (Table 1). From the data we collected, the mortality rate of pregnant women infected with HTNV was not significantly different from that of the general population, while that of those infected with SNV was lower than that of the general population (Zhang et al. 2010; Jonsson et al. 2010). We speculate that the sample size may primarily limit this. Moreover, after being infected by a hantavirus, the residual sequelae, which mainly consist of menstrual disorders, amenorrhea, no milk secretion after delivery, sexual dysfunction, hair loss, autonomic dysfunction and chronic renal insufficiency, were only seen with HTNV/SEOV infections (Table 1). Furthermore, no deaths have been reported in pregnant women infected with PUUV or DOBV (Hofmann et al. 2012; Ji et al. 2017; Partanen et al. 1993). However, PUUV and DOBV cannot be ruled out due to the limited number of cases (Table 1).
The clinical outcome for fetuses is also significantly different. Since the death of the mother causes the death of the fetus in most cases, this review will only discuss the cases where the mother survived the hantavirus infection. According to the available data, fetal deaths were only reported in HTNV/SEOV and SNV infections (Table 2). The mortality rate of fetuses delivered by pregnant women with HTNV/SEOV infections was 31.8% (71/223) (Table 2), and that of those delivered by pregnant women with SNV was 33.3% (2/6) (Table 2). Also, several sequelae, including congenital heart disease, necrotizing enteritis, restricted growth and development, as well as hydrocephalus, troubled these babies (Table 2). The incidence rate of sequelae in the babies, whose mothers had been infected with HTNV/SEOV and SNV was 3.6% (8/223) and 16.7% (1/6), respectively (Table 2).
Placental Passability and Hantavirus Species
There were no reports of fetal malformations in pregnant women with a hantavirus infection, though many vertically transmitted viral infections are likely to cause fetal malformations (Seferovic et al. 2018; Silasi et al. 2015).
Currently, there is no evidence that PUUV, DOBV and SNV can be transmitted through the placenta (Hofmann et al. 2012; Partanen et al. 1993; Howard et al. 1999). Partanen et al. (1990) presented a case, where a 29 year-old woman at 17 weeks gestation had suffered from acute abdominal pain with high fever and myalgia. As a result, she had been diagnosed with PUUV infection. After treatment, the woman had recovered within 4 weeks and delivered a healthy boy naturally. Besides, the postoperative serological testing showed no evidence of placental transmission. However, few cases of fetal death caused by PUUV infection were also reported (Partanen et al. 1993; Silberberg et al. 1993; Prebensen 1997; Tiilikainen and Jouppila 1989). Hofmann et al. (2012) conducted an immunological and molecular analysis of hantaviruses in the cord blood of four pregnant women, who had been infected with PUUV or DOBV. The results also showed no evidence of virus transmission through the placenta. Howard et al. (1999) reviewed five cases of HPS during pregnancy, and as expected, there was no evidence of vertical transmission, though SNV infections mostly had severe consequences, even death, in pregnant women and fetuses.
Therefore, it could be speculated that HTNV and SEOV pass through the placental barrier. Liu et al. (1987) suggested that there is a phenomenon of vertical transmission between the mother and the baby. Nonetheless, no evidence directly proves it. Lee (1989) detected the presence of IgM antibody against HTNV in the blood of fetuses, whose mothers had a HTNV infection, confirming the possibility of placental transmission. Kim et al. (1978) studied a pregnant woman, who had had a miscarriage due to hypotension after SEOV infection. After the autopsy of the fetus, researchers found bleeding throughout the body, including lungs, kidneys, and adrenal glands. This is in line with the symptoms of SEOV infection, but unfortunately, they did not perform further serological testing of the fetus (Kim et al. 1978). In addition Jing PT and Jing H (1994), isolated hantaviruses from fetal brain tissues of an aborted fetus. Unexpectedly, the results indicated that hantaviruses could pass not only the placental barrier but also the blood–brain barrier. However, it is a pity that the study failed to identify the species. Instead, it was based on the epidemiological analysis of the target area, which indicated that the virus was most likely SEOV. Most of the abortion and stillbirth cases in patients with HFRS occur when they have a fever, so further research is needed to clarify the specific mechanism (Chen 1994; Wang et al. 1992; Sha et al. 2000).
Influence of Age on the Clinical Outcome
The clinical outcomes of pregnant women with a hantavirus infection are also related to their age. The mortality rate of women aged 30 or older and infected with hantaviruses during pregnancy is 45.5% (5/11) (Table 3), while for women under 30, it is only 2.5% (1/40) (Table 3). Hjertqvist et al. (2010) found, in a study with 5,282 patients, that there is a significant correlation between mortality and the age of the hantavirus-infected patients. The mortality rates of pregnant women and others increase with age, and the trend remains consistent.
Incidence and Gestation
The incidence of hantavirus infection in pregnant women varies in different gestational periods. By reviewing the literature, we conducted a statistical analysis of the gestational age of women with a hantavirus infection during pregnancy. Among the reported cases of hantavirus-infected pregnant women, the incidence rate in the first trimester (< 13 weeks) was 7.7% (4/52) (Table 3), whereas that in the second trimester (≥ 13 weeks, < 28 weeks) and the third trimester (≥ 28 weeks) was 92.3% (48/52) (Table 3). Coincidentally, this phenomenon was also found in other placenta-transmissible viruses. Zhao (2017) conducted a controlled observation, including 288 cases of HPV-infected pregnant women at different stages of pregnancy, and found that the incidence of viral infection was the highest at the third stage. Moreover, having researched Epstein-Barr virus infection in pregnant women at different stages of pregnancy, Ming (2017) also reached the same conclusion.
Delivery Mode and the Outcome of the Fetus
Different mode of delivery may exert various influences on the transmission of the virus from mother to child. Lee (1989) detected hantaviruses in the serum of a vaginally delivered fetus. The fetus died within 12 h of delivery. However, there were no similar cases reported in fetuses born via cesarean section.
Diagnosis and Treatment
Early diagnosis is vital for pregnant women with a hantavirus infection since it leads to early and more effective treatment, as well as a better clinical outcome. Accurate diagnosis usually relies on clinical manifestations and laboratory tests. Exploring the history of exposure in the epidemic area is also essential for a precise diagnosis. The typical clinical manifestations of HFRS are fever, hemorrhage, hyperemia, hypotensive shock and kidney damage accompanied by clinical symptoms, such as hematuria, proteinuria, and disseminated intravascular coagulation (DIC) (Lazzerini et al. 2017). Similar to the general population, the typical clinical course of HFRS in pregnant women is divided into the following: Fever period (3–7 days), hypotensive period (hours to 2 days), oliguria (3–7 days), polyuria (several days to weeks), and recovery period (2–3 months) (Vaheri et al. 2013; Latus et al. 2015; Connolly-Andersen et al. 2014). The clinical symptoms of HPS include flu-like symptoms in the prodromal stage (5 days), subsequent acute respiratory distress syndrome (ARDS), bilateral diffuse interstitial pulmonary edema, respiratory failure, as well as non-cardiogenic shock (Duchin et al. 1994; Khan et al. 1996; Hallin et al. 1996; Macneil et al. 2011). Molecular, immunochemical, and serological examination contribute to routine laboratory tests (Zou et al. 2016). IgM/IgG antibodies against the Gn and Gc of hantaviruses are often used to aid the diagnosis (Hedman et al. 1991).
However, early diagnosis is not that simple and straightforward, although diagnostic criteria are clear. Misdiagnosis often happens because of inexperienced non-specialist clinicians, a limited number of cases, and atypical clinical manifestations. Misdiagnosis rate of pregnant patients with HFRS infection is as high as 50% according to several reports (Xia 2009; Yun 2007). Patients with HFRS that have mild or atypical presentations are most likely to be misdiagnosed, especially in the early period (Xia 2009; Yun 2007). Pregnant women with a hantavirus infection are often misdiagnosed with acute fatty liver of pregnancy (AFLP), hemolysis, and elevated liver enzymes and low platelets (HELLP), as they have similar clinical manifestations (Mace et al. 2013; Haram et al. 2009). Thus, accurate differential diagnosis is vital. A substantial reduction in non-selective proteinuria in a short period is conspicuous for HFRS, but rare for AFLP and HELLP (Clement et al. 2013).
Currently, there is no safe and effective treatment for pregnant women with hemorrhagic fever. Recently, new treatments have been developed, and satisfying medical results have been achieved. For example, pregnant women with a hantavirus infection during pregnancy can benefit from symptomatic treatment and supportive care, mainly including diuresis, hemodialysis, oxygen therapy, shock therapy, liver protection therapy and liquid therapy. However, increased cardiac load during pregnancy and pulmonary capillary damage caused by the hantavirus infection are more likely to cause ARDS and heart failure. Hence, it is essential to control the infusion rate during oliguria (Chen 1994). Furthermore, continuous renal replacement therapy (CRRT) plays a leading role, especially if hantavirus-infected pregnant patients display high blood volume and pulmonary edema (Ji et al. 2017). Last but not the least, glucocorticoids have strong anti-inflammatory effects and are widely used in patients with stubborn and unresolved renal failure. However, the question that needs to be studied further is whether glucocorticoids can improve the health of the patients (Kruger et al. 2015; Martinuč Bergoč et al. 2013).
In areas with high hantavirus prevalence, vaccination of women before pregnancy can effectively alleviate hantavirus infections (Dai 2004; Liu et al. 2013). However, for those who are already pregnant, the safety and effectiveness of vaccination have not been insightfully studied (Dai 2004; Liu et al. 2013).
Summary
Although there are not many cases of hantavirus infection reported in pregnant women, even in the endemic areas, clinicians are expected to thoroughly consider severe consequences when they receive patients with similar symptoms. Specific immune status of pregnant women might increase their susceptibility to hantavirus infection. Pregnant women have unique immunology and physiology, and their immune system can develop a particular immunosuppressive state by suppressing cellular immunity to tolerate fetal antigens of paternal origin (Beigi 2017; Jamieson et al. 2006). However, maintenance of this specific immune state during pregnancy has not been thoroughly explored. Gaunt and Ramin (2001) believe that this immunosuppressive state might be closely related to factors such as the absence of MHC-I antigens, the presence of unique HLA surface molecules, and nonspecific reduction of systemic immunoreactivity. Moreover, the blocking antibody, expressions of complement regulatory proteins, and reduced immunoreactivity might contribute to the formation of this unique immune state. These changes are likely to influence a systemic immune response to infections and lead to increased susceptibility to hantavirus infection.
After being infected with hantavirus, pregnant women and their fetuses seem to have worse clinical symptoms and outcomes (Sha et al. 2000; Liu et al. 2003) Nevertheless, currently, no theory can entirely explain the mechanism behind this phenomenon. In similar research of other viruses, this phenomenon is attributed to the unique immunological and physiological characteristics of pregnant women (Beigi 2017; Beigh 2012). However, patients’ immune response to a hantavirus infection, such as cytokine storms, is likely to harm the body (Schönrich et al. 2008). Pregnant women are in a specific state of immunosuppression, and their immune damage is much smaller than that of other people, which is inconsistent with the findings of this literature review. Therefore, it can be speculated that the cause for this difference might come from two aspects. Primarily, pregnancy increases the organ burden of patients with a hantavirus infection, worsens the health, and increases the risk of death (Guimarães et al. 2019). Additionally, the flow dynamics of patients might change during pregnancy. Consequently, the synthesis of clotting factors increases, and the placenta synthesizes a large number of thrombogenic substances (Avsic-Zupanc et al. 2019).
Early and timely diagnosis, comprehensive symptomatic treatment based on liquid therapy, and accurate judgments improve the patient’s life (Dai 2004). Moreover, the cooperation between emergency room physicians, infectious disease specialists, and obstetricians is also helpful for timely diagnosis and treatment. These measures are expected to improve the health of pregnant women and fetuses to a great extent.
Change history
08 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12250-021-00421-8
References
Avsic-Zupanc T, Saksida A, Korva M (2019) Hantavirus infections. Clin Microbiol Infect 21S:e6–e16
Beigh RH (2012) Influenza during pregnancy: a case of serious infection in obstetrics. Clin Obstet Gynecol 55:914–926
Beigi RH (2017) Emerging infections and pregnancy. Obstet Gynecol 129:896–906
Chen GH (2008) A case of pregnancy with epidemic hemorrhagic fever. Chin Gen Pract 11:1001 (in Chinese)
Chen GS (1994) Clinical analysis of 17 cases of epidemic hemorrhagic fever with pregnancy. J Hangzhou Med Coll 4:20–21 (in Chinese)
Choi EKM, Ro DY, Choi OC, Rho SH, Kim YW, Kim TE, Jung JK, Namkoong SE (2000) A case of hantavirus pulmonary syndrome complicating pregnancy. Obstet Gynecol 43:1282–1285
Chun SHCM, Kim YJ, Woo BH (1992) Two cases of Korean hemorrhagic fever complicated with pregnancy. Obstet Gynecol 35:778–782
Clement J, Vergote V, Laenen L, Van Ranst M (2013) Letter to the editor: distinguishing between hantavirus-induced haemorrhagic fever with renal syndrome and pregnancy-induced liver pathologies (aflp and hellp syndromes). Eurosurveillance 18:20493
Connolly-Andersen AM, Hammargren E, Whitaker H, Eliasson M, Holmgren L, Klingström J, Ahlm C (2014) Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: a self-controlled case series study. Circulation 129:1295–1302
Cui YJ (2005) Nursing for pregnant women with epidemic hemorrhagic fever. Mod Nurs 2:127 (in Chinese)
Dai JY (2004) A case of late pregnancy with epidemic hemorrhagic fever. J Binzhou Med Coll 6:467 (in Chinese)
Duan XY, Wang SH, Zhang HJ, Wang CL, Ma FZ (1996) Clinical and serological analysis of 48 patients with epidemic hemorrhagic fever during pregnancy. Prog Obstet Gynecol 2:46–48 (in Chinese)
Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET (1994) Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The hantavirus study group. N Engl J Med 330:949–955
Gao Y (2013) A case of pregnancy with epidemic hemorrhagic fever. Hainan Med 24:1704–1705 (in Chinese)
Gaunt G, Ramin K (2001) Immunological tolerance of the human fetus. Am J Perinatol 18:299–312
Georges CG, Artunc F, Weyrich P, Friedrich B, Wolf SC (2008) Nephropathia epidemica as the result of a Puumala virus infection in a pregnant patient. Dtsch Med Wochenschr 133:1830–1832 (in German)
Gilson GJ, Maciulla JA, Nevils BG, Izquierdo LE, Chatterjee MS, Curet LB (1994) Hantavirus pulmonary syndrome complicating pregnancy. Am J Obstet Gynecol 171:550–554
Guimarães T, Magalhães A, Veiga A, Fiuza M, Ávila W, Pinto FJ (2019) Heart disease and pregnancy: state of the art. Rev Port Cardiol 38:373–383
Hallin GW, Simpson SQ, Crowell RE, James DS, Koster FT, Mertz GJ, Levy H (1996) Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit Care Med 24:252–258
Hao CY (1997) A case of severe epidemic hemorrhagic fever in pregnancy. Prog Mod Obstet Gynecol 2:46 (in Chinese)
Haram K, Svendsen E, Abildgaard U (2009) The hellp syndrome: clinical issues and management. Rev BMC Pregnancy Childbirth 9:8
Hofmann J, Führer A, Bolz M, Waldschläger-Terpe J, Meier M, Lüdders D, Enders M, Oltmann A, Meisel H, Krüger DH (2012) Hantavirus infections by Puumala or Dobrava-Belgrade virus in pregnant women. J Clin Virol 55:266–269
Howard MJ, Doyle TJ, Koster FT, Zaki SR, Khan AS, Petersen EA, Peters CJ, Bryan RT (1999) Hantavirus pulmonary syndrome in pregnancy. Indian J Crit Care Med 29:1538–1544
Hedman K, Vaheri A, Brummer-Korvenkontio M (1991) Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet (Lond Engl) 338:1353–1356
Hjertqvist M, Klein SL, Ahlm C, Klingstrom J (2010) Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis 16:1584–1586
Jamieson DJ, Theiler RN, Rasmussen SA (2006) Emerging infections and pregnancy. Emerg Infect Dis 12:1638–1643
Jiang H, Zheng X, Wang L, Du H, Wang P, Bai XF (2017) Hantavirus infection: a global zoonotic challenge. Virol Sin 32:32–43
Ji F, Zhao W, Liu H, Zheng H, Wang S, He C, Wang W, Zhang R, Bai D, Tian C, Zhao W, Deng H (2017) Hemorrhagic fever with renal syndrome caused by Hantaan virus infection in four pregnant Chinese women. J Med Virol 89:1865–1870
Jing PT, Jing H (1994) Impact of pregnancy infection EHF virus on pregnant women, fetuses, newborns, infants. Shandong Med 34:3 (in Chinese)
Jonsson CB, Figueiredo LT, Vapalahti O (2010) A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 23:412–441
Kariwa H, Yoshimatsu K, Arikawa J (2007) Hantavirus infection in east Asia. Comp Immunol Microbiol Infect Dis 30:341–356
Kariwa H (2017) Rodent associated hantaviruses and hantavirus infections. Uirusu 67:25–32 (in Japanese)
Khan AS, Khabbaz RF, Armstrong LR, Holman RC, Bauer SP, Graber J, Strine T, Miller G, Reef S, Tappero J, Rollin PE, Nichol ST, Zaki SR, Bryan RT, Chapman LE, Peters CJ, Ksiazek TG (1996) Hantavirus pulmonary syndrome: the first 100 us cases. J Infect Dis 173:1297–1303
Kim CHRM, Moon YJ, Hwang JH, Kim SR, Hwang YY (1997) A case of pregnancy complicated with Korean hemorrhagic fever. Obstet Gynecol 40:2892–2897
Kim BN, Choi BD (2006) Hemorrhagic fever with renal syndrome complicated with pregnancy: a case report. Korean J Intern Med 21:150–153
Kim WD, Park YK, Lee HW, Moon GJ (1978) A case of fetal death after maternal infection of Korean hemorrhagic fever. Korean J Infect Dis 10:36–40
Kruger DH, Figueiredo LT, Song JW, Klempa B (2015) Hantaviruses—globally emerging pathogens. J Clin Virol 64:128–136
Latus J, Kitterer D, Kimmel M, Alscher MD, Braun N (2013) The case: fever, myalgia, visual disorders, and acute kidney failure in a pregnant woman. Diagnosis: severe acute hantavirus infection. Kidney Int 84:629–631
Latus J, Schwab M, Tacconelli E, Pieper FM, Wegener D, Dippon J, Müller S, Zakim D, Segerer S, Kitterer D, Priwitzer M, Mezger B, Walter-Frank B, Corea A, Wiedenmann A, Brockmann S, Pöhlmann C, Alscher MD, Braun N (2015) Clinical course and long-term outcome of hantavirus-associated nephropathia epidemica, Germany. Emerg Infect Dis 21:76–83
Lazzerini K, Gutierrez-Quintana R, Jose-Lopez R, McConnell F, Gonçalves R, McMurrough J, De Decker S, Muir C, Priestnall SL, Mari L, Stabile F, De Risio L, Loeffler C, Tauro A, Rusbridge C, Rodenas S, Añor S, de la Fuente C, Fischer A, Bruehschwein A, Penderis J, Guevar J (2017) Clinical features, imaging characteristics, and long-term outcome of dogs with cranial meningocele or meningoencephalocele. J Vet Intern Med 31:505–512
Lee HW, Lee PW, Johnson KM (1978) Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis 137:298–308
Lee HW (1989) Hemorrhagic fever with renal syndrome in Korea. Rev Infect Dis Suppl 4:S864–S876
Li G (2019) A case of pregnancy with epidemic hemorrhagic fever. Int J Obstet Gynecol 46:85–87 (in Chinese)
Liu YF, Yan PS, Wang BY, Liu J, Wang N-P, Zhu X-S, Huang M, Chen BQ (1987) Intrauterine infection of epidemic hemorrhagic fever (ehf) via placenta. Chin Med J 100:756–758 (in Chinese)
Liu X, He GW, Chen XQ, Li XH, Wang K, Huang J (2013) Epidemic hemorrhagic fever in pregnancy: a case report and literature review. Bio-IT World 2:86 (in Chinese)
Liu XL, Hao D, Wang XZ, Wang T, Lu F, Liu W, Lv LB, Lv CJ (2017) Epidemic hemorrhagic fever complicated with late pregnancy: a case report. Medicine 96:e8137
Liu ZF, Bai XF, He WG, Yang WS (2003) The 2263 cases of hemorrhagic fever with renal syndrome. Infect Dis 21:365–368 (in Chinese)
Lu ZJ, He QJ, Ming X (2018) Clinical analysis of 18 cases of hemorrhagic fever with renal syndrome during pregnancy. Endem Dis Control 33:461 (in Chinese)
Ma RM, Xiao H, Jing XT, Lao TT (2003) Hemorrhagic fever with renal syndrome presenting with intrauterine fetal death a case report. J Reprod Med 48:661–664
Mace G, Feyeux C, Mollard N, Chantegret C, Audia S, Rebibou JM, Spagnolo G, Bour JB, Denoyel GA, Sagot P, Reynes JM (2013) Severe Seoul hantavirus infection in a pregnant woman, France, October 2012. Euro Surveill 18:20464
Macneil A, Nichol ST, Spiropoulou CF (2011) Hantavirus pulmonary syndrome. Virus Res 162:138–147
Martinuč Bergoč M, Lindič J, Kovač D, Ferluga D, Pajek J (2013) Successful treatment of severe hantavirus nephritis with corticosteroids: a case report and literature review. Ther Apher Dial 17:402–406
Ming T (2017) Observation of EB virus infection and pregnancy outcome in pregnant women and newborns at different gestational weeks. Chin Med Sci 7:62–65 (in Chinese)
Murthy PR, Ucchil R, Shah U, Chaudhari D (2016) Hantavirus pulmonary syndrome in a postpartum woman. Indian J Crit Care Med 20:551–553
Nowakowska A, Heyman P, Knap JP, Burzynski W, Witas M (2009) The first established focus of hantavirus infection in Poland, 2007. Ann Agric Environ Med 16:79–85
Park SMKS, Kim HM, Son YS (1998) A case of Korean hemorrhagic fever complicated with pregnancy. Obstet Gynecol 41:1220–1224
Partanen S, Kahanpaa K, Peltola J, Lahdevirta J (1990) Infection with the Puumala virus in pregnancy. Case report. Br J Obstet Gynaecol 97:274–275
Partanen S, Sariola A, Lahdevirta J (1993) Lack of evidence of Puumala virus infection in patients with spontaneous abortion. Eur J Clin Microbiol Infect Dis 12:142–143
Pedrosa PB, Cardoso TA (2011) Viral infections in workers in hospital and research biosafety aspects. Int J Infect Dis 15:E366–E376
Prebensen D (1997) Nephropathia epidemica infection during first trimester of pregnancy—normal fetal outcome. Acta Obstet Gynecol Scand 76:884–885
Peters CJ, Simpson GL, Levy H (1999) Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med 50:531–545
Schneider F, Vidal L, Auvray C, Khider Y, Graesslin O (2009) The first French hemorrhagic fever with renal syndrome in pregnant woman. J Gynecol Obstet Biol Reprod 38:440–442
Schönrich G, Rang A, Lütteke N, Raftery MJ, Charbonnel N, Ulrich RG (2008) Hantavirus-induced immunity in rodent reservoirs and humans. Immunol Rev 225:163–189
Seferovic M, Sánchez-San Martín C, Tardif SD, Rutherford J, Castro ECC, Li T, Hodara VL, Parodi LM, Giavedoni L, Layne-Colon D, Tamhankar M, Yagi S, Martyn C, Reyes K, Suter MA, Aagaard KM, Chiu CY, Patterson JL (2018) Experimental Zika virus infection in the pregnant common marmoset induces spontaneous fetal loss and neurodevelopmental abnormalities. Sci Rep 8:6851
Sha Q, Cheng HZ, Cui FQ (2000) Clinical analysis of 21 cases of hemorrhagic fever with pregnancy renal syndrome. Shandong Med 40:3 (in Chinese)
Silberberg L, Rollin PE, Kerouani G, Courdrier D (1993) Haemorrhagic fever with renal syndrome and pregnancy: a case report. Trans R Soc Trop Med Hyg 87:65
Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G (2015) Viral infections during pregnancy. Am J Reprod Immunol 73:199–213
Tiilikainen T, Jouppila P (1989) Epidemic hemorrhagic fever during pregnancy. Duodecim 105:1916–1919 (in Finnish)
Todorovic Z, Canovic P, Gajovic O, Mijailovic Z (2010) Hemorrhagic fever with renal syndrome during pregnancy: case report. Med Pregl 63:280–284
Wang LZ, Zhang QD, Zhu KL, Gao WZ, Li SW, Wu SY, Chen SJ, Li TT, Hu GY (1992) Study on the influence of epidemic hemorrhagic fever on pregnancy outcome and childbirth. Shandong Med 7:1–2 (in Chinese)
Wang YL (2014) Epidemic hemorrhagic fever in pregnancy: report of 7 cases. J Jinan Univ Nat Sci Med 35:397–400 (in Chinese)
Watson DC, Sargianou M, Papa A, Chra P, Starakis I, Panos G (2014) Epidemiology of hantavirus infections in humans: a comprehensive, global overview. Crit Rev Microbiol 40:261–272
Vaheri A, Henttonen H, Voutilainen L, Mustonen J, Sironen T, Vapalahti O (2013) Hantavirus infections in Europe and their impact on public health. Rev Med Virol 23:35–49
Xia H (2009) Discussion on emergency screening for hemorrhagic fever with renal syndrome. Emerg Med 18:210–212 (in Chinese)
Xie XL (1994) Two cases of pregnancy with epidemic hemorrhagic fever. J Guiyang Med Coll 4:389 (in Chinese)
Ying K (1984) Epidemic hemorrhagic fever and pregnancy (1 case of stillbirth with autopsy). J Jiangxi Med Coll 4:93–94 (in Chinese)
Yun XH (2007) Analysis of 5 cases of atypical epidemic hemorrhagic fever. Chin J Misdiagnosis 7:6157–6158 (in Chinese)
Zhang XJ (1995) Pregnancy combined with epidemic hemorrhagic fever clinical features and treatment. Jiang Su Med Med 21:766–767 (in Chinese)
Zhang YZ, Zou Y, Fu ZF, Plyusnin A (2010) Hantavirus infections in humans and animals, China. Emerg Infect Dis 16:1195–1203
Zhao DN (2017) Human papillomavirus infection in pregnant women and its effect on maternal and infant outcomes. Nosocomiology 27:3554–3570 (in Chinese)
Zheng XH (1985) A case report of death from epidemic hemorrhagic fever in late pregnancy. Wuhan Med J 2:118 (in Chinese)
Zou LX, Chen MJ, Sun L (2016) Haemorrhagic fever with renal syndrome: literature review and distribution analysis in China. Int J Infect Dis 43:95–100
Acknowledgements
We would like to thank Jiamin Wang from Beijing Foreign Studies University, for improving the English writing. This work was supported by the National Scientific Research Program of China: New technology and project on intervention and elimination of cytokine storm and secondary infection in acute severe respiratory infectious diseases (2017ZX10204401-002-005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
The original online version of this article was revised: The original article can be found online at https://doi.org/10.1007/s12250-020-00300-8
Rights and permissions
About this article
Cite this article
Lu, DH., Jiang, H. & Lian, JQ. Hantavirus Infection during Pregnancy. Virol. Sin. 36, 345–353 (2021). https://doi.org/10.1007/s12250-020-00300-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-020-00300-8