Abstract
Purpose
There is a growing interest in continuous biopharmaceutical processing due to the advantages of small footprint, increased productivity, consistent product quality, high process flexibility and robustness, facility cost-effectiveness, and reduced capital and operating cost. To support the decision making of biopharmaceutical manufacturing, comparisons between conventional batch and continuous processing are provided.
Methods
Various process unit operations in different operating modes are summarized. Software implementation as well as computational methods used are analyzed pointing to the advantages and disadvantages that have been highlighted in the literature. Economic analysis methods and their applications in different parts of the processes are also discussed with examples from publications in the last decade.
Results
The results of the comparison between batch and continuous process operation alternatives are discussed. Possible improvements in process design and analysis are recommended. The methods used here do not reflect Lilly’s cost structures or economic evaluation methods.
Conclusion
This paper provides a review of the work that has been published in the literature on computational process design and economic analysis methods on continuous biopharmaceutical antibody production and its comparison with a conventional batch process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the monoclonal antibody market has been greatly growing [1,2,3,4]. Until 2014, 47 monoclonal antibodies (mAbs) had already been approved in the USA and Europe [5] and the approval rate is expected to be maintained or increased in coming years [6]. From the year 2008 to 2013, global sales of mAbs products rapidly grew from ~ $39 billion to ~ $75 billion, which was significantly larger than that of other recombinant protein therapeutics. Under this growth rate, the global sales of the mAbs are likely to reach $125 billion by 2020 [6] and $138.6 billion by 2024 [7].
Continuous production of mAbs becomes an increasingly promising alternative due to the increasing demand [8]. Continuous processing has multiple benefits including reduced capital cost, increased profitability, productivity, and equipment, buffer, and resin utilization, improved product quality, and flexibility [9,10,11]. Economic analysis can provide the industry with preliminary cost information that can be used at the production design stage [12]. Thus, to evaluate the cost-effectiveness of continuous processing, economic analysis methods that evaluate innovative processes and compare continuous and batch processes are needed.
Methods of cost analysis including both capital investment and prediction of Cost Of Goods (COG) have been introduced in the review paper [13] published in 2007. This work also compared software and risk analysis methods that can be used in economic evaluation of batch processes. Future trends and challenges were also highlighted in the paper. Seven years later, a book chapter [14] reviewed the prototype decision tool (based on discrete event simulations) developed at University College London, and explained its application through the economic analysis of both batch, continuous, and integrated biopharmaceutical processes. In order to improve the productivity and reduce the production cost, innovated techniques, such as aqueous two-phase systems (ATPS) for protein purifications, are designed. Torres-Acosta et al. [15] reviewed process economics of this new technology with case studies and showed its economic benefits compared to conventional purification process. It also mentioned that the ATPS had potential application in continuous biopharmaceutical processing. With increasing popularity of the topic, innovative processes and simulation software packages have been developed and updated. Economic analysis methods and simulation methods need to be further evaluated in combination with case studies of recent years. This review discusses economic analysis methods that specifically focus on biopharmaceutical manufacturing processes, highlights the important criteria in model building and cost analysis, reviews the case studies of economic assessment on continuous processing in recent years, and provides recommendations for future studies.
The review lists a general description of the steps in biologics manufacturing, and the assumptions introduced in various studies while presenting the software that can be applied in biopharmaceutical modeling. The important costs and methods necessary to be defined in economic analysis including specific cost analysis, decision-making tools, and stochastic analysis are summarized. This is then followed by a review of previous economic analysis publications in biopharmaceutical manufacturing, with the focus on continuous processing. In the final section, recommendations for future studies that can improve the understanding of the biopharmaceutical process economic structure and the impact of changes to the current process are presented.
Overview of Biologics Operations, Simulation Software Packages, and Economic Analyses
Biologics Operations
Biopharmaceutical processing is typically divided into two parts, upstream and downstream, and is mainly run in a batch mode. The scale of production ranges from the experimental bench scale [16] to the commercial manufacturing scale [17,18,19]. Biopharmaceutical processing transitions through three phases: preclinical, clinical, and commercial production. In the early phases of development, small quantities are produced (grams), but as of the protein therapeutics development progresses, kilograms of products are produced to support different operations including clinical trials. As the program becomes commercial, metric tons may be needed to cover the demand, especially for blockbuster therapies. There are specialized biologics like enzymes or therapies for rare diseases where commercial production is limited and the commercial batches are significantly smaller in size [19,20,21,22].
Unlike batch processing, continuous processing is not widely used and is not well defined, even in many research studies on continuous biopharmaceutical manufacturing. Table 1 presents the definitions of different operating modes provided by the FDA [23].
In mAbs production, a host cell is used to express the protein of interest (mAbs), and the choice of the host cell used has a great impact on process design. Host cells include microbials such as yeast and Escherichia coli, mammalian cells such as Chinese hamster ovary cells (CHO) and murine myeloma NS0 cells, or transgenic organisms such as plant cells [13, 24]. Protein expression in host cells can occur intracellularly, which is normally the case for prokaryotic cells [22]. Intracellular expression typically requires disintegration of the organism to release the protein, and in certain cases refolding of the protein is required to maintain the proper conformation [25]. On the other hand, in mammalian cells, protein expression is typically designed to occur extracellularly [26]. Different types of product-related and process-related impurities exist in host and media, such as DNA, unwanted proteins, and waste growth media. The impurities are removed through the manufacturing process which varies from product to product [27]. In transgenic organisms, solid and fat need to be also removed. A general schematic of a biopharmaceutical process using an intracellular and extracellular protein expression is shown in Fig. 1. In upstream operations, both microbial and mammalian cells are cultured in bioreactors or fermenters. In downstream, ion exchange methods need to be applied in all the processes for protein purification. Over the years, mammalian cell culture became the most competitive among the three hosts because the post-translational modification profile of CHO generates proteins that resemble human proteins the most, thus optimizing activity, reducing the occurrence of immunogenicity related adverse events, and leading to a favorable PK/PD profile. Both batch and continuous operations have been developed for mAbs production in mammalian cells, which are summarized in Fig. 2.
In upstream mammalian mAbs production, there are typically two steps: first, inoculation and cultivation, also called seed expansion, which occurs in seed bioreactors, and second, cell culture production which occurs in the production bioreactors [28]. Four types of cell culture conditions have been used in upstream mAbs production: batch, fed-batch, concentrated fed-batch, and perfusion bioreactors. Biopharmaceutical companies typically use batch or fed-batch bioreactors ranging in size between 5000 and 25,000 L with a duration of 10–17 days. The titer of the product proteins usually varies from 1 to 5 g/L, but some have reported reaching 10–13 g/L [29]. Concentrated batch and perfusion bioreactors are operated by continuously adding fresh media and removing exhaust media to maintain high cell concentration and long-term cultural process. In the case of concentrated fed-batch bioreactors, only the waste media is removed from the bioreactor, while both the proteins and cells are retained in the bioreactor. Thus, the titer of this process can reach up to 25–30 g/L [30]. Unlike concentrated fed-batch bioreactors, perfusion bioreactors only retain cells inside of bioreactor and harvest the protein produced with waste media in the filtrate. Thus, perfusion process can be applied in continuous protein production and can achieve long-term steady state cell growth in small scales. However, many researchers indicate that the titer of perfusion bioreactors is less than that of fed-batch bioreactors. For example, Pollock et al. [21] used the concentration of perfusion culture protein to be 20 and 45% of fed-batch culture in their economic analysis. In order to maintain cells in the perfusion bioreactor, cell retention devices are used. Cell retention can sometimes be referred to as the product clarification step that can exist either inside or outside of a perfusion bioreactor. Internal cell retention devices include spin filtration (SPIN), submerged membrane filtration, and external cell retention devices containing gravitation settlers, acoustic settlers, tangential flow filtration (TFF), and alternating tangential flow filtration (ATF) [9, 28, 31]. Single-pass tangential flow filtration which can be used in downstream formulation process is also investigated for cell culture harvest [32]. In ATF perfusion system, the cell density can reach as high as 130 million cells/mL at the steady state [33, 34]. The concentrated fed-batch reactors are mainly operated with filtration cell retention devices with microfiltration. Perfusion bioreactor can be operated with all the cell retention methods above. Based on the level of purity of perfusate, an extra filtration step may be necessary for the following downstream processing. Seed bioreactors can be either batch, fed-batch, or perfusion bioreactor. In this operation, the goal of a perfusion seed bioreactor is not to achieve continuous production, but to provide higher cell concentration for production bioreactor.
The downstream processing typically starts at the clarification step, but some companies consider it to be part of upstream operations. Traditionally, centrifuges followed by filtration setups are most widely used in batch processing. As for the continuous operations, this clarification step is included in the bioreactor as explained above. In addition, flocculation and precipitation are two methods that can be applied in both batch and continuous clarification steps to reduce impurities [9, 35]. Following the clarification process is the primary capture step, where protein A chromatography is widely used in traditional batch production to remove host cell protein DNA and other impurities [9]. Non-chromatography processes have also been developed, such as ATPS in batch mode [36]. The process has been integrated with cell harvest and capture step in batch manufacturing process for mAb production [37]. However, this process has not been used in large-scale manufacturing of mAbs [38, 39]. In recent years, several continuous or semi-continuous methods have been developed for primary capture including periodic counter-current chromatography (PCC) [40], multi-column countercurrent solvent gradient purification (MCSGP) [9, 28], continuous countercurrent tangential chromatography (CCTC) [9, 28, 41,42,43,44], and continuous precipitation process [18]. ATPS can also be modified for continuous operation with packed columns [45, 46]. The primary capture is typically followed by the viral inactivation unit operation. During this step, the capture step eluate is exposed to low pH conditions to inactivate viruses. In batch process, this step can be performed using blending tanks for incubation and then neutralization. As for continuous operation, the methods developed include using multi-virus inactivation tanks in parallel or in series pattern [47], using static mixer with staging tanks or incubation vessels [48], or using straight tube reactor, plug flow reactor, or coiled flow inverter to achieve continuous virus inactivation [49, 50]. Additional purification, also called polishing, of the product is achieved through ion exchange chromatography, hydrophobic interaction chromatography, or a combination of both. The polishing steps are typically performed in bind and elute or flow through modes in batch processing. Those methods can be achieved continuously by using multi-column methods [9]. In addition, other methods like simulated moving bed (SMB) chromatography, six-, three-, and two-column MCSGP processes [51], annular chromatography, radial flow chromatography, protein crystallization, and tangential flow filtration can also be used in continuous purification steps [28]. For continuous viral filtration, two viral filters running alternatively can be used [9]. The last step in the drug substance (API) biopharmaceutical manufacturing is the formulation step, which includes buffer exchange and protein concentration. Instead of traditional ultrafiltration and diafiltration processes, single-pass tangential flow filtration (SPTFF) [52], cascade diafiltration, countercurrent staged diafiltration [53], and crystallization can also be used to achieve continuous protein production [9, 28]. Rucker-Pezzini et al. [52] integrated SPTFF with other continuous upstream and downstream unit operations and built an end to end continuous process operation. A non-protein A purification platform for continuous processing is developed by using precipitation in novel coiled flow inverter reactor (CFIR). Integrated with cation exchange chromatography, multimodal chromatography, and salt-tolerant anion exchange adsorber, the proposed platform is tested to purify three types of mAbs successfully [54]. Somasundaram et al. [55] reviewed the key factors, benefits, and challenges in the development of each continuous downstream unit operations in detail. The paper showed a current trend for implementation of continuous downstream processing in manufacturing scale and most of methods listed above are explained in the paper. In order to build an integrated continuous manufacturing system, three approaches: modular, adaptation, and merger, are proposed. Real-time monitoring and control methods such as measurement of product quality, impurity, and concentration are also available [56].
For microbial expression, batch and fed-batch cultures can both be used for upstream production. Microbial-based upstream operations are typically significantly shorter in duration than mammalian-based operation. In order to achieve continuous processing in microbial operations, the product in perfusate is continuously harvested. Both the perfusion culture and the bleed stream are the production alternatives [57] [58]. Downstream processing for intracellular products is more complicated than that for extracellular products because of more impurities contained in the production stream. Instead of progressing directly into purification after clarification, the cells need to go through lysis and removal of the debris [59]. The purification steps can also be different from that of mammalian/extracellular products as extra refolding or extraction steps might be applied [28]. Continuous disc stack centrifuge can be used for continuous cell harvesting. To operate homogenization continuously, two homogenizers can be installed in series to achieve continuous cell lysis. Continuous chemical lysis with hollow fiber filters has been shown as an alternative process. The downstream refolding process can also be converted to continuous by using paddle reactor, CSTR, annular and simulated moving bed chromatography, or expanded bed in continuous mode. Review papers from Jungbauer [28] and Jozala et al. [60] provide a detailed discussion of the advantages of different methods of continuous intracellular/microbial production and their applications in different cases.
Simulation Software
This section focuses on the different simulation software that can be used for economic analysis in continuous biopharmaceutical processing. The three simulation software packages that have been commonly used for cost analysis are BioSolve (Biopharm Services, UK), SuperPro Designer (Intelligen, Scotch Plains, NJ), and Aspen Batch Process Developer (Aspen Technology, Burlington, MA).
Table 2 presents the software used in selected recent published papers on economic analysis of biopharmaceutical manufacturing, with focus on continuous processing. It can be noted that BioSolve software is more commonly used in the recent years especially for continuous biopharmaceutical manufacturing whereas SuperPro Designer and Aspen have not yet been applied for published continuous processing simulation.
BioSolve is an Excel-based process and cost modeling software (Biopharm Services, UK). It can be used to calculate COG with a breakdown of cost drivers in both batch and continuous process operations based on a scalable process model, as the scale can be varied from lab scale to manufacturing scale. It can also be used for cash flow analysis, sensitivity analysis, and Monte Carlo analysis. The software can perform multiple process comparisons and can be used to identify the most cost-effective technologies [70]. The software contains yearly updated built-in cost data library, including equipment, consumables, and solution components. The software allows users to customize cost data based on models [18, 35]. However, this software is not able to capture the dynamic nature of the process. The latest BioSolve software shows ability to map batch, continuous, and perfusion process into a Gantt chart [70].
SuperPro Designer (Intelligen, Scotch Plains, NJ) is a flowsheet-driven simulator and can be used to integrate both process and business modeling. With this software, material and energy balances can be automatically calculated based on the mathematical model built by the user [63]. Thus, equipment sizing, debottlenecking, cost, and economic evaluation can be performed both in batch and continuous process operations. Combined with SchedulePro, scheduling in batch and semi-continuous processes can be achieved in both SuperPro and SchedulePro Designer. However, the simulation model can only be specified with fixed resource, time delay, and failure events in bioprocess. In the continuous mode, there is limited continuous equipment that exists in the current library and they do not have scheduling option. Cost analysis and project economic evaluation can be done by SuperPro Designer. The software can show the basic cost analysis including direct and indirect costs, and also do the profitability and cash flow analysis. Sensitivity analysis and Monte Carlo analysis can also be done with SuperPro model but the data need be transferred to Excel and distribution of parameters should be provided in Excel spreadsheet. On the Excel spreadsheet, the add-ons packages need to be used, for example Crystal Ball (Oracle).
Similar to SuperPro Designer, Aspen Batch Process Developer (Aspen Technology, Burlington, MA) is a recipe-driven simulator that can also be used to simulate biopharmaceutical processes with economic analysis. The software can be used to obtain material balances, cycle-times calculation, perform scale-up, alternative process evaluation, and process debottlenecking. The software can also simulate single or multi-product campaigns and track the in-plant emission such as CO2 emission for environmental purpose [71]. However, for Aspen Batch Process Developer, the simulation is limited to building batch processes. In addition to Aspen Batch, Aspen Technology also developed Aspen Chromatography that is used to simulate batch and continuous chromatographic separation processes that are applied in pharmaceutical and biotechnology [72]. One of the advantages of Aspen Process is Aspen Chromatography and Aspen Batch process developer can be combined with Aspen Plus Dynamic so that the process can not only be analyzed under steady state but also evaluated under dynamic process for both batch and continuous process operations built in the software.
To understand the dynamic behavior of the process including process failure, delay, and rescheduling, a simulator such as ExtendSim should be used [17, 21]. By using ExtendSim, discrete event simulation can be applied to both discrete and continuous biopharmaceutical simulation. ExtendSim is a simulator that can be used for resource management, a mass balance analysis, in-process testing and costing analysis. A detailed discussion and its application can be found in Paige Ashouri’s thesis [73]. The thesis also provides the discussion of previous research that applied the software to biopharmaceutical manufacturing [73, 74].
Instead of using simulation packages, some publications performed economic analysis and optimization of biopharmaceutical production by using programming software such as GAMS. Liu et al. [62] applied mixed integer nonlinear programming (MINLP) to provide sizing strategies, process debottlenecking and decision making of facility configuration in order to build cost-effective chromatography. In 2016, Liu et al. [66] applied stochastic mixed integer linear programming (MILP) model to optimize both upstream and downstream equipment sizing, and their working cycle determinations. Combined with chance-constrained programming (CCP) techniques, the most cost-effective decision was made with the consideration of upstream titer and chromatography resin yield. However, these methods have been only applied on mAbs production in batch mode.
Economic Analysis Methods
The economic analysis method includes deterministic cost calculation, sensitivity cost analysis, and stochastic analysis. Since many of the cost calculations of biopharmaceutical manufacturing are the same as those of chemical plants [75] including labor cost, maintenance cost, and even profitability cost, their cost calculations are not going to be covered in detail in this review. This section only presents the calculation of biopharmaceutical manufacturing cost.
For capital cost calculation, there are three main items: working capital, start-up and validation cost, and direct fixed capital (DFC). In biopharmaceutical plants, working capital is 10–20% of the DFC, while start-up and validation are typically 20–30% of DFC. For single-use system, the processing flexibility increases and validation, start-up, and commercialization times become shorter [22]. The calculation of capital cost is similar to the calculation done for chemical plants using available scaling relations [22] as in Eq. (1).
Where C2 is the target cost of the equipment; C1 is the reference cost; size1 and size2 are sizes of reference equipment and target equipment; a is a scale-up factor and ranges between 0.5 and 1. In biopharmaceutical calculation, two equipment have been used [62]:
V is the bioreactor volume; D is chromatography diameter; SUF is a scale-up factor.
Cost of goods per gram (COG/g) combines direct and indirect costs of the process and is widely used in analyzing the impact of investment and operating decisions. The COG/g analysis can be applied in both single steps comparison and integrated manufacturing process.
In Eq. (4), labor cost, material cost, and consumables cost belong to direct cost. In economic analysis, labor cost is usually assumed independent of facility scales [67]. In biopharmaceutical process, material cost includes media, buffer, and cleaning cost (for example, waste for injection cost) [22]. Liu et al. [62] built cost calculation model for batch biopharmaceutical facilities and provided the equations to calculate media cost:
θ is media over-fill allowance, and Nbatch is number of batches of bioreactor, while Pcmedia is the price of media, α is bioreactor working volume ratio, and Vbior represents the volume of the bioreactor.
Consumables are the items that need to be replaced during processing, for example, filters and membranes in filtration, diafiltration, and resins in chromatography. In some studies, the resin cost is also considered as a material cost. In most of the cases, it is considered as a consumable cost. When calculating the cost of consumables, lifetime and reusable cycles need to be considered. For example, in Liu et al. [62] study, the resin cost in chromatography column was calculated as:
A is an over-packing factor for resin, and \( P{c}_s^{resin} \) is the resin price.Nbatch is number of batches, while \( {N}_s^{cyc} \) is number of cycles per batch. \( Tot{V}_s^{col} \) is total column volume, and L is the resin lifetime, while n represents the number of chromatography steps.
Profit analysis, such as global cash flow analysis with the calculation of net present value (NPV), internal rate of return (IRR), return on investment (ROI) and add and payback time, can help to understand the overall picture of the manufacturing process from economic aspect, by considering both capital cost, operating cost with product launch scenarios. NPV is the sum of the present values of the future cash flow and it is a strong function of interest rate. Compared to ROI and payback time, it is a better economic measurement because it represents time value of money and annual variation in expenses and revenues [75]. It is an important tool for capital investment prediction and process decision making. To calculate NPV and other economic profits of mAbs production, the mAbs selling price need to be defined. Figure 3 shows the mAbs market prices from 1997 to 2016. The average is around at $20 mg [76].
Monoclonal antibody price approved from 1997 to 2016 (The figure is plotted based on literature data) [76]
Sensitivity and Monte Carlo analysis can also be applied in economic evaluation. Sensitivity analysis can help with decision making regarding testing solutions, identifying variables, finding the optimal solutions, and evaluating the risk [77]. In economic analysis, it can observe the most sensitive parameters relative to the cost and help to design the most cost-effective process under the existing conditions.
Monte Carlo analysis can help predict the system response to certain events with specific probabilities. By defining the parameters’ minimum, maximum, and probabilistic distributions, this analysis helps to understand the real manufacturing behavior with two or more key parameters’ variation. It can also help to analyze uncertainty, such as product failure, process contaminations, and technology transfer delay existing in real manufacturing process [19].
Since the output of analysis is the cost, the sensitivity and Monte Carlo analysis from economic aspect of biopharmaceutical production can be calculated by RiskAMP (structured Data LLC, NY), Visual Basic Analysis or Crystal Ball (Oracle). In sensitivity analysis, the variation of parameters can be determined from literature resources or experimental data and define best, base, and worst case scenarios [78]. As for Monte Carlo analysis, random values need to be generated based on the distribution defined by user: uniform, normal, and triangular distribution are usually used. In bioprocess with known best, worst, and base case scenarios, triangular distribution is normally used [21, 78]. In terms of process failure (filter clogging) or process contamination, a failure rate needs to be defined and their correlated consequence, for example batch loss, a percentage yield loss, also needs to be considered in the simulation.
Overview of Integrated Continuous Process Operation Model Building and Economic Analysis
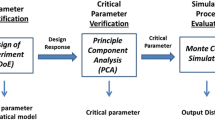
Figure 4 shows the general steps and methods that are used in the literature to compare batch and continuous processing by economic analysis.
General method to evaluate economic analysis of biopharmaceutical process [13]
Model Building
Table 3 lists different publications regarding the comparison between batch and continuous biopharmaceutical production based on economic analysis in the last decade. The table shows that the analysis on upstream, downstream, and integrated processing is all built in a fully integrated process. In upstream or downstream analysis, models are built in a hybrid mode, meaning that there is a combination of batch and continuous operations. For the integrated line, the whole system is fully continuous. Equipment sizing and number were calculated based on annual production rate of the final product.
The upstream analysis mainly focuses on choosing a cost-effective operating mode between batch and continuous and selection of cell retention devices when continuous perfusion bioreactors are applied. The process model contains three parts: seed bioreactor, production bioreactor, and batch downstream operations. Seed bioreactors are operated in batch mode and need to be built for the completeness of the process [20, 21]. The cells are transferred from seed bioreactor when they are in exponential growth phase. Perfusion bioreactors need to be initiated by batch culture at the beginning of continuous processing. Repligen, a bioprocessing company that produces XCELL™ ATF perfusion bioreactor, recommends to start perfusion with XCELL™ ATF system 2 to 3 days after inoculation [80]; then adjust perfusion and cell bleeding rate to maintain the system in a steady state. Vermasvuori and Hurme [79] also stated that during the first 8–10 days, the perfusion bioreactor operates in batch mode for cells to grow to production concentration. To compare different upstream processing options, a downstream model is also needed and usually is considered in batch mode. Equipment choice and size depend on the types of upstream operations. When comparing different perfusion bioreactors, the downstream model should consider the same equipment. When the analysis compares batch to continuous operations, downstream equipment is operated differently. For batch mode, downstream includes clarification and purification steps, whereas for continuous processing, a centrifuge is not required but pooling tanks are needed to collect perfusate from bioreactor [21].
To compare different downstream processes properly, the upstream process has to stay constant and should equally contribute to the different downstream process scenarios, while maintaining productivity and economic cost [35, 63, 65]. Li and Venkatasubramanian [63] applied statistical methods such as design of experiments and principal component analysis to find the most cost-effective parameters in different batch downstream operations. From their deterministic analysis, parameters of protein A chromatography loading and the second ion exchange yield were the major influencers on downstream cost. Another way to analyze downstream processing is to compare integrated processes with batch and continuous downstream processing. Xenopoulos [35] compared cost and operation advantages of each of the main operations in batch and continuous downstream process. The work evaluated operations including (1) clarification unit operation: hybrid, depth filtration, flocculation; (2) primary capture unit operation: protein A, CEX, precipitation; and (3) polishing step: flow through polishing and traditional polishing. The paper also compared fully integrated batch process and continuous process operations by deterministic cost analysis.
For the integrated process, three operating modes are usually compared: batch mode, continuous mode, and hybrid mode. The batch mode contains all the conventional operations in the system and is usually set as a base case. As for building integrated continuous process operation, both upstream and downstream are running continuously. Seed bioreactors still need to be included in the process; however, the centrifuge and pooling tanks are not necessary to be applied [17,18,19, 64]. From the batch and continuous processing comparison, the researchers also created hybrid operating mode in order to take full advantage of both batch and continuous operations within the system.
Deterministic Cost Analysis
Deterministic cost analysis mainly includes calculation of investment capital cost, COG/g, and cost in contributions in different categories, including material, depreciation, and utilities cost. By comparison of categories, the cost bottleneck of each operation can be found [16, 20, 21, 79].
In both upstream and downstream processing, capital cost, material cost, consumable and labor cost are important categories of the overall cost and thus have the highest weight compared to other cost categories. Continuous processing results in smaller footprint by using smaller perfusion bioreactors with high cell concentration, smaller size of chromatography columns, and elimination of hold tanks between operations [81]. In upstream, understanding and optimizing labor cost and material cost are very important since labor cost constitutes a large portion of the overall perfusion COG. Vermasvuori and Hurme [79] showed that among different cost categories, approximately 30% of the cost is from labor cost for stirred tank perfusion and hollow fiber perfusion bioreactors in small production scale. Regarding the material cost, there are great differences between batch and continuous processing. In the continuous perfusion process, media is continuously fed into and collected out of the bioreactor, and in batch or fed-batch process, media is maintained in the bioreactor. Pollock et al. [21] showed that material costs take 38% of total cost in continuous processing and only take 24% in batch processing. The material cost including media, buffer, and consumable cost of SPIN bioreactor is 1.8-fold higher than that of batch with annual production rate 500 kg/year whereas ATF bioreactor is 1.2-fold higher than that of batch bioreactor. The specific cell line used and the media composition greatly affect product titer and productivity. Under the assumption of keeping the same production rate, selecting a cost-effective media is an important consideration [81]. Xu et al. [16] compared the productivity, media cost among fed-batch, concentrated fed-batch, and perfusion bioreactor under same cell line and similar media conditions. The result showed that media cost for perfusion process (2.29 ± 0.28 g/L/day) was similar or lower than that in fed-batch (on day 14, titer 6.8 ± 0.2 g/L) and concentrated fed-batch process (on day 18, titer 27.5 g/L). This comparison was based on an initial assessment, and media optimization can also be explored in a future study [16]. Klutz et al. [64] discussed the comparison between fed-batch operation in fully integrated batch process and perfusion operation in fully integrated continuous process operation at 200 kg/year manufacturing scale. The result showed the COG/g from perfusion upstream process is 1.2–3.2 higher than fed-batch process due to higher media cost.
In commonly used batch biopharmaceutical manufacturing, up to 80% of total manufacturing cost is due to downstream processes [82]. More than 70% of this downstream cost is dominated by chromatography and the material cost [83]. In addition, the decision of resin and filter reuse plays important role in downstream material or consumables cost. Especially in protein A chromatography, the resin cost is higher than other chromatography options and accounts for a large portion of the overall raw material cost [84]. From previous literatures, the cost of protein A resin is 8000–14,000 $/L [61, 85] and AEX resin cost is 1500 $/L [61]. Thus, many researches focus on the study of protein A chromatography in order to reduce the resin use in the process or find another substitute equipment. Torres-Acosta et al. [65] compared the economic cost between chromatographic purification and aqueous two-phase extraction (ATPS) of uricase, an intracellular product enzyme, purification in batch mode. The deterministic analysis shows that the chromatographic option has higher capital cost, consumables cost (where resin cost takes a high percentage of consumables cost), and lower raw material cost compared to the ATPS method. The ATPS process can also be applied in continuous mode [86]; however, no economic analysis has been performed yet. Many continuous operations are also developed, such as periodic countercurrent chromatography. Pollock et al. [61] evaluated the periodic counter-current (PCC) semi-continuous chromatography including column design, operation, and economic analysis. They applied economic analysis to compare PCC chromatography and conventional batch process. The results showed that at the stage of PoC (proof-of-concept) 4 kg/batch scale model, the PCC method reduced resin use by 50% leading to reduction of batch manufacturing direct costs by 31%. The three columns system resulted in buffer usage reduction by 39% in protein A chromatography operation and reduced overall buffer usage by 12%. However, the investigators pointed out that these benefits become less significant as the production scale increases. In addition to PCC, SMB is another primary capture method for mAbs purification. Angarita et al. [87] compared CaptureSMB with protein A column for protein primary capture. CaptureSMB showed a higher productivity, capacity utilization and had higher loading capability and product concentration (24, 44%). It can also be integrated with continuous upstream process. Economic analysis showed the CaptureSMB process can save up to 28% of the resin cost compared to batch process at PoC, Phase III, and commercial scales. With only two columns, the CaptureSMB operation is also less complex and decreases the downtime risk compared to PCC operation. Hummel et al. [68] built integrated continuous bioprocessing (ICB) for mAbs production with Pall’s existing and planned disposable and continuous technology, which includes Cadence acoustic separator continuous clarification, Cadence BioSMB primary capture and continuous polishing, and Cadence final formulations. The author compared the ICB platform to SS batch and SU batch platforms under both clinical scale (0.1–19 kg/year) and commercial scale (17–1600 kg/year). Stainless steel batch had the highest COG compared to other operation modes among all the design space. Single-use batch system had the lowest COG at the lowest throughput in clinical scale. ICB platform is least sensitive to different production scales which has the lowest COG at the high throughput in clinical scale and lowest COG among all the design space at commercial scale. As a result, continuous process operations took 78% of lowest COG among all the design spaces which are mainly due to capital cost saving from acoustic separator and the reduced resin and buffer utilization from BioSMB primary capture. Different from upstream and downstream processing, the analysis of integrated processing not only focuses on overall cost but also analyzes different cost categories in order to evaluate the contribution of each unit operations to the whole process. Cash flow and net present value are also analyzed for future decision making. Walther et al. [19] compared integrated fully continuous biomanufacturing (ICB) with conventional batch process in mAbs and non-mAbs production. The publication showed integrated continuous process operations reduced operation cost by 21% and capital cost by 47% in mAbs production and 80 and 72% cost for non-mAb production, respectively. The breakdown cost analysis showed the ICB process has higher upstream filters and media cost but less upstream labors and downstream resins cost. In every unit operation, the capital cost is lower compared to conventional process. This is mainly due to the reduced facility footprint, less frequent process turnaround, and single-use system used. In the NPV analysis, the paper assumed the production began 5 years after investment in stainless steel process and 3.5 years after investment in single-use facilities. The results provided information that the ICB platform can obtain benefits immediately after the manufacturing start and showed the advantage (∆NPV) of $371 M ($64 M for mAbs and $306 M for non-mAbs) during a 15 years’ investment. Arnold et al. [67] compared integrated continuous antibody production with batch processing using experimental and computational results. By keeping the same annual production rate, the continuous processing has 15% lower operating cost in COG/g and the capital cost in continuous processing reduces 50% comparing to that in batch.
Instead of converting the traditional batch downstream process operations to continuous process operations, some other researches invest their research on alternative batch operations. For example, Grilo et al. [69] suggested a novel downstream purification processing that used phenylboronate multimodal chromatography followed by monolithic AEX chromatography and packed bed HIC. The researchers built two integrated mAb production line with the same perfusion upstream and two different downstream processes by using SuperPro Designer software. Under annual production rate 2000 kg/year and upstream titer 25 g/L mAb, results showed the new technology maintained similar capital cost and reduced 20% operating cost. From profit analysis, the new process had higher NPV (~ $500 M), shorter payback time (0.02 year), and higher IRR (1%) which also indicated the new process was more advisable compared to the conventional one. Varadaraju et al. [88] used SuperPro Designer and designed a downstream mAbs process with membrane-based purification operations. Instead of using protein A, IEX, and HIC chromatography columns, the authors used nanofibrous adsorption membranes, which reduced labor working hours, medium consumptions, and thus the overall downstream COG/g expenses by 23%.
Economic analysis can also be used to process optimization and unit operations screening to aid technology selections. Liu et al. [66] integrated upstream and downstream batch process and optimized bioreactor sizes, downstream column sizes, and upstream downstream ratios. The authors used MILP model and aimed to minimize COG/g considering the operating uncertainty. To extend this work, Liu, S. and Papageorgiou, L. [89] considered uncertainty in the biopharmaceutical manufacturing such as upstream titer, chromatography downstream yield, and level of impurity reduction and addressed multi-objective optimization problem to determine chromatography sequencing and column strategy. Popva, D. et al. [90] applied multi-attribute decision making method (MADM) and economic analysis to select among alternative primary recovery options (three types of centrifugation, depth filtrations, and two tangential flow filtrations). MADM analysis evaluated the selected criteria such as yield and purity and economic analysis helped to select cost-effective operations.
Sensitivity and Stochastic Cost Analysis
Sensitivity analysis can be applied to analyze the effects of process parameters in order to determine the most important parameters in terms of cost. Bunnak et al. [20] compared fed-batch and perfusion operation for mAbs production using sensitivity analysis to identify the most sensitive parameters in each system. The results indicated that titer, bioreactor working volume, and perfusion rate are three crucial process parameters to overall COG. It also indicated that increasing the pooling duration in the process can decrease the overall COG. Longer pooling duration brings lower frequency of both upstream and downstream processes, but larger downstream equipment needs to be applied as a trade-off. Another highlight of this work is that the paper applied life-cycle assessment and used sensitivity analysis to investigate water consumption, solid waste generation, and energy requirement of the process. Table 4 summarizes the main variables of perfusion bioreactor, multi-column chromatography, and integrated process that have been used in sensitivity analysis.
Most commonly, sensitivity analysis is applied using deterministic methods, which involves the evaluation of cost at a few points of independent variables in a certain range. The method is used to analyze protein titer and production capacity effects on upstream, downstream, and integrated processing cost in order to compare the flexibility of various operating modes. Bunnak et al. [20] found as the annual production rate increases from 28 to 1000 kg/year, the capital cost contribution to overall COG decreases in both batch and perfusion process but consumables and materials cost increase. Pollock et al. [21] analyzed production scales and product titers effects on overall cost with the use of fed-batch and perfusion bioreactors with different cell retention devices (ATF with single-use perfusion bioreactor and SPIN filter with stainless steel perfusion bioreactor). The production scales increased from 100 to 1000 kg/year and titers increased from 2 to 10 g/L. The results showed that SPIN strategy only had COG benefit over fed-batch system at low production scale (100 kg/year). ATF strategy showed similar COG savings (20%) across all the titers and production scales. The paper also analyzed the working conditions of bioreactor such as viable cell density. It shows that as long as the viable cell density in perfusion bioreactor can be maintained 3-folds of that in fed-batch bioreactor, the ATF perfusion process will save cost in all the scales.
Hammerschmidt et al. [18] compared the integrated hybrid and fully continuous precipitation process with traditional batch chromatography process. Three operation modes were tested under different scales, including development and fully commercial production scales. The result showed that as the scale increases, the highest cost of batch process shifted from consumable cost to capital cost. For continuous and hybrid process (batch process upstream with continuous precipitate downstream), the highest cost shifted from labor cost to material costs. In all scales, hybrid process has the lowest cost comparing to other operation modes. The study showed that with an increase in titer capacity from 2 to 10 g/L, the cost of downstream cost contributions increases from 48 to 73% of the overall cost. A similar analysis is also applied to integrated analysis with multi-column chromatography downstream process. Pollock et al. [17] showed the comparison among batch, hybrid, and continuous integrated process shown in Table 3. The work showed the trend of media and buffer costs, single-use component cost, chromatography resin, and QC/QA cost as the operation increase from Phase I development scale to commercial scale. In addition to compare break down cost of continuous and batch process, the author also analyzed the impact of manufacturing scales with company sizes in COG of different operation modes. The work provided the analysis of operational feasibility and evaluated the process environmental effect. It also applied sensitivity analysis on decision making by calculating an overall aggregated score that is a multi-attribute decision-making methodology to represent environmental economical and operational robustness of the system changed by the importance of economic benefits.
Stochastic cost analysis such as Monte Carlo simulation can be used to evaluate variability and robustness of the process. Pollock et al. [21] took the possibility of culture contaminations, filter failure, and filtration failure in the real manufacturing process into the consideration and showed that under 500 kg/year annual production rate, and 5 g/L titer condition, the amount of annual output of ATF perfusion process was higher comparing to batch process; however, it had lower robustness with high standard deviations. This results mainly due to ATF system has higher probability of failure rate and fewer number of batches per year. However, ATF perfusion process still shows lower COG/g than fed-batch, even at the worst-case situation. Walther et al. [19] applied Monte Carlo analysis to estimate the overall, mAbs, and non-mAbs ∆NPV of continuous facility with the possibility of product or technology transfer success with varying product demand. Sub-distributions with different product types and cost types were also calculated in order to understand the economical drivers. In mAbs production, distribution of ∆NPV had mean $64M with standard deviations $6M which is dominated by capital investment reduction.
Instead of using sensitivity and Monte Carlo analysis in comparison between batch and continuous process operations, those methods can be applied to evaluate the effect of parameter optimization. Examples are found in batch process with ATPS systems. Torres-Acosta et al. [78] applied economic modeling tool to evaluate royalactin production with aqueous two-phase systems. Through sensitivity analysis, the upstream titer was found as one of the key parameters affecting the cost of production. By evaluating the optimized process, Monte Carlo analysis showed the distribution of COG/g and decided the most feasible option to investigate. These two analyses also played an important role in process selection and decision making. Torres-Acosta et al. [91] designed four types of ATPS processes for tetracycline purification and then optimized the process by sensitivity analysis. For process decision making, Monte Carlo analysis presented that downstream process yield and upstream titers’ effected on COG/g distribution in all the scenarios. As the result, ATPS formed by cholinium chloride/K3PO4 were selected. These two examples show the application of uncertainty analysis in batch biopharmaceutical process for process evaluation which has a potential application in continuous operations.
Single-Use System
Single-use systems reduce capital costs, labor cost, the risk of cross-contaminations, and validations. The single-use system has smaller footprint, simplified classified area, reduced project timescale and facility construction times [92]. It is also easy for process transfer and faster product turn-over [93]. They can be used in both batch and continuous process operations, including media preparation, bioreactor, and filtration [94]. In continuous process operations, the application of the single-use technology mainly focuses on upstream processing [95]. Whitford, W. [10] introduced single-use (SU) systems in application of continuous perfusion cultures including SU bioreactors and clarification methods. Different SU bioreactors with their production companies are listed, and their advantages and operating conditions are detailed explained. Single-use systems can also be utilized in downstream processing, for example, single-use prepacked countercurrent multi-column chromatography [96], and single-use tangential flow filtration [97].
In recent years, biopharmaceutical companies have been slowly adapting single-use technology for production. Langer and Rader [95] showed that in 2013, 44% of the industry anticipated to apply basic single-use devices and 34% had a desire to apply single-use bioreactors, however, concerns also associated with the single-use development. Jacquemart et al. [98] reviewed the impact of single-use strategy on manufacturing operating cost and showed the single-use facility reduced operating cost by 22%. The paper pointed out the advantages and limitations of the single-use system. The single-use system is majorly built by plastic materials which would affect the quality of the final product and produce large number of disposables that would cause environmental problems. Lopes, A. [92] also pointed out that single-use system had challenges such as limited material strength, low capacities, and lack of automation. However, the benefits of the single-use system such as reducing consumptions of energy and water, improving the productivities compensate those drawbacks and the cost-effectiveness is still obvious comparing to stainless steel process [98].
Shirahata et al. [99] provided a decision-support method to evaluate single-use and multi-use systems in drug manufacturing both from economic and environmental points of view. The study evaluated the application of single-use technology and multi-use technology in single type of drug production with different batch sizes, and highlighted a case study with multi-products with the same production volume. It showed that the single-use system had significant cost savings in producing small batches and multiple small-scale products. However, the evaluation has been limited to batch processing mode. Walther et al. [19] first compared batch and continuous integrated processes with either single-use batch or single-use perfusion bioreactor. The results showed in both cases that the continuous system had less capital cost and operating cost. However, the single-use system evaluation in this study is only limited to upstream processing. Klutz et al. [64] compared a batch and a continuous integrated process. For the batch process, only upstream applied single-use technology. As for the continuous operations, some unit operations like viral inactivation (single-use CSTRs) and final storage of bulk drug substance were single-use. Xenopoulos, A. [35] built a novel integrated semi-continuous process with single-use bioreactor, regular PCC column, and membrane-based polishing and final formulation steps. The novel process cost was 19 to 33% lower compared to conventional batch process and furthermore reduced buffer volume. As mentioned in Section 3.2, Hummel et al. [68] compared stainless steel batch, single-use batch, and single-use ICB downstream platforms under different production scales. Results showed continuous process operations reduced the consumable cost, labor cost in the single-use system especially in downstream purification processes which were the most promising method in building cost-effective biopharmaceutical manufacturing. However, this evaluation is mainly focused on downstream processing. Hammerschmidt et al. [18] compared three operation modes with stainless steel (SS) batch process, single-use hybrid and continuous precipitation process including all the upstream and downstream operations. The results showed that the batch process has the highest cost because more cleaning procedures are required with stainless steel equipment. An abstract from ECI Symposium proposed that Bayer’s technology services GmbH is going to build fully integrated continuous single-use pilot plant to produce monoclonal antibodies, with a yearly production of 150 kg [100]. Due to the capacity limitation of the single-use system, applying single-use system to continuous process operation is more feasible and economic effective than batch and single-use batch processes. However, limitations such as the lack of automation still exist. The failure rate of single-use system due to contaminations and material strength also need to be taken into the consideration.
Discussion
Economic analysis has been applied for process evaluation, optimization, and decision making in biologics manufacturing facilities. However, limitations still exist due to model building and software choice for continuous processing design.
In traditional biopharmaceutical manufacturing, the batch seed bioreactor is commonly used for inoculation and expansion prior to the production bioreactor. Seed bioreactors can be batch, fed-batch, or perfusion bioreactors. It is technically challenging to sustain high cell density in batch and fed-batch bioreactors which could affect the product quality and operation time in the production bioreactor. Since perfusion bioreactors maintain higher cell density, they demonstrate a potential advantage in the production stage. Yang et al. [101] showed that a perfusion seed expansion bioreactor with ATF cell retention prior to the production bioreactor can shorten the protein production period from 17 to 12 days in CHO upstream batch processes. However, this method has not been applied in continuous upstream process and has not been economically evaluated and compared with batch protein production. For the development of continuous production bioreactor, most of the existing publications focus on mammalian cell culture as the most widely used method in antibody productions [102], with the simplest downstream processing approach. However, continuous prokaryotic protein production does exist in biopharmaceutical processing [28, 103]. In addition, the upstream processing requires strict cell culture conditions in order to maintain desired product quality. It has been shown that robust mAbs production with consistent glycan distribution can be achieved in the batch bioreactor. Glycosylation profile and product titer can also be controlled by adjusting the time of adding media supplementation [104, 105]. To maintain long-term cell culture in continuous operations, dynamic control of perfusion bioreactor is an important consideration both in plant design and economic analysis.
However, the traditional live cell process has limitations including the following: (1) long cell line development and validation time; (2) product substance sensitive to process conditions; (3) batch-to-batch variation [106]; (4) production cost-intensive [107]. Cell-free protein synthesis can also be used in antibody and antibody fragments production from eukaryotic or prokaryotic cell extracts and has the capability to achieve translational machinery, protein synthesis reaction, protein folding, and post-translational modification without using the living cells. Stech and Kubick [107] provided an overview of the cell-free synthesis system and disulfide bond formation using in vitro protein production. The review also summarized the advances and development of antibody production in both eukaryotic and prokaryotic based cell-free systems. The production condition can also be optimized to increase the antibody production yield, titers and minimize protein aggregation. Martin et al. [108] used a cell-free protein synthesis system to synthesize soluble and active mAbs by using CHO cells lysate. In addition to optimize the protein synthesis conditions, the authors also provided the analysis of rapid screening of mAbs to reduce the screening bottleneck for producing new mAbs therapeutics. Instead of time-consuming cell cultural process (usually 2 days to 2 weeks), cell-free protein synthesis system provides short reaction times (4–12 h) with low product variability [106]. Production efficiency and product yield also increase along with high throughput screening and process cost-effectiveness. Due to the process simplification, the cell-free system suited for automation which allows for reaction environment manipulation, direct monitoring, and rapid sampling during the protein synthesis [106, 108]. In addition, semi-continuous or continuous exchange bioreactors are also available to the cell-free system to further improve productivity and scalability [106]. As early as in 1988, Spirin et al. introduced continuous cell-free translation method that performs the addition of reaction substrates such as amino acid and energy factors with continuous removal of reaction products which reached high yield of polypeptides production. Shirokov et al. [109] reviewed continuous cell-free methodologies in protein production and compared continuous-flow cell-free systems and continuous-exchanged cell-free systems in protein production. In recent years, Stech et al. [110] developed the synthesis of complex antibody formats in antibody production from CHO cell lysate and demonstrated antibody production in batch and continuous-exchange cell-free reactions system. The result shows that by changing from batch to continuous, the IgG protein yield increased 9-fold and scFv-Fc yield increased 11-fold without changing the product quality. Thus, from the discussion above, the cell-free system has a great potential in continuous antibody production. However, detailed economic analysis need to be performed for such systems.
With the development of downstream processing, many alternative methods for each of the unit operations have been developed as reviewed in the previous section of this review paper. However, the analysis and comparison among those different methods have not received enough attention in the literature. Periodic countercurrent chromatography system can reach a high production yield in contrast to continuous precipitation. However, this process is under semi-continuous operation mode and it is hard to achieve steady state because concentration in elution changes with time. It is worth mentioning though that this approach seems to be the most commonly used in downstream continuous operations. The optimized continuous countercurrent tangential chromatography method increases yield and productivity compared to traditional batch protein A chromatography and also reduces the protein aggregation with the same amount of contamination removal in the outlet product [41, 111]. There are plans to scale up to a commercial scale system [112], but the larger static mixer and filtration membranes [41] have to be further developed to maintain the consumable costs competitively. For non-chromatography methods, ATPS can also achieve continuous protein primary capture with up to 80% global recovery yield and can achieve protein purity more than 99% [113]. Rosa et al. [114] stated that continuous ATPS system reduces annual operating cost by at least 39% and the economic benefits become more obvious as titer increases comparing to traditional protein A chromatography. However, the capability, flexibility, contamination potential, and economical sustainability of the continuous ATPS [115] still need to be evaluated in comparison to other continuous primary capture systems. In addition, more advanced process analytical technology (PAT), quality by design (QbD), multivariate data analysis (MVDA) need to be develped and utilized to achieve direct and real-time drug measurement and bioproducts production [116]. Viral safety and formulation buffers can also be considered in the downstream processing in order to achieve a robust continuous manufacturing process [11, 117].
The economic feasibility of integrated continuous process operation, especially comparing different operating modes in terms of their economic feasibility has not received enough attention in the literature. The comparison between batch and continuous processing mostly focuses on main operations. Buffer preparation, process automation and control, inline processing that have significant differences between the two production modes also need to be considered. Cost evaluation of buffer preparation methods for continuous processing, the cost of automation, and system complexity in batch and continuous process operations are also important to the total cost of the process. The number of tanks that used in buffer preparation will affect the facility cost and labor cost. CIP, clean in place, contains CIP buffers and non-processing water that will affect the material cost and environmental impact. SIP, sterilization in place, affects the energy consumption and water usage in the process system. The number of SIP and CIP skids used account for equipment cost. In continuous process operation, the CIP and SIP operations are reduced due to a smaller number of batches applied. However, when comparing the hybrid process (with perfusion operation in upstream and batch operations in downstream) to integrated batch process, the water consumptions in hybrid process is 85% higher than that of batch [20]. It is mainly due to the perfusion activity increases twice of the downstream activities than batch. Thus, even though the CIP and SIP activities are reduced in upstream, the total activities are still increased. In addition, the single-use system eliminated the usage of CIP and SIP skids which also reduces the overall costs. In upstream process, the amount of CIP water that applied to the stirred tank bioreactor is considered as four times of reactor volumes [79]. However, in the hollow fiber bioreactor, CIP and SIP are not considered due to the consumables used in this operations, such as roller bottles and HF cartridges. Single-use system also reduced the failure rate that is caused by CIP/SIP operation, installation, and maintenance [67]. During the process design, the number of CIP and SIP skids usage will affect the scheduling of the process since different unit operations share the same CIP or SIP skids. With adding more details such as auxiliary systems, the facility designed will be closer to the reality, thus the economic analysis will be more accurate.
Considering existing software to perform economic analysis, those include simulation software and programming methods. Simulation software such as BioSolve, SuperPro, and Aspen are user-friendly and can be easily adapted by industry. However, except Aspen Dynamic, the available software packages are only limited to steady-state simulation, and have low flexibility comparing to programming models. Using Aspen simulation software only downstream separation processes can be modeled for continuous process systems. In order to model biopharmaceutical processes, GAMS, MATLAB, and other modeling software can be used to model continuous manufacturing, especially to enable process optimization. Raftery et al. [118] use GAMS to optimize the process operation of multi-feed bioreactor by increasing the productivity and decreasing operating costs for beta-carotene production process. Using these software platforms, users have more flexibility on building process models, but modeling is more complicated and difficult to adapt to different processes.
Summary
This paper focuses on the economic analysis of mAbs biopharmaceutical manufacturing production, especially in continuous process operations. The paper reviews the biopharmaceutical processes including upstream batch and perfusion processes, as well as downstream batch and continuous chromatography, in addition to non-chromatography methods. A review of the existing simulation packages and the methods that can be applied to economic analysis is also provided. The paper also summarizes the common assumptions in model building and reviews case studies that appear in the literature in the last decade. Finally, the paper highlights the limitations that exist based on the previous work.
References
Newswire PR. Monoclonal antibodies (mAbs) market analysis by source (chimeric, murine, humanized, human), by type of production, by indication (cancer, autoimmune, inflammatory, infectious, microbial, viral diseases), by end-use and segment forecasts, 2013–2024. LON-Reportbuyer: Y; 2017.
Gjoka X, Gantier R, Schofield M. Transfer of a three step mAb chromatography process from batch to continuous: optimizing productivity to minimize consumable requirements. J Biotechnol. 2017;242:11–8. https://doi.org/10.1016/j.jbiotec.2016.12.005.
Gjoka X, Rogler K, Martino RA, Gantier R, Schofield M. A straightforward methodology for designing continuous monoclonal antibody capture multi-column chromatography processes. J Chromatogr A. 2015;1416:38–46. https://doi.org/10.1016/j.chroma.2015.09.005.
Ng CKS, Rousset F, Valery E, Bracewell DG, Sorensen E. Design of high productivity sequential multi-column chromatography for antibody capture. Food Bioprod Process. 2014;92(C2):233–41. https://doi.org/10.1016/j.fbp.2013.10.003.
America PRaMo. Medicines in development: biologics 2013 report. Pharmaceutical Research and Manufacturers of America. 2013. http://phrma-docs.phrma.org/sites/default/files/pdf/biologics2013.pdf. Accessed 01 June 2018.
Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. Mabs-Austin. 2015;7(1):9–14. https://doi.org/10.4161/19420862.2015.989042.
Newswire PR. Monoclonal antibodies (mAbs) market Size worth $138.6 billion by 2024: grand view research, Inc. bc-Grand-View-Research: Y; 2016.
Gronemeyer P, Ditz R, Strube J. Trends in upstream and downstream process development for antibody manufacturing. Bioengineering. 2014;1(4):188–212. https://doi.org/10.3390/bioengineering1040188.
Zydney AL. Continuous downstream processing for high value biological products: a review. Biotechnol Bioeng. 2016;113(3):465–75.
Whitford WG. Single-use systems support continuous bioprocessing by perfusion culture. In: Subramanian G, editor. Continuous processing in pharmaceutical manufacturing. 2014, .
Godawat R, Konstantinov K, Rohani M, Warikoo V. End-to-end integrated fully continuous production of recombinant monoclonal antibodies. J Biotechnol. 2015;213:13–9. https://doi.org/10.1016/j.jbiotec.2015.06.393.
Taracena FL. An economic analysis for product and process design. Qual Eng. 2006;18(1):33–7. https://doi.org/10.1080/08982110500403474.
Farid SS. Process economics of industrial monoclonal antibody manufacture. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(1):8–18. https://doi.org/10.1016/j.jchromb.2006.07.037.
Farid SS, Pollock J, Ho SV. Evaluating the economic and operational feasibility of continuous processes for monoclonal antibodies. Continuous Processing in Pharmaceutical Manufacturing. Wiley-VCH Verlag GmbH & Co. KGaA; 2014;433–56. https://doi.org/10.1002/9783527673681.ch17.
Torres-Acosta MA, Ruiz-Ruiz F, Benavides J, Rito-Palomares M. Process economics: evaluation of the potential of ATPS as a feasible alternative to traditional fractionation techniques. Food Eng Ser. 2017;1:161–78. https://doi.org/10.1007/978-3-319-59309-8_9.
Xu S, Gavin J, Jiang R, Chen H. Bioreactor productivity and media cost comparison for different intensified cell culture processes. Biotechnol Prog. 2016;33:867–78. https://doi.org/10.1002/btpr.2415.
Pollock J, Coffman J, Ho SV, Farid SS. Integrated continuous bioprocessing: economic, operational, and environmental feasibility for clinical and commercial antibody manufacture. Biotechnol Prog. 2017;33:854–66. https://doi.org/10.1002/btpr.2492.
Hammerschmidt N, Tscheliessnig A, Sommer R, Helk B, Jungbauer A. Economics of recombinant antibody production processes at various scales: industry-standard compared to continuous precipitation. Biotechnol J. 2014;9(6):766–75. https://doi.org/10.1002/biot.201300480.
Walther J, Godawat R, Hwang C, Abe Y, Sinclair A, Konstantinov K. The business impact of an integrated continuous biomanufacturing platform for recombinant protein production. J Biotechnol. 2015;213:3–12. https://doi.org/10.1016/j.jbiotec.2015.05.010.
Bunnak P, Allmendinger R, Ramasamy SV, Lettieri P, Titchener-Hooker NJ. Life-cycle and cost of goods assessment of fed-batch and perfusion-based manufacturing processes for mAbs. Biotechnol Prog. 2016;32(5):1324–35. https://doi.org/10.1002/btpr.2323.
Pollock J, Ho SV, Farid SS. Fed-batch and perfusion culture processes: economic, environmental, and operational feasibility under uncertainty. Biotechnol Bioeng. 2013;110(1):206–19. https://doi.org/10.1002/bit.24608.
Petrides D. Bioprocess design and economics. In: Roger G. Harrison PWT, Scott R. Rudge and Demetri P. Petrides, editor. Bioseparations Science and Engineering 2ed., 2015.
Chatterjee S, editor. FDA perspective on continuous manufacturing. IFPAC Annual Meeting; 2012; Baltimore: FDA U.S. Food and Drug Administration Protecting and Promoting Public Health.
Jain E, Kumar A. Upstream processes in antibody production: evaluation of critical parameters. Biotechnol Adv. 2008;26(1):46–72. https://doi.org/10.1016/j.biotechadv.2007.09.004.
Novais JL, Titchener-Hooker NJ, Hoare M. Economic comparison between conventional and disposables-based technology for the production of biopharmaceuticals. Biotechnol Bioeng. 2001;75(2):143–53.
Hutterer KM, Hong RW, Lull J, Zhao X, Wang T, Pei R, et al. Monoclonal antibody disulfide reduction during manufacturing: untangling process effects from product effects. Mabs-Austin. 2013;5(4):608–13. https://doi.org/10.4161/mabs.24725.
Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. Mabs-Austin. 2010;2(5):480–99.
Jungbauer A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013;31(8):479–92. https://doi.org/10.1016/j.tibtech.2013.05.011.
Kelley B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. Mabs-Austin. 2009;1(5):443–52.
Yang WC, Minkler DF, Kshirsagar R, Ryll T, Huang Y-M. Concentrated fed-batch cell culture increases manufacturing capacity without additional volumetric capacity. J Biotechnol. 2016;217:1–11. https://doi.org/10.1016/j.jbiotec.2015.10.009.
Patil R, Walther J. Continuous manufacturing of recombinant therapeutic proteins: upstream and downstream technologies. Adv Biochem Eng Biotechnol. 2017. https://doi.org/10.1007/10_2016_58.
Arunkumar A, Singh N, Peck M, Borys MC, Li ZJ. Investigation of single-pass tangential flow filtration (SPTFF) as an inline concentration step for cell culture harvest. J Membrane Sci. 2017;524:20–32. https://doi.org/10.1016/j.memsci.2016.11.007.
Clincke MF, Molleryd C, Zhang Y, Lindskog E, Walsh K, Chotteau V. Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactor. Part I. effect of the cell density on the process. Biotechnol Prog. 2013;29(3):754–67. https://doi.org/10.1002/btpr.1704.
Bielser JM, Wolf M, Souquet J, Broly H, Morbidelli M. Perfusion mammalian cell culture for recombinant protein manufacturing—a critical review. Biotechnol Adv. 2018;36(4):1328–40. https://doi.org/10.1016/j.biotechadv.2018.04.011.
Xenopoulos A. A new, integrated, continuous purification process template for monoclonal antibodies: process modeling and cost of goods studies. J Biotechnol. 2015;213:42–53. https://doi.org/10.1016/j.jbiotec.2015.04.020.
Eggersgluess JK, Richter M, Dieterle M, Strube J. Multi-stage aqueous two-phase extraction for the purification of monoclonal antibodies. Chem Eng Technol. 2014;37(4):675–82. https://doi.org/10.1002/ceat.201300604.
Schmidt A, Richter M, Rudolph F, Strube J. Integration of aqueous two-phase extraction as cell harvest and capture operation in the manufacturing process of monoclonal antibodies. Antibodies. 2017;6(4):21. https://doi.org/10.3390/antib6040021.
Gonzalez-Valdez J, Mayolo-Deloisa K, Rito-Palomares M. Novel aspects and future trends in the use of aqueous two-phase systems as a bioengineering tool. J Chem Technol Biot. 2018;93(7):1836–44. https://doi.org/10.1002/jctb.5434.
Torres-Acosta MA, Mayolo-Deloisa K, González-Valdez J, Rito-Palomares M. Aqueous two-phase systems at large scale: challenges and opportunities. Biotechnol J. 0(0):1800117. https://doi.org/10.1002/biot.201800117.
Godawat R, Brower K, Jain S, Konstantinov K, Riske F, Warikoo V. Periodic counter-current chromatography—design and operational considerations for integrated and continuous purification of proteins. Biotechnol J. 2012;7(12):1496–508. https://doi.org/10.1002/biot.201200068.
Dutta AK, Tan J, Napadensky B, Zydney AL, Shinkazh O. Performance optimization of continuous countercurrent tangential chromatography for antibody capture. Biotechnol Prog. 2016;32(2):430–9. https://doi.org/10.1002/btpr.2250.
Napadensky B, Shinkazh O, Teella A, Zydney AL. Continuous countercurrent tangential chromatography for monoclonal antibody purification. Sep Sci Technol. 2013;48(9):1289–97. https://doi.org/10.1080/01496395.2013.767837.
Shinkazh O, inventor Countercurrent tangential chromatography methods, systems, and apparatus. US2011.
Shinkazh O, Kanani D, Barth M, Long M, Hussain D, Zydney AL. Countercurrent tangential chromatography for large-scale protein purification. Biotechnol Bioeng. 2011;108(3):582–91. https://doi.org/10.1002/bit.22960.
Espitia-Saloma E, Vazquez-Villegas P, Aguilar O, Rito-Palomares M. Continuous aqueous two-phase systems devices for the recovery of biological products. Food Bioprod Process. 2014;92(C2):101–12. https://doi.org/10.1016/j.fbp.2013.05.006.
Rosa PAJ, Azevedo AM, Sommerfeld S, Bäcker W, Aires-Barros MR. Continuous aqueous two-phase extraction of human antibodies using a packed column. J Chromatogr B. 2012;880(Supplement C):148–56. https://doi.org/10.1016/j.jchromb.2011.11.034.
Ransohoff TC. Bisschops MAT. Google Patents: Continuous processing methods for biological products; 2013.
Coffman J, Goby J, Godfrey S, Orozco R, Vogel JH. Methods, apparatuses, and systems for continuously inactivating a virus during manufacture of a biological product. Google Patents; 2017.
Klutz S, Lobedann M, Bramsiepe C, Schembecker G. Continuous viral inactivation at low pH value in antibody manufacturing. Chem Eng Process: Process Intensif. 2016;102(Supplement C):88–101. https://doi.org/10.1016/j.cep.2016.01.002.
Orozco R, Godfrey S, Coffman J, Amarikwa L, Parker S, Hernandez L, et al. Design, construction, and optimization of a novel, modular, and scalable incubation chamber for continuous viral inactivation. Biotechnol Prog. 2017;33(4):954–65. https://doi.org/10.1002/btpr.2442.
Steinebach F, Muller-Spath T, Morbidelli M. Continuous counter-current chromatography for capture and polishing steps in biopharmaceutical production. Biotechnol J. 2016;11(9):1126–41. https://doi.org/10.1002/biot.201500354.
Rucker-Pezzini J, Arnold L, Hill-Byrne K, Sharp T, Avazhanskiy M, Forespring C. Single pass diafiltration integrated into a fully continuous mAb purification process. Biotechnol Bioeng. 2018;115(8):1949–57. https://doi.org/10.1002/bit.26708.
Nambiar AMK, Li Y, Zydney AL. Countercurrent staged diafiltration for formulation of high value proteins. Biotechnol Bioeng. 2018;115(1):139–44. https://doi.org/10.1002/bit.26441.
Kateja N, Kumar D, Sethi S, Rathore AS. Non-protein A purification platform for continuous processing of monoclonal antibody therapeutics. J Chromatogr A. 2018;1579:60–72. https://doi.org/10.1016/j.chroma.2018.10.031.
Somasundaram B, Pleitt K, Shave E, Baker K, Lua LHL. Progression of continuous downstream processing of monoclonal antibodies: current trends and challenges. Biotechnol Bioeng. 2018;115:2893–907. https://doi.org/10.1002/bit.26812.
Rathore AS, Kateja N, Kumar D. Process integration and control in continuous bioprocessing. Curr Opin Chem Eng. 2018;22:18–25.
Flickinger MC. Upstream industrial biotechnology. John Wiley & Sons.
Castilho LR. Continuous animal cell perfusion processes: the first step toward integrated continuous biomanufacturing. In: Subramanian G, editor. Continuous Processing in Pharmaceutical Manufacturing 2015.
Lee R, Mikol V, Allen E, Ruetsch N, Cameron B, Oligino T et al. Humanized anti-CXCR5 antibodies, derivatives thereof and their use. Google Patents; 2011.
Jozala AF, Geraldes DC, Tundisi LL, Feitosa VD, Breyer CA, Cardoso SL, et al. Biopharmaceuticals from microorganisms: from production to purification. Braz J Microbiol. 2016;47:51–63. https://doi.org/10.1016/j.bjm.2016.10.007.
Pollock J, Bolton G, Coffman J, Ho SV, Bracewell DG, Farid SS. Optimising the design and operation of semi-continuous affinity chromatography for clinical and commercial manufacture. J Chromatogr A. 2013;1284:17–27. https://doi.org/10.1016/j.chroma.2013.01.082.
Liu S, Simaria AS, Farid SS, Papageorgiou LG. Designing cost-effective biopharmaceutical facilities using mixed-integer optimization. Biotechnol Prog. 2013;29(6):1472–83. https://doi.org/10.1002/btpr.1795.
Li YF, Venkatasubramanian V. Integrating design of experiments and principal component analysis to reduce downstream cost of goods in monoclonal antibody production. J Pharm Innov. 2016;11(4):352–61. https://doi.org/10.1007/s12247-016-9263-8.
Klutz S, Holtmann L, Lobedann M, Schembecker G. Cost evaluation of antibody production processes in different operation modes. Chem Eng Sci. 2016;141:63–74. https://doi.org/10.1016/j.ces.2015.10.029.
Torres-Acosta MA, Aguilar-Yanez JM, Rito-Palomares M, Titchener-Hooker NJ. Economic analysis of uricase production under uncertainty: contrast of chromatographic purification and aqueous two-phase extraction (with and without PEG recycle). Biotechnol Prog. 2016;32(1):126–33. https://doi.org/10.1002/btpr.2200.
Liu S, Farid SS, Papageorgiou LG. Integrated optimization of upstream and downstream processing in biopharmaceutical manufacturing under uncertainty: a chance constrained programming approach. Ind Eng Chem Res. 2016;55(16):4599–612. https://doi.org/10.1021/acs.iecr.5b04403.
Arnold L, Lee K, Rucker-Pezzini J, Lee JH. Implementation of fully integrated continuous antibody processing: effects on productivity and COGm. Biotechnol J. 2018. https://doi.org/10.1002/biot.201800061.
Hummel J, Pagkaliwangan M, Gjoka X, Davidovits T, Stock R, Ransohoff T, et al. Modeling the downstream processing of monoclonal antibodies reveals cost advantages for continuous methods for a broad range of manufacturing scales. Biotechnol J. 2018. https://doi.org/10.1002/biot.201700665.
Grilo AL, Mateus M, Aires-Barros MR, Azevedo AM. Monoclonal antibodies production platforms: an opportunity study of a non-protein-A chromatographic platform based on process economics. Biotechnol J. 2017;12(12). https://doi.org/10.1002/biot.201700260.
The biopharmaceutical industry’s leading process analysis and economic modelling package. In: BioSolve Process 7. Biopharm. 2016. https://biopharmservices.com/wp-content/uploads/2016/02/Process-2pp-A4-2016-2-FINAL.pdf. Accessed 8/9 2017.
Aspentech. Aspen Batch Process Developer https://www.aspentech.com/en/products/pages/aspen-batch-process-developer. Accessed 12/19 2018.
Aspentech. Aspen Chromatogrpahy. https://www.aspentech.com/en/products/pages/aspen-chromatography. Accessed 12/19 2018.
Ashouri P. A dynamic simulation framework for biopharmaceutical capacity management: University College London; 2011.
Sachidananda M, Erkoyuncu J, Steenstra D, Michalska S. Discrete event simulation modelling for dynamic decision making in biopharmaceutical manufacturing. Procedia CIRP. 2016;49:39–44. https://doi.org/10.1016/j.procir.2015.07.026.
Towler G, Sinnott RK. Chemical engineering design: principles, practice and economics of plant and process design: Elsevier; 2012.
Hernandez I, Bott SW, Patel AS, Wolf CG, Hospodar AR, Sampathkumar S, et al. Pricing of monoclonal antibody therapies: higher if used for cancer? Am J Manag Care. 2018;24(2):109–+.
Pannell DJ. Sensitivity analysis: strategies, methods, concepts, examples. Agric Econ. 1997;16:139–52.
Torres-Acosta MA, Aguilar-Yanez JM, Rito-Palomares M, Titchener-Hooker NJ. Economic analysis of Royalactin production under uncertainty: evaluating the effect of parameter optimization. Biotechnol Prog. 2015;31(3):744–9. https://doi.org/10.1002/btpr.2073.
Vermasvuori R, Hurme M. Economic comparison of diagnostic antibody production in perfusion stirred tank and in hollow fiber bioreactor processes. Biotechnol Prog. 2011;27(6):1588–98. https://doi.org/10.1002/btpr.676.
Repligen inspiring advances in bioprocessing. Frequently asked questions—perfusion. Repligen inspiring advances in bioprocessing official web-site. 2017. https://www.repligen.com/products/upstream-solutions/xcell-atf-cell-retention-system/faq-perfusion/#steady. Accessed 1 June 2018.
Konstantinov KB, Cooney CL. White paper on continuous bioprocessing. May 20–21, 2014 continuous manufacturing symposium. J Pharm Sci. 2015;104(3):813–20. https://doi.org/10.1002/jps.24268.
Roque ACA, Lowe CR, Taipa MÂ. Antibodies and genetically engineered related molecules: production and purification. Biotechnol Prog. 2004;20(3):639–54.
Azevedo AM, Rosa PAJ, Ferreira IF, Aires-Barros MR. Chromatography-free recovery of biopharmaceuticals through aqueous two-phase processing. Trends Biotechnol. 2009;27(4):240–7. https://doi.org/10.1016/j.tibtech.2009.01.004.
Farid SS. Economic drivers and trade-offs in antibody purification processes. BioPharm International. 2009; 2009(7).
Karst DJ, Serra E, Villiger TK, Soos M, Morbidelli M. Characterization and comparison of ATF and TFF in stirred bioreactors for continuous mammalian cell culture processes. Biochem Eng J. 2016;110:17–26. https://doi.org/10.1016/j.bej.2016.02.003.
Espitia-Saloma E, Vazquez-Villegas P, Rito-Palomares M, Aguilar O. An integrated practical implementation of continuous aqueous two-phase systems for the recovery of human IgG: from the microdevice to a multistage bench-scale mixer-settler device. Biotechnol J. 2016;11(5):708–16. https://doi.org/10.1002/biot.201400565.
Angarita M, Muller-Spath T, Baur D, Lievrouw R, Lissens G, Morbidelli M. Twin-column CaptureSMB: a novel cyclic process for protein A affinity chromatography. J Chromatogr A. 2015;1389:85–95. https://doi.org/10.1016/j.chroma.2015.02.046.
Varadaraju H, Schneiderman S, Zhang L, Fong H, Menkhaus TJ. Process and economic evaluation for monoclonal antibody purification using a membrane-only process. Biotechnol Prog. 2011;27(5):1297–305. https://doi.org/10.1002/btpr.639.
Liu SS, Papageorgiou LG. Multi-objective optimisation for biopharmaceutical manufacturing under uncertainty. Comput Chem Eng. 2018;119:383–93. https://doi.org/10.1016/j.compchemeng.2018.09.015.
Popova D, Stonier A, Pain D, Titchener-Hooker NJ, Farid SS. Integrated economic and experimental framework for screening of primary recovery technologies for high cell density CHO cultures. Biotechnol J. 2016;11(7):899–909. https://doi.org/10.1002/biot.201500336.
Torres-Acosta MA, Pereira JFB, Freire MG, Aguilar-Yanez JM, Coutinho JAP, Titchener-Hooker NJ, et al. Economic evaluation of the primary recovery of tetracycline with traditional and novel aqueous two-phase systems. Sep Purif Technol. 2018;203:178–84. https://doi.org/10.1016/j.seppur.2018.04.041.
Lopes AG. Single-use in the biopharmaceutical industry: a review of current technology impact, challenges and limitations. Food Bioprod Process. 2015;93:98–114. https://doi.org/10.1016/j.fbp.2013.12.002.
Berthold Boedeker JM. Opportunities and limitations of continuous processing and use of disposables. Am Pharm Rev. 2017.
Shukla AA, Gottschalk U. Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013;31(3):147–54. https://doi.org/10.1016/j.tibtech.2012.10.004.
Langer ES, Rader RA. Single-use technologies in biopharmaceutical manufacturing: a 10-year review of trends and the future. Engineering in Life Sciences. 2014;14(3):238–43. https://doi.org/10.1002/elsc.201300090.
Marc Bisschops LF, Scott Fulton, Tom Ransohoff. Single-use, continuous-countercurrent, multicolumn chromatography. BioProcess International. 2009.
Michael LaBreck MP. An economic analysis of single—use tangential flow filtration for biopharmaceutical applications. BioProcess International. 2010;2010 Supplement(8).
Jacquemart R, Vandersluis M, Zhao MC, Sukhija K, Sidhu N, Stout J. A single-use strategy to enable manufacturing of affordable biologics. Comput Struct Biotec. 2016;14:309–18. https://doi.org/10.1016/j.csbj.2016.06.007.
Shirahata H, Hirao M, Sugiyama H. Decision-support method for the choice between single-use and multi-use technologies in sterile drug product manufacturing. J Pharm Innov. 2017;12(1):1–13. https://doi.org/10.1007/s12247-016-9264-7.
Magnus J, Temming M, Schwan P, Micovic J, Lobedam M, Sievers S, editors. A biomanufacturing facility based on continuous processing and single use technology2015.
Yang WC, Lu J, Kwiatkowski C, Yuan H, Kshirsagar R, Ryll T, et al. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol Prog. 2014;30(3):616–25. https://doi.org/10.1002/btpr.1884.
Farid SS. Established bioprocesses for producing antibodies as a basis for future planning. In: Hu W-S, editor. Cell Culture Engineering. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 1–42.
Rathore AS, Agarwal H, Sharma AK, Pathak M, Muthukumar S. Continuous processing for production of biopharmaceuticals. Prep Biochem Biotechnol. 2015;45(8):836–49. https://doi.org/10.1080/10826068.2014.985834.
St Amand MM, Hayes J, Radhakrishnan D, Fernandez J, Meyer B, Robinson AS, et al. Identifying a robust design space for glycosylation during monoclonal antibody production. Biotechnol Prog. 2016;32(5):1149–62. https://doi.org/10.1002/btpr.2316.
Radhakrishnan D, Robinson AS, Ogunnaike BA. Controlling the glycosylation profile in mAbs using time-dependent media supplementation. Antibodies. 2018;7(1):1. https://doi.org/10.3390/antib7010001.
Ogonah OW, Polizzi KM, Bracewell DG. Cell free protein synthesis: a viable option for stratified medicines manufacturing? Curr Opin Chem Eng. 2017;18:77–83. https://doi.org/10.1016/j.coche.2017.10.003.
Stech M, Kubick S. Cell-free synthesis meets antibody production: a review. Antibodies. 2015;4(1):12–33. https://doi.org/10.3390/antib4010012.
Martin RW, Majewska NI, Chen CX, Albanetti TE, Jimenez RBC, Schmelzer AE, et al. Development of a CHO-based cell-free platform for synthesis of active monoclonal antibodies. ACS Synth Biol. 2017;6(7):1370–9. https://doi.org/10.1021/acssynbio.7b00001.
Shirokov VA, Simonenko PN, Biryukov SV, Spirin AS. Continuous-flow and continuous-exchange cell-free translation systems and reactors. In: Spirin AS, editor. Cell-free translation systems. Berlin, Heidelberg: Springer Berlin Heidelberg; 2002. p. 91–107.
Stech M, Nikolaeva O, Thoring L, Stocklein WFM, Wustenhagen DA, Hust M, et al. Cell-free synthesis of functional antibodies using a coupled in vitro transcription-translation system based on CHO cell lysates. Sci Rep-Uk. 2017;7:12030. https://doi.org/10.1038/s41598-017-12364-w.
Dutta AK, Tran T, Napadensky B, Teella A, Brookhart G, Ropp PA, et al. Purification of monoclonal antibodies from clarified cell culture fluid using Protein A capture continuous countercurrent tangential chromatography. J Biotechnol. 2015;213:54–64. https://doi.org/10.1016/j.jbiotec.2015.02.026.
Dutta AK, Fedorenko D, Tan J, Costanzo JA, Kahn DS, Zydney AL, et al. Continuous countercurrent tangential chromatography for mixed mode post-capture operations in monoclonal antibody purification. J Chromatogr A. 2017;1511:37–44. https://doi.org/10.1016/j.chroma.2017.06.018.
Rosa PA, Azevedo AM, Sommerfeld S, Mutter M, Backer W, Aires-Barros MR. Continuous purification of antibodies from cell culture supernatant with aqueous two-phase systems: from concept to process. Biotechnol J. 2013;8(3):352–62. https://doi.org/10.1002/biot.201200031.
Rosa PAJ, Azevedo AM, Sommerfeld S, Bäcker W, Aires-Barros MR. Aqueous two-phase extraction as a platform in the biomanufacturing industry: economical and environmental sustainability. Biotechnol Adv. 2011;29(6):559–67. https://doi.org/10.1016/j.biotechadv.2011.03.006.
Rosa PAJ, Ferreira IF, Azevedo AM, Aires-Barros MR. Aqueous two-phase systems: a viable platform in the manufacturing of biopharmaceuticals. J Chromatogr A. 2010;1217(16):2296–305. https://doi.org/10.1016/j.chroma.2009.11.034.
Fisher AC, Kamga MH, Agarabi C, Brorson K, Lee SL, Yoon S. The current scientific and regulatory landscape in advancing integrated continuous biopharmaceutical manufacturing. Trends Biotechnol. 2018. https://doi.org/10.1016/j.tibtech.2018.08.008.
Fisher AC, Lee SL, Harris DP, Buhse L, Kozlowski S, Yu L, et al. Advancing pharmaceutical quality: an overview of science and research in the U.S. FDA’s Office of Pharmaceutical Quality. Int J Pharm. 2016;515(1–2):390–402. https://doi.org/10.1016/j.ijpharm.2016.10.038.
Raftery JP, DeSessa MR, Karim MN. Economic improvement of continuous pharmaceutical production via the optimal control of a multifeed bioreactor. Biotechnol Prog. 2017;33(4):902–12. https://doi.org/10.1002/btpr.2433.
Funding
This work was supported by Eli Lilly and company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, O., Qadan, M. & Ierapetritou, M. Economic Analysis of Batch and Continuous Biopharmaceutical Antibody Production: a Review. J Pharm Innov 15, 182–200 (2020). https://doi.org/10.1007/s12247-018-09370-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-09370-4