Abstract
The presence of impurities is the primary cause of safety related drug product recall. Clinical data generated during the drug discovery process may not be able to conclude the long-term safety of a drug. The best way to ensure drug safety is to control the associated impurities. It can be achieved by a clear understanding of the route and factors for impurity formation. Tight control on manufacturing, storage, and transport process can provide a beneficial effect on minimization of impurity generation. Control strategies employing quality by design and risk assessment-based approaches potentially enhance drug safety profile and, thereby, minimize product recalls. Prior understanding of key aspects of the safety profile of impurities, post-marketing surveillance criticalities, and establishment of constructive preventive strategies is the best way to minimize unwanted product recall due to associated safety issues. Effective preventive strategies should be designed and executed during the product manufacturing and supply chain process. The novelty of this article can be justified by the unavailability of any similar type of reports discussing the functional pathway of regulatory bodies aiming for effective control of drug impurities. This article contains novel, critical, and constructive prevention strategies to establish a robust safety profile of pharmaceutical products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tremendous advancement and innovation in the assessment and control strategies of unwanted drug impurities have been reported in the recent past. Risks associated with impurities present in pharmaceutical drug products lead to serious health hazards. The drug discovery process is thought to be a success if the molecule does not produce any adverse events during post-marketing surveillance. To ensure the reliability of drug products, various regulatory bodies monitor the post-marketing safety of the molecule. If the drug product shows any toxicity in this phase, it can be recalled or withdrawn from the market based on the severity of adverse events [1, 2].
Introducing a drug product into the market is a tedious process. It is a result of the efforts of decades and the expense of huge capital costs. The maintenance of balance between safety and potency is the primary challenge during the drug discovery and development process. This ensures that the drug product would not show any unprecedented adverse effect in the post-marketing phase. Despite all the efforts to reduce adverse events, product recalls and withdrawals remain a major concern for the pharmaceutical industry. A recall is the withdrawal of products from the market due to one or multiple causes. Non-compliance with the Good Manufacturing Practices (GMP) in the manufacturing sites is one of the prime reasons for drug recall. Majority of the drug recalls in the past were due to a poor understanding of the product manufacturing criticality. The risk associated with drug product recall and withdrawal can be a potential loss of capital and market image [3]. The frequency of the recall increases when generic industries compete for marketing a product after the expiry of a patent. Often the generic companies deviate or do not follow the GMP principles and practice many irregularities in product manufacturing. For example, the reuse of solvents and equipment for several products within the same manufacturing premises leads to the generation of impurities. Such products may be of substandard quality with compromised safety and efficacy profiles. The United States (US) Food and Drug Administration (FDA) inspection reports on the manufacturing sites reported many data irregularities which indicate that manufacturers do violate the GMP protocol. The 21 code of federal regulation (CFR) 483 warning letters issued to manufactures were increased significantly in recent years citing the violation in the basic functionality of manufacturing premises [4]. In 2019, a record number of warning letters were issued to the pharmaceutical industries from the USA, China, and India. This indicates that violations in the manufacturing premises frequently take place in both the developing as well as developed countries.
The product recalls may adversely affect the availability of the products in the market. Recent nitrosamine impurities-related withdrawal of several over-the-counter drug products from different manufacturers is an example of serious crisis of few drugs in the global market [5]. Several over-the-counter drug products were found to contain nitrosamine impurities above their specification limit. Different marketed drug products of metformin, ranitidine, nizatidine, and angiotensin II receptor blockers were recalled from the market worldwide due to the presence of nitrosamines at a higher level than the specified limit. This emphasizes pharmaceutical industries to empower their process and analytical control strategy to avoid product recalls in future.
Keeping all these facts in mind, this review briefly discussed impurity type, regulatory perspectives, case studies on significant recalls, and preventive measures to minimize drug product recalls.
Drug Impurities
Pharmaceutical impurities in drug substances or drug products need to be controlled as they can cause severe adverse events in patients. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) defines impurity as “any component of the new drug substance that is not the chemical entity defined as the new drug substance” [6].
Sources of Impurities
The presence of impurities in pharmaceutical drug products is a major issue that should be addressed to minimize product recalls in the post-marketing phase. The source of impurities for various reasons should be investigated thoroughly and logically. The critical factors affecting the purity of the final drug substances should be identified. The prior understanding of the type of impurities and their source helps to design an effective control strategy during the drug development process.
Organic Impurities
Organic impurities can be categorized into process-related and drug-related impurities. Unwanted side reactions between the reactants during the synthesis process may lead to the formation of impurities [6].
Starting Materials or Raw Materials
Impurities from starting materials are the most common form of impurities found in drug substances. Some amount of the starting material may remain unreacted and present in the final product as an impurity [7, 8].
By-products or Related Substances
Varieties of phenomenons like incomplete or over-reaction, isomerization, dimerization, rearrangement, or unwanted reaction between starting materials or intermediates with chemical reagents or catalysts may lead to the formation of by-products [7]. Such by-products may adversely affect the final product quality being the impurity.
Intermediates
Intermediate is a new chemical entity formed in the reaction which reacts further to generate a product. Intermediates can be isolated according to the requirement of the reaction and can be used in the synthesis of the final product. Some amount of intermediate may remain unreacted during the reaction and appear as an impurity in the finished final product [7, 8].
Degradation Products
During storage or shipment, drugs may undergo chemical or physical breakdown leading to the formation of degradation products as impurities. Such impurities are produced in drug substances or drug products on exposure to light, temperature, humidity, pH, water, moisture, reaction with container, or excipients used during manufacturing, storage of new drug substances, or drug products [7, 8].
Inorganic Impurities
Inorganic impurities are introduced in the final product during any of the steps in the manufacturing process. These impurities are normally known and identified and include reagents, ligands, catalysts, heavy metals, residual metals, inorganic salts, filter aids, and charcoal [7].
Reagents, Ligands, and Catalysts
Impurities can arise from residues of reagents, ligands, or catalysts that are used in the manufacturing process. They do not provide any therapeutic benefit but may cause harm to the patient [9].
Elemental Impurities
The presence of elemental impurities such as lead and arsenic in drug products can cause severe toxicity in patients. Hence, it is necessary to control the concentration of metal impurities in drug substances and drug products. Elemental impurities can arise from residual catalysts or inorganic reagents used during synthesis, leaching from container closure systems, and manufacturing equipment [10].
Residual Solvents
Residual solvents are organic volatile impurities produced during the synthesis of active pharmaceutical ingredients (API), excipients, and formulation. It is very difficult to remove these solvents completely from the final product. High exposure to these solvents can cause serious adverse events. ICH Q3C guideline recommends to control the residual solvents used in manufacturing processes of drug substances, intermediates, and starting materials. To assure the safety of pharmaceuticals, the permissible daily exposure (PDE) for different residual solvents in drug substances is provided in the guideline [11].
Other Impurities
Besides the above, other impurities include those that arise due to container closure interaction, cross-contamination, etc. Moreover, particulate matter, packaging related impurities, polymorphic impurities, genotoxic impurities, etc. can significantly contribute to the poor quality of pharmaceuticals.
Particulate Matter
Recall of most of the intravenous solutions occurs due to the presence of particulate matter. They could lead to serious adverse events such as local irritation, vasculitis/phlebitis, antigenic or allergic reactions, microvascular obstruction, and pulmonary embolism [12]. Table 1 summarizes few examples of product recall due to the presence of particulate matter.
Packaging-Related Impurity
Impurities could be generated due to the interaction of the drug or excipients with packaging material. Leaching of components of packaging material under certain conditions can incorporate impurity into the drug product. For instance, traces of phthalidomide derivative impurity were found in amlodipine besylate even though no phthalidomide derivative was incorporated in the synthesis of amlodipine besylate. Further study confirmed that amlodipine besylate reacted with bis(2-ethylhexyl) phthalate released from process equipment material and plastic packaging to generate the said impurity [13].
Impurities due to Cross-Contamination
Cross-contamination is the contamination of the API, intermediate, or finished product with another starting material or product. The use of common equipment/ancillary facilities can lead to cross-contamination. According to the Medicines and Healthcare products Regulatory Agency (MHRA), cross-contamination is the second highest reason for recalls in the United Kingdom (UK) in recent years [14]. Cross-contamination can arise due to insufficient cleaning, inappropriate design of the heating, ventilation, and air conditioning (HVAC) system, poor facility design, or handling of a product without a proper container closure system [15]. Table 2 summarizes the instances of drugs recalled due to cross-contamination.
Microbial Contamination
Microbial contamination refers to the undesired introduction of fungi, yeast, mold, and bacteria such as gram-positive rod (from environment and soil), gram-negative rod (from water and soil), gram-positive cocci (from personnel), virus, protozoa, and their toxins and by-products which can lead to adverse reactions, infections, and decrease in the efficacy of drug products. Control and monitoring of microbial contamination are some of the important requirements in Current Good Manufacturing Practice (cGMP). There are various sources of microbial contamination such as raw materials, excipients, equipment, the environment of premises, and facility having inadequate air handling, poor sanitization procedures, personnel with exposed skin, hair, and clothing. Numerous drugs have been recalled because of microbial contamination [16]. Table 3 provides a list of drug products recalled because of microbial contamination.
Potential Foreign Substance
These impurities are not related to synthesis. They can be present in drug products due to contamination or due to adulteration [9]. They can enter into the formulation during manufacturing through people, materials, equipment, and the surrounding environment. One lot of alprazolam tablets (USP C-IV 0.5 mg) was voluntarily recalled by Mylan Pharmaceuticals in 2019 due to the potential of foreign substances. The recall was conducted with the knowledge of the USFDA [17].
Polymorphic Impurity
Different polymorphic forms have different solubilities, dissolution, and thermodynamic stability. They may show variation in bioavailability and, hence, it is important to monitor them during formulation development. Polymorphic impurities are due to the incorporation of undesired polymorphs of a substance into a drug product [18]. Ritonavir is a protease inhibitor class of an antiviral drug used in the treatment of HIV-1 infections. Initially, only one form of ritonavir was known. After two years of the launch of the soft gel capsules of ritonavir, several lots failed to achieve the dissolution requirements due to the generation of a new polymorph (form II) which had reduced solubility compared to the original crystal form (form I). It became difficult for the manufacturer to manufacture the formulation because of the generation of form II, which was dominant, thermodynamically stable, and less soluble having less bioavailability. Also, there was a risk to store the oral solution at 2–8 °C because of the risk of crystallization. The manufacturer recalled the original formulation from the market and reformulated it in an oily vehicle [19].
Genotoxic Impurities
Certain impurities alter genetic material and are toxic at even very low levels. Genotoxic impurities are generated mostly due to the use of reagents like methyl sulphonic acid or toluene sulphonic acid during manufacturing. The examples of reported genotoxic impurities include N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) [20]. Recently, the drugs like metformin, ranitidine, and sartans are recalled due to the presence of genotoxic impurities. Figure 1 shows the various potential genotoxic structural alerts.
Limits of Drug Impurities Set by Guidelines
Regulatory bodies have specified certain limits for impurities to control and monitor their presence in pharmaceuticals. Various specifications for impurities by the regulatory bodies are described hereafter.
ICH
Several ICH guidelines defined the allowable limit of impurities in pharmaceuticals.
ICH Q3A (R2): Impurities in New Drug Substances
Based on the daily dosage and daily exposure, the ICH Q3A guideline has specified impurity levels at which an impurity must be reported, identified, and qualified. As per the guideline, if the maximum daily dose of a drug is less than or equal to 2 g/day, the reporting, identification, and qualification threshold of the impurities in the drug substance should be 0.05%, 0.10%, or 1.0 mg/day (whichever is less) and 0.15% or 1.0 mg/day (whichever is lower), respectively. If the maximum daily dose of the drug is greater than 2 g/day, the threshold for the reporting, identification, and qualification of the impurities in the drug substance has been set as 0.03%, 0.05%, and 0.05%, respectively [6].
ICH Q3B (R2): Impurities in New Drug Products
The ICH Q3B guideline has set specific criteria for impurities in pharmaceutical formulations. However, it does not take into account the extractables and leachables. The guideline specified that if the maximum daily dose of the new drug product is less than or equal to 1 g/day, the reporting threshold is 0.1%. If the maximum daily dose surpasses 1 g, then the reporting threshold is 0.05%. For the identification threshold, if the maximum daily dose is less than 1 mg, then the threshold is 1.0% or 5 µg total daily intake (TDI), whichever is less. If the maximum daily dose is in the range of 1 mg–10 mg, then the identification threshold is 0.5% or 20 µg TDI, whichever is less. If the highest daily dose is more than 10 mg but less than 2 g, then the threshold is 0.2% or 2 mg TDI, whichever is smaller, and if it exceeds 2 g, then the threshold has been set at 0.10%. For qualification threshold, if the maximum daily dose of the new drug product is less than 10 mg, the threshold is 1.0% or 50 µg TDI, whichever is lower. If the maximum daily dose ranges from 10 to 100 mg, then the qualification threshold is 0.5% or 200 µg TDI, whichever is lesser. If the maximum daily dose of the drug product is less than 100 mg but greater than 2 g, then the threshold is set as 0.2% or 3 mg TDI, whichever is smaller, and if it exceeds 2 g, then the threshold has been set at 0.15% [21].
ICH Q3C (R6): Impurity Guidelines for Residual Solvents
The ICH Q3C guideline advises to use solvents that are less toxic and defines the limits of certain residual solvents deemed to be toxicologically acceptable. As per the guideline, class 1 solvents are those which are toxic and are recommended to avoid in the manufacturing process of drug substances, excipients, and drug products. Their use should be rationalized based on risk–benefit assessment. These compounds are classified as potential human carcinogens and may cause environmental hazards. Solvents like benzene, carbon tetrachloride, 1,2-dichloroethane, 1,1-dichloroethene, and 1,1,1-trichloroethane are of class 1 categories. The solvent of the class 2 category has limited toxicity as compared to class 1, but still, their use should be limited as they may cause severe adverse effects and irreversible toxicities in humans. Examples of class 2 solvents are dichloromethane, 1,2-dimethoxyethane, N, N-dimethylformamide, 1,4-dioxane, ethylene glycol, hexane, methanol, etc. Class 3 solvents like acetic acid, acetone, anisole, ethyl acetate, formic acid, butanol, and triethylamine possess less toxicity. Wherever applicable, class 3 solvents should be preferred. These solvents do not require a health-based exposure limit. As per the ICH Q3C guideline, the PDE of class 3 solvents can be considered as 50 mg or more [22].
ICH Q3D (R1): Guideline for Elemental Impurities
The ICH Q3D guideline has set limits and acceptance criteria for elemental impurities in drug products. The guideline mainly provides a recommendation regarding estimation of toxicity of elemental impurities, determination of their allowable maximum daily exposure, and application of a risk-dependent approach to monitor impurity levels in the drug product. The guideline states that if the elemental impurities do not go beyond PDE in drug products, then the manufacturer is not required to tighten its limits. The elemental impurities have been classified into four classes (class 1, 2A, 2B, and 3). Class 1 elements include arsenic, cadmium, mercury, and lead. They are unique elements as they have very limited or no use in pharmaceuticals. They are categorized as human toxicants. Various sources of these impurities are drug substances, container closure systems, manufacturing equipment, water, mined excipients, etc. They require a risk assessment to ensure that they are well below their PDE limit. Class 2 elements are route-dependent human toxicants. Based upon their relative abundance in the drug product, class 2 elements are subdivided into class 2A and 2B. Class 2A elements require risk assessment as they have a high probability of presence. Elements included in class 2A are cobalt, nickel, and vanadium. Class 2B elements are low in natural abundance and are not likely to be co-isolated with other products. They have comparatively lower chances of presence in the drug product. Therefore, they may be waived off from risk assessment unless added intentionally. Examples include silver, gold, iridium, osmium, palladium, platinum, rhodium, ruthenium, selenium, and thallium. Class 3 elements have lower toxicities if administered by the oral route. Their PDE are greater than 500 µg/day. They also may not require risk assessment unless added intentionally. But they require risk assessment when administered by inhalational and parenteral routes. Elements in this class include barium, chromium, copper, lithium, molybdenum, antimony, and tin [23].
ICH M7
ICH M7 guideline focuses primarily on impurities, which can cause deoxyribonucleic acid mutations and can lead to cancer. For mutagenic impurities, the total daily intake depends on the duration of treatment. For individual impurities, the recommended TDI for a span of less than or equal to 1 month is 120 µg/day, greater than 1 month but lesser than 12 months is 20 µg/day, duration ranging from 1 to 10 years is 10 µg/day, and greater than 10 years until the lifetime is 1.5 µg/day. In case of multiple mutagenic impurities, the TDI for a duration of less than or equal to 1 month, greater than 1 month but lesser than 12 months, duration ranging from 1 to 10 years, and greater than 10 years until the lifetime is recommended as 120, 60, 30, and 5 µg/day, respectively [24].
FDA
Centre for Drug Evaluation and Research (CDER) under FDA published “Manual of Policies and Procedures” document entitled as “Establishing impurity acceptance criteria as part of specifications for New Drug Applications, Abbreviated New Drug Applications, and Biological Licence Applications based on clinical relevance.” The objective was to understand what type of data is required while defining impurity acceptance criteria [25].
New Drug Applications and Abbreviated New Drug Applications Acceptance Criteria
If the acceptance criteria of the impurities are less than the qualification threshold, ICH Q3A and Q3B guidelines are followed, unless there is any concern regarding safety such as a structural alert for mutagenicity or the impurity is toxic or has immunological concerns. For the opposite instance information from in silico, and in vitro studies, scientific trials and other information from the public domain can be taken. If no risk is identified, then the proposed acceptance criteria recommended in the guidelines can be considered. If a potential risk is identified, justification of impurity levels needs to be provided. Impurity levels need to be evaluated if there is any concern related to clinical safety [25].
European Medicines Agency
Specifications for thresholds and impurity levels are the same in ICH and European Medicines Agency (EMA). Unlike ICH, apart from drug substances and products, EMA provides additional specifications for impurities in antibiotics. Table 4 describes impurity thresholds for fermentation products (single, family of compounds), semi-synthetic compounds, and peptides. The first threshold values should be considered for the impurities, which have already been characterized. Otherwise, the second threshold values need to be considered. If active substances are manufactured by fermentation (family of compounds), the qualification threshold of 0.50% for structurally closely related impurities is set in combination with a qualification threshold of 0.2% for other related impurities [26].
Pharmacopeia
Various compendial tests are recommended in pharmacopeia on the analysis of drug impurities. The impurity specification for the pharmaceuticals is provided in the individual monographs for both drug substances and drug products.
United State Pharmacopoeia
As per United State Pharmacopoeia (USP), the listing of impurities along with analytical procedures should be performed before quantification is done. For toxic impurities, irrespective of their values, analytical procedures should be established. Degradation products obtained from over-the-counter (OTC) drugs should also be reported, identified, and qualified. For the drugs of complex impurity profile, a combined limit for impurity needs to be established [27].
Indian Pharmacopoeia
Chapter 5.5 in Indian Pharmacopoeia (IP) provides a recommendation to assure the identity, quality, strength, and purity of pharmaceuticals. As per the IP, acceptance limits for identified and specified impurities are less than or equal to 0.5% for drug substances and less than or equal to 1% for the drug product. Similarly, for unidentified impurities, the limits are 0.3% and 0.5% for drug substance and drug product, respectively. The limits for total impurities present in the drug substance and drug product are 1% and 0.2%, respectively [28].
British Pharmacopoeia
British Pharmacopoeia (BP) does not provide any limit of impurity specification in detail. It focuses on limiting the amount of impurities present in drug substances or products originating from starting materials or intermediates. Limits for impurities in BP are set on a case-to-case basis based on impurities present in the final product and also based on toxicity data of the impurity. Analytical procedures for quantification have been provided in BP for the impurities that are toxic and potent [29].
European Pharmacopoeia
The transparency list given after each monograph in European Pharmacopoeia (EP) provides a list of impurities and the techniques for their control. In EP monograph 2034, threshold values for drug substances excluding synthetic peptides have been provided. As per the monograph, the threshold for reporting, identification, and qualification of impurities in any drug substance having a maximum daily intake of less than or equal to 2 g/day is less than 0.05%, more than 0.10%, or greater than 1 mg (whichever is lesser) and more than 0.15% or greater than 1 mg (whichever is lower), respectively. For drug substances, having a maximum daily intake of greater than or equal to 2 g/day, the reporting, identification, and qualification threshold in case of drug substance is 0.03%, 0.05%, and 0.05%, respectively [30].
Toxicity due to Impurities
The presence of impurities in drug substances or drug products can cause serious toxic effects. Over the past decades, it has been observed that the majority of the impurities are responsible for changes in genetic material and the major part affected by impurity is DNA. Genotoxic impurities like NDMA, NDEA, methyl sulphonate, and epoxides increase the risk of cancer in humans. It is essential to identify, evaluate, prevent, and control the impurities to eliminate the toxicological problems associated with the drugs. Apart from genotoxic impurities, toxicity can be caused by the presence of metals, particulate matter present in the finished product [20, 31].
Post-marketing Surveillance of Drugs
Post-marketing surveillance (PMS) is the fourth phase of clinical trials. When drugs reach the market after clinical trials, they are monitored through the PMS process. The population that is exposed to a new drug during clinical trial is significantly smaller than the actual population exposed after marketing. During clinical trials, the drug may not be exposed to the population having children, elder people, pregnant women, and people with other medical conditions taking different kinds of medications. PMS trials are conducted over a long period to verify the impact of the drug on a wide variety of populations under a wide range of conditions. Such surveillance detects the effects of drugs that were previously unrecognized during clinical trials [32].
Many times, drugs are approved for therapeutic use and are then found to cause serious adverse effects. It can cause long-term illness and even death after prolonged use. A voluntary worldwide withdrawal of rofecoxib is an example of the advantage of such PMS observation. Rofecoxib, a cyclooxygenase-2 (COX-2) inhibitor, was approved as a pain killer and anti-inflammatory agent for osteoarthritic patients. It was also used for menstrual pain and later it got approval for use in rheumatoid arthritis. Later, it was found that consumption of Vioxx leads to an increased risk of heart attack compared to those receiving the placebo [33]. There are many such cases where drugs did not show any harmful or unexpected effects during clinical trials but became evident once they reached the market and were used by a diverse patient population for longer periods.
Regulatory Bodies for Post-marketing Surveillance Monitoring
Different types of organizations and entities, including pharmaceutical companies, universities, government agencies, private enterprises, and consumer advocacy groups, monitor PMS of pharmaceuticals. Regulatory bodies like the United States Food and Drug Administration (USFDA), EMA, and World Health Organization (WHO) performs different international drug surveillance program. Similarly, regulatory bodies of different countries implement PMS monitoring for the approved drugs in the respective regions.
FDA
FDA investigates the post-marketing safety of every product that is marketed in the USA. It has the authority to inspect the manufacturing facility outside the USA where the API was synthesized and later used for the products marketed in the USA.
Canada
Health Canada’s “Marketed Health Products Directorate” (MHPD) is the regulatory body of Canada that looks into PMS and runs the Canada Vigilance Program or Canada MedEffect. It monitors the prescription and non-prescription medicines, natural health products, biologicals, and radiopharmaceuticals. Canada vigilance regional offices collect data at the regional level and make contact with adverse drug reaction (ADR) reporters of the Canada National Office for further analysis. The MedEffect emphasizes facilitating access to databases containing drug safety data, thereafter, increasing awareness of the need to report ADR to Health Canada, recognizing and communicating serious risks associated, and creating a clear and effective framework for ADR reports in health care professionals. It makes it possible to file ADR reports through phone, email, and mail. Drug Safety and Effectiveness Network (DSEN), a part of Health Canada, is working hand to hand with the Institute for Health Research and is collectively involved in the various surveillance activities. Canadian Network for Observational Drug Effect Studies (CNODES), a program initiated by DSEN, has set up an inter-linking framework of researchers and databases across Canada to monitor drug safety and efficacy-based testing for drugs sold in Canada. It has the accessibility into Clinical Practical Research Datalink of UK for the drugs already marketed in the UK but is to be approved in Canada. The Vaccine and Immunization Surveillance in Ontario (VISION), primarily a vaccine monitoring program, preceded CNODES. It is run by the Institute for Clinical Evaluative Sciences [34]. The Canadian Adverse Drug Reaction Information System (CADRIS) is a computerized database of filed reports which is maintained by Canada’s Adverse Drug Reaction Monitoring Programme (CADRMP). It is the legal responsibility of the manufacturer to submit reports of adverse effects within 15 days [32].
United Kingdom
Medicines and Healthcare Products Regulatory Agency (MHRA) and Committee of Human Medicines (CHM) are collectively implementing Yellow Card Scheme to monitor the safety of medicines in the UK. Any adverse effects witnessed after the use of the drug may be reported to MHRA Yellow Card reports. The data is then analyzed by scientists in MHRA and the decision on the requirement of withdrawal of the drug is taken by CHM and Pharmacovigilance Expert Advisory Group. MHRA introduced one more scheme “Black Triangle” in 2009 to increase public awareness about the newly entered drug in the market requiring intensive monitoring [35].
Patient Group in the Risk of Exposure to Drug Impurities
Impact of exposure to impurities differs significantly among different categories of the population. Some specific populations may be more susceptible to develop the risk of carcinogenicity than others due to exposure to carcinogenic impurities. Hence, it is important to consider different populations during the toxicological study. It is recommended by FDA to have a lower threshold of impurities for pediatrics as they have high susceptibility. It is noteworthy that children who are exposed to carcinogens and are between 0 and 16 years of age have an elevated risk compared to adults. The risk of developing cancer before 2 years of age is increased by a factor of 10 while it will be 3 when the carcinogen exposure occurs between 2 and 16 years of age. Hence, it is of utmost importance to consider the pediatric population in the context of determining the acceptable impurity level for drug products [36].
In Silico Study to Ascertain the Absence of Impurities
As per ICH Q3A and Q3B guidelines, it is important to evaluate the genotoxic potential of impurities beyond the qualification threshold. The structure–activity relationship and structure toxicity relationship help to predict the safety and efficacy of the molecule. The structure toxicity relationship predicts the structural soft spots which might be responsible for the toxicity of the molecule. These soft spots may be carry forwarded in the impurities which will lead to serious side effects. If any genotoxic or carcinogenic substance is used in the chemical process or any intermediate or side products show potential to cause genotoxicity, it should be mentioned by the applicant.
Some specific structures can be genotoxic and are known as “structural alerts” [37]. The computational platform provides the necessary data to chemical scientists to synthesize a molecule devoid of structural alerts without altering the efficacy of the molecule. There are some external databases such as TOXNET, SciFinder, and VITIC where data of genotoxicity or carcinogenicity is available for compounds. This genotoxic data, before initiation and investigation of clinical trials, helps to understand the genotoxic potential [38]. The various databases with chemical entities having established toxicity profiles were utilized to establish the prediction. In the simulation process, various mathematical algorithm was employed in the validation of the software to provide an accurate platform for prediction. Table 5 provides examples of structurally alerting Ames negative compounds.
Analytical Techniques for Determination of Impurities
For the safe and effective use of pharmaceutical products, impurity profiling of the drug substance and drug products is necessary. Analytical techniques facilitate the identification and characterization of impurities which helps in understanding the safety and efficacy profile of the drugs. High-performance liquid chromatography (HPLC) is one of the most popular techniques used for the identification and separation of impurities. It performs the identification by matching the retention time of the impurities with known standards [39, 40]. A very useful technique for the detection of organic volatile impurities is gas chromatography. It provides good resolution, ease of quantitation, and selectivity [41, 42]. FTIR assists in the identification of impurities by checking the presence or absence of chemically related impurities if the structure of the impurity is known [43]. Capillary electrophoresis is used when the sample available for analysis is very low and a higher resolution is required. The only limitation it has is that the method is not reproducible [44, 45]. Supercritical fluid chromatography being one of the most recent tools is an orthogonal technique for impurity profiling of pharmaceuticals [46].
Hyphenated Techniques
The conventional techniques for impurity profiling possess various limitations as they are time-consuming and not sensitive enough. They become very complex when the number of impurities is more and present in trace quantities. Hyphenated techniques which combine separation capabilities of gas chromatography (GC), liquid chromatography (LC), or capillary electrophoresis (CE) with specific or sensitive detection phenomenon of mass spectrometry (MS), nuclear magnetic resonance (NMR), or infrared spectroscopy (IR) offer several benefits compared to the conventional methods [47].
GC–MS technique is widely used for the estimation of volatile impurities and residual solvents [48]. It has been used in the determination of impurities in ecstasy tablets containing amphetamine derivatives [49, 50]. The components which do not show fragmentation under electro spray ionization and atmospheric pressure chemical ionization source of LC–MS can undergo fragmentation in electron impact and chemical ionization source of GC–MS. LC–MS being the most popular technique has become a vital part of analysis during impurity characterization nowadays. LC–MS is the most widely used technique as it gives unequivocal structural determination alone. The various variants of LC–MS include LC–MS (Single Quad), LC–MS-MS (Triple Quad), LC-TOF (Time of Flight), LC–MS-TOF (Q-TOF, Triple TOF), LC–MS-3DTRAP (MSn), LC–MS-2DTRAP (Q-Trap), LC-Hybrid Trap TOF Systems (LCMS-IT-TOF), LC-Orbitrap, and LC-FTICR (Fourier Transform Ion Cyclotron Resonance). The major utilities of LC–MS for structure elucidation are high-resolution mass spectrometry (HRMS), hydrogen deuterium exchange mass spectrometry, and multi-stage mass spectrometry (MSn). Interpretation of mass spectrometric data is carried out employing nitrogen rule, isotopic behavior, number of rings, and double bonds (RDB) for establishing the structure of unknown substances [51]. Another recent technique CE-MS and its CEC variant are in the exploratory phase and is an effective orthogonal technique for impurity separation. CEC combines the separation efficiency of CE with stationary and mobile phase selectivity of LC [52]. Vassort et al. developed a CZE-MS method for the impurity profiling of six pharmaceuticals which was also found to be orthogonal with the LC–MS method [53]. SFC-MS instrument technique has the advantage of non-requirement of LC solvents. Many studies reported the use of SFC-MS for impurity characterization [54]. LC-NMR technique is specifically useful for isomer analysis for which the mass and fragmentation patterns are identical. It has the additional advantage of high resolution and improved sensitivity. The major limitations are that a large volume of expensive deuterated solvents is required and difficult to have C13 spectra due to low sample concentration in LC effluent [55]. Murakami et al. reported characterization of impurities in olmesartan medoxomil tablets using LC-NMR [56]. CE-NMR provides the same benefits as provided by LC-NMR. The limitation is that the sample output from CE is very low resulting in reduced residence time in NMR. This leads to a decrease in sensitivity [57]. Very few reports have been published reporting the use of CE-NMR for analysis of impurities. Wolters et al. characterized atenolol as an impurity in sucrose [58]. LC-FTIR technique is not very popular as IR requires 1–5 mg of sample for analysis, which is not possible when impurities are generated in minute quantities. In one study, MS, NMR, and IR were hyphenated with LC and collectively used for the identification of three degradation products of cefpodoxime proxetil [47, 59].
Techniques for Determining Elemental Impurities
There are various techniques available for the detection of elemental impurities. A laser-induced breakdown spectrometer (LIBS) is utilized for the detection of inorganic contaminants [60]. UV/visible spectrophotometry offers various advantages as it is relatively cheap, simple, and rapid. It measures the concentration of several metals in colored samples. For the samples that are not colored, a UV/visible spectrophotometer incorporates the use of ligands that bind with copper and iron and other metals to increase the sensitivity of detection by forming colored complexes. The use of UV–visible spectrophotometry for the detection of elemental impurities has been demonstrated by Yadav et al. for the determination of molybdenum in pharmaceutical samples [61]. Techniques like atomic absorption spectrometry (AAS) are used for the detection of metal impurities in pharmaceuticals and drugs at microgram to nanogram levels. It has better sensitivity and requires only a small amount of solution for the analysis. It has been used for the determination of Cu impurity in Wenglitong capsules [62]. X-ray fluorescence spectrometry (XRF) is an attractive technique for the detection of elemental impurities because of its ease of sample preparation requirement. Various types of XRF techniques include wavelength dispersive XRF (WD-XRF), energy dispersive XRF (ED-XRF), and total reflection XRF [63]. Metals such as Zn, Fe, and Ni were easily detected in API when the abovementioned techniques were employed [64]. Samples of lecithin, insulin, procaine, and tryptophan have been investigated by Wagner et. al. using these techniques [65]. The only limitation of these techniques is their low sensitivity. Instrumental neutron activation analysis (INAA) is proved to be a powerful technique in the determination of elemental abundance in a variety of materials. The principle of this technique involves neutron irradiation of sample followed by measurement of radiation from the radionuclides formed either directly or indirectly. The various advantages of INAA technique include less sample preparation, possibility of simultaneous multi-elemental analysis of up to 40 elements, requirement of very little amount of sample, higher sensitivity, and its non-destructive nature. Chen et. al. reported the analysis of fourteen trace elements in drug samples of natural origin by using INAA [66]. It has certain limitations including the requirement of a reactor and needs for a longer time for cooling. Another technique is inductively coupled plasma (ICP) atomic emission spectrometry (AES). The principle is based on the emission of light by elements in an ICP source. It has become popular as it can simultaneously analyze up to 60 elements with high sensitivity. It has a dynamic range of detection and high sensitivity. USP general chapter < 233 > describes two techniques, ICP-AES and ICP-MS for the estimation of elemental impurities [67]. Celina et. al reported ICP-AES-based analysis on different medicinal tablets to determine elemental impurities such as As, Cd, Cu, Cr, Fe, Hg, Ir, Mn, Mo, Ni, Os, Pb, Pd, Pt, Rh, Ru, V, and Zn [68]. The other technique ICP-MS can offer sensitivity for detection up to one part in 1015 for many elements [69]. This technique was utilized for the determination of Pt with a detection limit of 15 ng/g in enalapril maleate [70]. Microwave plasma atomic emission spectrometry (MP-AES) was introduced in 2011 and is utilized in the pharmaceutical industry as an inorganic content analysis. Laser ablation ICP-MS (LA-ICP-MS) has become a powerful elemental analysis technique as it does not require much preparation of samples. The technique is capable to analyze the elemental impurities present in both solid and liquid samples. The only disadvantage of LA-ICP-MS is the unavailability of a matrix reference standard for calibration and validation. It has recently been introduced for the analysis of elemental impurities in the pharmaceutical industry to regulate inorganic contaminants [71, 72].
Control Strategy to Minimize the Risk of Impurities
Control of impurities is one of the critical concerns in the pharmaceutical industry. There are various control strategies to minimize the risk of impurities such as material control, process control, and end-product testing [73].
Material Control
It is essential to check the quality and purity of the raw materials used for the manufacturing of drug substances and drug products. Impurity levels can significantly affect the safety profile and, therefore, are considered to be important critical quality attributes. For raw materials or intermediates, certain specifications for impurity content need to be set. There should be a robust method for the detection of impurities in drug products. Impact of the changes in the manufacturing site or process and raw material on impurity content in the final product should be critically evaluated. In a report, it was reported that after switching to a new supplier for purchasing the coupling agent dicyclohexylcarbodiimide (DCC), a new impurity was observed in repaglinide. This was due to the presence of an impurity (cyclohexylamine) in the coupling agent supplied by the new vendor [74].
Process Control
During the manufacturing process, it is of utmost importance to obtain knowledge regarding the source of impurities. It is required to assess whether the impurities can react further and convert into other structures and whether suitable purification processes such as crystallization and extraction are available for their removal. In-process quality control testing should be performed for the detection of impurities. Reaction mixtures, intermediates, and filtrates should be analyzed to obtain information on impurities during the synthesis process of the drugs. Prior understanding of the origin of the generation of impurities and its subsequent identification can help to modify the reaction conditions to minimize the formation of impurities. To minimize the impurities in drug substances to a negligible limit, there should be tight control over impurities in the intermediate steps [9]. The equipment used in the process should be of good quality, properly maintained, and cleaned as per the relevant SOPs. Solvents and reagents used in the process should meet the specifications. Standard methods should be followed to remove the residual solvents from the drug substance or drug products. To ensure the complete removal of solvents, pharmaceutical companies have started to use in-process analyzers. GC–MS is used for this purpose in which the mass system analyzes the headspace above the material and measures the concentration of solvent [75]. Interaction of an API with excipients leads to the formation of degradation products. The presence of a trace amount of reactive impurity can influence safety and efficacy of the final product. Therefore, it is important to understand API-excipient interaction. For instance, polysaccharides containing reducing sugars like glucose and lactose can undergo Millard’s reaction with amine drugs. Primary amine drug such as vigabatrin undergoes Millard reaction with lactose and monosaccharide [76]. The presence of reducing sugars in mannitol can lead to oxidative degradation of cyclic heptapeptide from a lyophilized formulation. Excipients such as MCC, starch, cross povidone, and PEG contain impurities of formaldehyde, acetaldehyde, and furfuraldehyde. Breakdown of the polymeric chain of PEG and polysorbates could form an impurity, i.e., formaldehyde. In the long-term stability of film-coated tablets of irbesartan, it was found that the drug degraded to hydroxymethyl derivative [76]. Polymeric excipients such as povidone, crosspovidone, PEG, polyethylene oxide, and hydroxypropyl cellulose contain trace levels of hydroperoxide impurities. The drying process can build up peroxides more rapidly as it is conducted at a high temperature. Nitrite and nitrate impurities in an excipient can lead to the formation of N-nitroso compounds. Hence, it is important to identify the incompatibilities between excipients, impurities in excipients, and drugs. This can avoid undesirable events in late-stage development. Stability studies of excipients also should be performed with the drug to form a robust drug product. The risk of impurities due to excipients can be minimized by controlling the crystal properties of active pharmaceutical ingredients, manufacturing processes, packaging, and storage conditions. The final formulation should contain excipients that are highly stable and completely inert. There should be proper communication between pharmaceutical companies and the excipient vendor [76].
Water is extensively used in the pharmaceutical industry as raw material, starting material, in manufacturing, for preparation of reagents, solvent systems, and cleaning purposes. Contaminants present in water can adversely affect every step of the formulation. It can react with drug substances and products to generate undesirable impurities which can lead to serious health hazards. To prevent these risks, proper precautions and controls should be taken. Throughout the entire process of production, storage, and distribution, the chemical and microbial quality of water should be monitored. Different grades of water are available depending on its intended use. The right quality of water meeting the required specifications should only be used. Sources of water should be investigated and monitored for chemical, microbial, and endotoxins [77]. The cleaning procedure should effectively remove residues of drug products, degradation products, excipients, preservatives, and cleaning agents to prevent cross-contamination with impurities and to prevent microbial growth. It is essential to assure that the equipment is clean to protect the quality and safety of products. To check the effectiveness of the procedure, cleaning validation should be carried out. There are levels of cleaning which are dependent on the usage of equipment (whether the equipment is dedicated or not), stages of manufacturing (early, intermediate or final step), nature of the potential contaminant, etc. [78]. Additionally, evaluation of equipment design is another important consideration. Equipment having a blind area that is difficult to clean must be evaluated. Equipment should be easy to disassemble and easy to move [78]. Environmental conditions at the manufacturing site such as temperature, humidity, and air ventilation should be in control following the requirements of manufacturing conditions to prevent drug degradation. It is difficult to handle powder at high humidity as it can capture moisture from the environment. There should be an airlock system to separate the areas of high and low humidity. According to the requirement of the product, humidity should be controlled. Humidifiers are one of the sources of microbial contamination. Hence, it should be avoided wherever possible. Humidification can be achieved by injection of steam into the air stream. For the manufacturing of protein drug formulation, temperature becomes a critical aspect. For sterile products, clean room classification is critical. Therefore, it is essential to have a proper air ventilation system in the manufacturing plant. The design facility should include proper heat ventilation and air conditioning system (HVAC) to prevent air born contamination. Laminar airflow system consisting of HEPA filters works effectively in grade A areas. Packaging is one of the major sources of elemental impurities.
Leaching of elements from packaging in drug products is responsible for elemental impurities. Many times, the manufacturing of plastic and rubber materials does not take place according to cGMP, and even small changes in process or technique can attract leachables in the finished product. There are various methods to test different packaging materials for extractables and leachables. Regulatory agencies such as WHO, GMP, USFDA, and ICH provide guidelines and specifications on packaging materials testing [79]. The glass containers should be tested by crushed glass test, water attack test, whole container test, chemical resistance test, etc. Closure materials should be tested by transparency test, self-seal test, extractive test, and penetrability fragmentation test. Plastics are also tested by leakage test, collapsibility test, water vapor permeability test, and transparency test. The work area, packaging machine, and printing machine should be clean, and free from contaminants. Moreover, packaging material and labeling should be checked for quality, quantity, and conformity [79].
End Product Testing
End product testing is a mandatory requirement before dispatching a drug substance or drug product. It ensures the purity, integrity, efficacy, and safety of pharmaceuticals [73]. Storage conditions should be defined by stability testing of the end product. Possible degradation products are required to be predicted by performing forced degradation studies. Moreover, in silico studies can predict the possible degradation impurities of the drugs. Based on all such studies, storage conditions such as temperature, humidity, pH, and light can be recommended for the end products.
Transport Validation
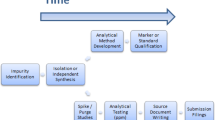
Transport validation of pharmaceuticals is carried out to ensure that the product will remain unaffected by environmental conditions. It generates important data on the performance of packaging during transport. The protocol should include validation process design, transport process qualification, and continuous monitoring of transport. Laboratory tests such as vibration testing, basic drop testing, shock impact testing, basic drop testing, UV-radiation, and temperature cycles should be performed to mimic the actual conditions. It is necessary to monitor and control the transport conditions during the shipping of products. There is great variation in environmental conditions from origin to destination such as temperature excursions and humidity. Transport plans should consider distance, handovers, environmental conditions, and extreme exposures during shipping. Small changes, even within ± 2 °C in temperature, can affect the quality of the drug. For biomaterials, cryogenic temperature (−150 °C) is required to prevent them from degradation. To minimize the risk, real-time monitoring devices should be used constantly. Passive packaging can maintain stability over extreme temperature conditions but it should be selected depending on the time required for transportation, as it gets expired after a certain period. For active packaging, it must be ensured that the temperature is preserved across the supply chain, which can be accomplished by introducing procedures and educating workers on how to manage active packaging solutions when they need to manage [80]. Figures 2, 3, and 4 shows the various industrial criticality that can generate impurity and make the product unsafe. Figure 5 represents control strategies for impurities that can be applied during the manufacturing process.
Control of Impurities in Biologics
Apart from small molecules, protein-based therapeutics such as monoclonal antibodies, blood derived therapeutics, allergenic extract, cellular therapies, vaccines, and gene therapies are available for the treatment of various life-threatening diseases. These biologics may produce some unfortunate adverse effects like anaphylaxis, immune complex diseases, and off-target binding. The contaminants and impurities present in the products may also have serious adverse effects. Unlike small molecule, the quality control strategy for biologics is not simple and consists of numerous challenges. The conventional approaches like capillary electrophoresis, reverse and normal phase HPLC, ion exchange chromatography, and hydrophilic interaction chromatography are used by the manufacturer at every drug development phase to ascertain the quality of biologics. These techniques may not always have the required specificity and accuracy to ascertain the efficacy and safety of a biological product. The prime obstacle for quality control of biologics is the difficulty to detect the site-specific structural changes in the amino acid sequences. ICH Q6B dictates the quality specification for biologics intended for market uses. Physicochemical characterization, potency test, and immunological aspects of biologics are taken into consideration for the product quality surveillance. To achieve a sustained quality attribute of the biologics, different quality control measures must be practiced during the manufacturing process, storage period, and after the marketing of the product. Post-marketing surveillance for biologics is of utmost importance as the product quality can be compromised with a minor variation in the storage or shipping conditions. The complex structure of the product with a high risk of contamination makes it difficult to establish the purity profile of these products. The implement of orthogonal methods like LC–MS-based multiple-attribute method along with quality by design phenomenon identifies the critical quality attributes. The variation critical process parameters of manufacturing process and critical material parameters of the raw material, intermediates, and reagents eventually affect the critical quality attributes of the product. The control strategy involves the interplay among different process and material parameters and attributes of materials and intermediates, in-process control strategy, facility and equipment operation monitoring, finished product specification, and associated methods and frequency of monitoring and control [81, 82].
Expert Opinion
The presence of unwanted and unforeseen impurities is the primary cause of product recall due to the inferior safety profile of drugs. Non-compliance with the regulatory guidelines and deviation from the cGMP protocol leads to the generation of impurities in a pharmaceutical product. Insufficient knowledge and ill-trained personnel are other aspects of the irregularities which aid to the product failure. These factors are collectively responsible for the post-marketing product recall. Strategies like spiking and purging of impurities are implemented at each step of the synthesis scheme to monitor the purging capability of the process. This ensures that the analytical approaches are capable to analyze the specified impurity limit at each step. This early stage control strategy improves the product quality and can reduce the incidents of post-marketing withdrawals of pharmaceuticals. The best possible way to ensure the good safety profile of a drug is to control its associated impurities. This can be achieved by a clear understanding of the route and factors for their formation. Impurities can be generated during the synthesis process of actives, manufacturing process of their formulations, storage of starting material, raw material, intermediates, excipients, reagents, packaging material, or from associated apparatus and equipment. Establishing tight control on manufacturing, storage and transport can have a beneficial effect on minimization of impurity generation. This in turn will enhance the safety profile of the drugs and can minimize the risk of product recalls. Control strategies can be developed by employing quality by design (QbD), and risk assessment-based approaches. Prior understanding of key aspects of safety profile of the impurities, post-marketing surveillance criticalities, and establishment of constructive preventive strategies is always the best way to minimize unwanted product recall due to associated safety issues. The testing methods employed in the quality control of the drugs must be validated. However, designing and executing such control strategies may not eliminate the generation of drug impurities. The impurities can be restrained to a minimum by designing stringent protocols on manufacturing and storage parameters. Preventive actions against all the critical aspects of the manufacturing and post-manufacturing criticality can ensure the highest standard of safety of a drug product. The incorporation of process analytical technology during drug discovery and development phase eliminates the risk of product failure at a late stage. Early stage identification of critical quality attributes and critical material attributes by empowering quality target product profile as well as risk assessment and their correlation in the desired DoE by QbD approach with multivariate analysis generates a product with high quality. These approaches cumulatively strengthen the continued process verification by which the time and cost for the production of high-quality products can be minimized. Moreover, the integrity of the quality can be maintained throughout the product life cycle. In conclusion, the novelty of this article can be justified by the unavailability of any similar type of reports which briefly discussed the potential sources of drug impurities including the functional pathway of regulatory bodies aiming their effective control. This article also contains a novel, critical, and constructive prevention strategy to establish a robust safety profile of pharmaceutical products.
References
Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA. 1999;281(9):824–9.
Dart RC. Monitoring risk: post marketing surveillance and signal detection. Drug Alcohol Depend. 2009;105:S26–32.
Lorenz E. The butterfly effect. World Scientific Series on Nonlinear Science Series A. 2000;39:91–4.
Bai HK, Ahearn JD, Bartlett MG. Over-the-counter drugs: regulatory analysis of warning letters between fiscal years 2015–2019. Ther Innov Regul Sci. 2020:1–11.
Ni JZ, Flynn BB, Jacobs FR. Impact of product recall announcements on retailers׳ financial value. Int J Prod Econ. 2014;153:309–22.
ICH. Q3A(R2) : Impurities in new drug substances ICH harmonised tripartite guideline; 2006. p. 1–11.
Rao NR, Kiran S, Prasanthi N. Pharmaceutical impurities: an overview. Indian J Pharm Educ Res. 2010;44(3):301–10.
Wadekar KR, Bhalme M, Rao SS, Reddy KV, Kumar LS, Balasubrahmanyam E. Evaluating impurities in drugs (Part I of III). Pharm Technol. 2012;36(2):46–51.
Prajapati P, Agrawal YK. Analysis and impurity identification in pharmaceuticals. Rev Anal Chem. 2014;33:123–33.
Goyer RAC, Thomas W. Toxic effects of metals, Casarett and Doull’s toxicology: the basic science of poisons. The McGraw-Hill Companies. 1996; 811–867.
International Council for Harmonisation. Q3C(R6): Guideline for residual solvents. ICH harmonized guideline; 2016; 1–32.
Tawde SA. Particulate matter in injectables: main cause for recalls. J Pharmacovigil. 2014;3:1–2. https://doi.org/10.4172/2329-6887.1000e128.
Zmitek J, Grahek R, Zizek T, Furlan B. Sources of impurities-investigation of 4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-2-[(2-phtalim ido)-methyl]-1, 4-dihydropyridine traces formation during the synthesis of amlodipine besylate. Acta Chim Slov. 2000;47:63–8.
Choudhary A. Mix up and cross contamination in pharmaceutical manufacturing [7–12–20]. Available from: https://www.pharmaguideline.com/2011/09/mix-up-and-cross-contamination-in.html.
White paper: Prevention of contamination and cross-contamination in medicinal manufacturing facilities: PharmOut; 2016 [18–12–20]. Available from: https://www.pharmout.net/downloads/white-paper-prevention-of-contamination-and-cross-contamination.pdf.
Kushwaha P. Microbial contamination: a regulatory perspective. J Pharm Res. 2010;3:124–31.
FDA. Company announcement. Mylan pharmaceuticals initiates voluntary nationwide recall of one lot of Alprazolam tablets, USP C-IV 0.5 mg, due to the potential of foreign substance 2019 [18–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/mylan-pharmaceuticals-initiates-voluntary-nationwide-recall-one-lot-alprazolam-tablets-usp-c-iv-05.
Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20(10):18759–76.
Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, Morris J. Ritonavir: an extraordinary example of conformational polymorphism. Pharm Res. 2001;18(6):859–66.
Pokar D, Rajput N, Sengupta P. Industrial approaches and consideration of clinical relevance in setting impurity level specification for drug substances and drug products. Int J Pharm. 2020;576:119018.
ICH. Q3B(R2) : Impurities in new drug products ICH harmonised tripartite guideline; 2006. p. 1–12.
ICH. Q3C(R6) : Guideline for residual solvents. ICH harmonised guideline; 2016. p. 1–32.
ICH. Q3D(R1) : Guideline for elemental impurities ICH harmonised guideline; 2019. p. 1–82.
ICH. M7(R1) : Assessment and control of dna reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. ICH harmonised guideline; 2017. p. 1–116.
MAPP C. Establishing impurity acceptance criteria as part of specifications for NDAs, ANDAs, and BLAs based on clinical relevance. Office of pharmaceutical quality; 2020. p. 1–10.
CVMP. Guideline on setting specifications for related impurities in antibiotics. European medicines agency; 2012. p. 1–15.
USP. <1086> Impurities in drug substances and drug products; 2014. p. 1–13.
IP. Impurities In: Welfare MoHaF, editor.: Indian Pharmacopoeia; 2010. p. 656–8.
BP. Control of impurities. British pharmacopoeia; 2018. p. 666–8.
EP. 5.10. Control of impurities in substances for pharmaceutical use; 2005. p. 559–61.
Cok I, Emerce E. Overview of impurities in pharmaceuticals: Toxicological aspects. Asian chem lett. 2012;16:87–97.
Fuller C, Saibil D. Post-market1 surveillance of therapeutic drugs in Canada. 2005 [cited 2020 09–12]. Available from: https://whp-apsf.ca/en/documents/adrBackground.html#top.
Ullrich P. Vioxx recall and NSAID side effects. 2005 [cited 2020 12–12]. Available from: https://www.spine-health.com/treatment/pain-medication/vioxx-recall-and-nsaid-side-effects.
Raj N, Fernandes S, Charyulu NR, Dubey A, GS R, Hebbar S. Postmarket surveillance: a review on key aspects and measures on the effective functioning in the context of the United Kingdom and Canada. Ther Adv Drug Saf. 2019;10:2042098619865413.
Gough S. Post-marketing surveillance: a UK/European perspective. Curr Med Res Opin. 2005;21(4):565–70.
McGovern T, Jacobson-Kram D. Regulation of genotoxic and carcinogenic impurities in drug substances and products. TrAC, Trends Anal Chem. 2006;25(8):790–5.
Sahu AK, Goswami A, Kate AS, Sengupta P. Identification and structural characterization of potential degraded impurities of ribociclib by time of flight -tandem mass spectrometry, and their toxicity prediction. J Pharm Biomed Anal. 2021;197:113933.
Sharma MK, Shah RP, Sengupta P. Amalgamation of stress degradation and metabolite profiling in rat urine and feces for characterization of oxidative metabolites of flibanserin using UHPLCQ-TOF-MS/MS, H/D exchange and NMR technique. J Chromatogr B. 2020;1139:121993.
Sharma MK, Pandey K, Shah RP, Kumar D, Sengupta P. A mechanistic explanation on degradation behavior of flibanserin for identification and characterization of its potential degradants using LC-DAD/ESI/APCI-Q-TOF-MS/MS. Microchem J. 2021;167:106281.
Swamy N, Reddy VK, Thorat P, Sengupta P. Development and validation of a stability indicating high performance liquid chromatography method for trimethobenzamide. Braz J Pharm Sci. 2020;56:e18817.
Qiu F, Norwood DL. Identification of pharmaceutical impurities. J Liq Chromatogr Relat Technol. 2007;30(5–7):877–935.
Tulshiram DG, Umamaheshwari D. Novel analytical techniques used in identification and isolation of impurities in pharmaceuticals an overview. J Pharm Sci Res. 2020;12(1):37–42.
Kupcewicz B, Ronowicz J, Balcerowska-Czerniak G, Walasek A, Budzisz E. Evaluation of impurities in simvastatin drug products with the use of FT-IR spectroscopy and selected chemometric techniques. Open Chem. 2013;11(8):1320–9.
Suntornsuk L. Capillary electrophoresis in pharmaceutical analysis: a survey on recent applications. J Chromatogr Sci. 2007;45:559–76.
Shen Y, Liu H, Rong S, Li Y, Hu C. Simultaneous determination of Cephradine, L-Arginine, and Cephalexin in Cephradine for injection by capillary zone electrophoresis. Anal Lett. 2006;39(3):569–78.
Dhangar KR, Jagtap RB, Surana SJ, Shirkhedkar AA. Impurity profiling of drugs towards safety and efficacy: theory and practice. J chil chem soc. 2017;62(2):3543–57.
Pokar D, Sahu AK, Sengupta P. LC-Q-TOF-MS driven identification of potential degradation impurities of venetoclax, mechanistic explanation on degradation pathway and establishment of a quantitative analytical assay method. J Anal Sci Technol. 2020;11(54):1–13.
Lee H, Shen S, Grinberg N. Identification and control of impurities for drug substance development using LC/MS and GC/MS. J Liq Chromatogr Relat Technol. 2008;31(15):2235–52.
Bonadio F, Margot P, Delémont O, Esseiva P. Optimization of HS-SPME/GC–MS analysis and its use in the profiling of illicit ecstasy tablets (Part 1). Forensic Sci Int. 2009;187:73–80.
Gimeno P, Besacier F, Chaudron-Thozet H, Girard J, Lamotte A. A contribution to the chemical profiling of 3, 4-methylenedioxymethamphetamine (MDMA) tablets. Forensic Sci Int. 2002;127:1–44.
Panday NK, Thakkar D, Patel S, Shard A, Sengupta P. Metabolite profiling of IMID-2, a novel anticancer molecule of piperazine derivative: in silico prediction, in vitro and in vivo metabolite characterization using UPLC–QTOF–MS/MS. Biomed Chromatogr. 2021;35:e5082.
Visky D, Jimidar I, Ael WV, Vennekens T, Redlich D, Smet MD. Capillary electrophoresis-mass spectrometry in impurity profiling of pharmaceutical products. Electrophoresis. 2005;26:1541–9.
Vassort A, Barrett DA, Shaw PN, Ferguson PD, Szucs R. A generic approach to the impurity profiling of drugs using standardised and independent capillary zone electrophoresis methods coupled to electrospray ionisation mass spectrometry. Electrophoresis. 2005;26(9):1712–23.
Brondz I, Ekeberg D, Bell DS, Annino AR, Hustad JA, Svendsen R, et al. Nature of the main contaminant in the drug primaquine diphosphate: SFC and SFC–MS methods of analysis. J Pharm Biomed Anal. 2007;43(3):937–44.
Phadke R, Mali R, Mundhe A, Gosar A. Drug impurity profiling an emerging task to pharmaceutical industries now days - a review. Am J Pharm Tech Res. 2019;9(2):94–111.
Murakami T, Konno H, Fukutsu N, Onodera M, Kawasaki T, Kusu F. Identification of a degradation product in stressed tablets of olmesartan medoxomil by the complementary use of HPLC hyphenated techniques. J Pharm Biomed Anal. 2008;47(3):553–9.
Singh S, Handa T, Narayanam M, Sahu A, Junwal M, Shah RP. A critical review on the use of modern sophisticated hyphenated tools in the characterization of impurities and degradation products. J Pharm Biomed Anal. 2012;69:148–73.
Wolters AM, Jayawickrama DA, Larive CK, Sweedler JV. Capillary isotachophoresis/NMR: extension to trace impurity analysis and improved instrumental coupling. Anal Chem. 2002;74(10):2306–13.
Fukutsu N, Kawasaki T, Saito K, Nakazawa H. Application of high-performance liquid chromatography hyphenated techniques for identification of degradation products of cefpodoxime proxetil. J Chromatogr A. 2006;1129(2):153–9.
Lal B, Yueh F-Y, Singh J. Laser induced breakdown spectroscopy for quality control in pharmaceuticals industry. J Opt. 2005;34:181–92.
Yadav R, Shirish P, Janwadkar S. Solvent extraction and spectrophotometric determination of Mo(VI) by using acetophenone 2’,4’-dihydroxy semicarbazone as an analytical reagent. Int J Pharm Bio Sci. 2012;3(4):309–14.
Zhang M, Wang M. FAAS determination of copper in Wenglitong capsules. Chinese J Pharm Anal. 2008;28(10):1755–6.
Chowdhury AR, Maheshwari N, Soni J, Kapil M, Mehta T, Mukharya A. Quantitative X-ray fluorescence analysis: trace level detection of toxic elemental impurities in drug product by ED-XRF spectrometer. J Pharm Biomed Anal. 2020;189:113292.
Gonçalves LM, Costa IM, Brito JA. Assessment of metal elements in final drug products by wavelength dispersive X-ray fluorescence spectrometry. Anal Methods. 2011;3(7):1468–70.
Wagner M, Rostam-Khani P, Wittershagen A, Rittmeyer C, Kolbesen B, Hoffmann H. Trace element determination in drugs by total-reflection X-ray fluorescence spectrometry. Spectrochim Acta, Part B. 1997;52(7):961–5.
Keh-Shaw C, Te-Hsien L. Trace elements in natural drugs determined by INAA. J Radioanal Nucl Chem. 1993;170(1):265–80.
USP. <233> Elemental impurities—procedures. Second supplement to USP 38–NF 33; 2020. p. 1–5.
Celina Støvinga HJ, Bente G, Stefan S. Development and validation of an ICP-OES method for quantitation of elemental impurities in tablets according to coming US pharmacopeia chapters. J Pharm Biomed Anal. 2013;84:209–14.
Rao R, Talluri MVN. An overview of recent applications of inductively coupled plasma-mass spectrometry (ICP-MS) in determination of inorganic impurities in drugs and pharmaceuticals. J Pharm Biomed Anal. 2007;43:1–13.
Lasztity A, Varga I, Zih-Perenyi K, Bertalan E. Development of atomic spectrometric methods for trace metal analysis of pharmaceuticals. Microchem J. 2002;73:59–63.
Vita V, Andris A. Application of LA-ICP-MS as a rapid tool for analysis of elemental impurities in active pharmaceutical ingredients. J Pharm Biomed Anal. 2014;91:119–22.
Balaram V. Recent advances in the determination of elemental impurities in pharmaceuticals – status, challenges and moving frontiers. Trends Anal Chem. 2016;80:83–95.
Price E. Controlling impurities in drug manufacturing. 2016 [8–12–20]. Available from: https://www.pcisynthesis.com/controlling-impurities-in-drug-manufacturing/.
Olsen BA, Sreedhara A, Baertschi SW. Impurity investigations by phases of drug and product development. TrAC, Trends Anal Chem. 2018;101:17–23.
Lewis G. Maximizing solvent removal efficiency: Pharma manufacturing; 2014 [15–12–20]. Available from: https://www.pharmamanufacturing.com/articles/2014/maximizing-solvent-removal-efficiency/.
Wu Y, Levons J, Narang AS, Raghavan K, Rao VM. Reactive impurities in excipients: profiling, identification and mitigation of drug–excipient incompatibility. AAPS PharmSciTech. 2011;12(4):1248–63.
WHO. Good manufacturing practices: Water for pharmaceutical use. Annex; 2005.
Lodhi B, Padamwar P, Patel A. Cleaning validation for the pharmaceuticals, biopharmaceuticals, cosmetic and neutraceuticals industries. J Innov Pharm Biol Sci. 2014;1(1):27–38.
Sahil Jasuja M. Quality control testing of packaging materials [8–12–20]. Available from: https://www.pharmatutor.org/articles/quality-control-testing-packaging-materials.
Markarian J. Understanding risks in pharmaceutical shipping. 2015 [08–12–20]. Available from: https://www.pharmtech.com/view/understanding-risks-pharmaceutical-shipping.
Rogers RS, Abernathy M, Richardson DD, Rouse JC, Sperry JB, Swann P, et al. A view on the importance of “multi-attribute method” for measuring purity of biopharmaceuticals and improving overall control strategy. AAPS J. 2018;20(1):1–8.
Control of biologics. Available from: https://www.ema.europa.eu/en/documents/presentation/presentation-control-biologics-kowid-ho-afssaps_en.pdf. Accessed 10 Nov 2021.
Food and drug administration. Hospira issues a voluntary nationwide recall for one lot of Vancomycin Hydrochloride for injection, USP, 750 mg/vial due to the presence of particulate matter within a single vial. 2017a [18–12–2020]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/hospira-issues-voluntary-nationwide-recall-one-lot-vancomycin-hydrochloride-injection-usp-750-mgvial.
White ME. Aspirin tablets recalled. 2017 [11–12–20]. Available from: https://www.managedhealthcareconnect.com/index.php/content/aspirin-tablets-recalled.
MoneyWorks4me. Sun Pharma recalls Testosterone Cypionate injection from US market. 2017 [cited 2020 12–12]. Available from: https://www.moneyworks4me.com/company/news/index/id/245550.
Food and drug administration. ICU medical issues a voluntary nationwide recall of one lot of 0.9% sodium chloride injection due to the presence of particulate matter. 2017b [12–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/icu-medical-issues-voluntary-nationwide-recall-one-lot-09-sodium-chloride-injection-due-presence.
BAXTER. Baxter Issues a voluntary nationwide recall for one lot of Nexterone injection due to presence of particulate matter. 2017 [12–12–20]. Available from: https://www.baxter.com/baxter-newsroom/baxter-issues-voluntary-nationwide-recall-one-lot-nexterone-injection-due-presence.
Anesthesiologists. Ampicillin and Sulbactam for injection USP 1.5 g/vial by Auromedics: Recall - Presence of glass particles in vial. 2018 [12–12–20]. Available from: https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/fda-alerts/2018/01/ampicillin-and-sulbactam-for-injection-usp-1-and-half-g-vial-by-auromedics-recall-presence-of-glass.
FDA. AuroMedics recalls Ampicillin and Sulbactam for injection. 2018 [12–12–20]. Available from: https://www.ormanager.com/briefs/fda-auromedics-recalls-ampicillin-and-sulbactam-for-injection/.
Pathan A. Lamotrigine and phenytoin indicated for epilepsy recalled by the manufacturer in America. NeuroPharmac Journal. 2020;5:118–20.
Health G. Drug recall list. 2018 [18–12–20]. Available from: https://fm.formularynavigator.com/FormularyNavigator/DocumentManager/Download?clientDocumentId=KW9btDDkAEKmRemwQkM9UQ.
Kansteiner F. Fresenius Kabi recalls 2 lots of scarce ICU sedative over potential lidocaine contamination. 2020 [19–12–20]. Available from: https://www.fiercepharma.com/manufacturing/fresenius-kabi-recalls-sedative-over-lidocaine-contamination.
Safemedicines. Nigerian children killed by contaminated teething medicine [19–12–20]. Available from: https://www.safemedicines.org/nigerian-children-killed-by-contaminated-teething-medicine.
Palmer E. Updated: GSK recalls more than 425K tubes of antibiotic cream. 2015 [19–12–20]. Available from: https://www.fiercepharma.com/partnering/updated-gsk-recalls-more-than-425k-tubes-of-antibiotic-cream.
USFDA. SD import issues voluntary nationwide recall of aphrodisiac capsules due to presence of undeclared Sildenafil. 2019 [19–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/sd-import-issues-voluntary-nationwide-recall-aphrodisiac-capsules-due-presence-undeclared-sildenafil.
USFDA. Ata Int. Inc. Issues voluntary nationwide recall of bluefusion capsules, due to presence of undeclared Sildenafil, Tadalafil, Desmethyl carbodenafil, Dithiodesmethyl carbodenafil, Scutellarin and Daidzein. 2019 [19–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ata-int-inc-issues-voluntary-nationwide-recall-bluefusion-capsules-due-presence-undeclared.
USFDA. EZ weight loss TX LLC issues voluntary nationwide recall of atomic and xplode capsules due to the presence of undeclared Sibutramine. 2017 [19–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ez-weight-loss-tx-llc-issues-voluntary-nationwide-recall-atomic-and-xplode-capsules-due-presence.
Food and drug administration. Marcas USA, LLC issues voluntary nationwide recall of pasta de lassar andromaco skin protectant 25% zinc oxide 60g due to potential contamination. 2018b [12–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/marcasusa-llc-issues-voluntary-nationwide-recall-pasta-de-lassar-andromaco-skin-protectant-25-zinc.
Food and drug administration. Lannett issues voluntary nationwide recall of Levetiracetam oral solution, 100mg/ml due to microbial contamination. 2019b [12–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/lannett-issues-voluntary-nationwide-recall-levetiracetam-oral-solution-100mgml-due-microbial.
Performrx. Drug information update. 2019 [19–12–20]. Available from: https://www.performrx.com/sites/default/files/downloads/Drug%20Information%20Update%20June%202019.pdf.
Health G. 2019 Drug recall list. 2019 [19–12–20]. Available from: https://fm.formularynavigator.com/FormularyNavigator/DocumentManager/Download?clientDocumentId=oHi502QKvUqwjozPdyO6NQ.
Food and drug administration. Pfizer Inc. issues a voluntary nationwide recall for 2 lots of relpax® (eletriptan hydrobromide) 40 mg tablets due to potential microbiological contamination of non-sterile products. 2019c [12–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/pfizer-inc-issues-voluntary-nationwide-recall-2-lots-relpaxr-eletriptan-hydrobromide-40-mg-tablets.
Food and drug administration. Sagent pharmaceuticals issues voluntary nationwide recall of ketorolac tromethamine injection, USP, 60mg/2ml (30mg per ml) due to lack of sterility assurance. 2019d [12–12–20]. Available from: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/sagent-pharmaceuticals-issues-voluntary-nationwide-recall-ketorolac-tromethamine-injection-usp.
Acknowledgements
We are thankful to NIPER-A and the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, New Delhi, India, for providing the necessary facilities and supports for compiling this review.
Author information
Authors and Affiliations
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tapkir, N., Soni, F., Sahu, A.K. et al. A Comprehensive Review on Assessment and Key Control Strategies for Impurities in Drug Development with a Special Emphasis on Post-marketing Surveillance. J Pharm Innov 17, 1510–1529 (2022). https://doi.org/10.1007/s12247-021-09607-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09607-9