Abstract
The patchy nature of landscapes drives variation in the extent of ecological processes across space. This spatial ecology is critical to our understanding of organism-environmental interactions and conservation, restoration, and resource management efforts. In fisheries, incorporation of the spatial ecology of fishes remains limited, despite its importance to fishery assessment and management. This study quantified the effects of variation in headwater river stage, as an indicator of freshwater inflow, on the distribution and movement of a valuable recreational fishery species in Florida, common snook (Centropomus undecimalis). The hypothesis tested was that variation in river stage caused important habitat shifts and changes in the movement behavior of Snook. A combination of electrofishing and acoustic telemetry was used to quantify the distribution and movement patterns of snook in the upper Shark River Estuary, Everglades National Park. Negative relationships with river stage were found for all three variables measured: electrofishing catch per unit effort, the proportion of detections by upstream acoustic receivers, and movement rates. Snook were up to 5.8 times more abundant, were detected 2.3 times more frequently, and moved up to 4 times faster at lower river stages associated with seasonal drawdowns in water level. These findings show how seasonal drawdowns result in local aggregations of consumers, largely driven by improved foraging opportunities, and emphasize the importance of maintaining the natural variance in managed hydrological regimes. Results also highlight the importance of understanding the nature of flow-ecology relationships, especially given projected changes in freshwater availability with climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The patchiness of landscapes and resources produces heterogeneity in the extent of ecological processes across space (Levin 1994). This spatial ecology is known to affect all ecological and evolutionary processes and is fundamental for understanding the structure of populations and communities, patterns and processes in biodiversity and ecosystem function, and the provisioning of ecosystem services (Legendre and Fortin 1989; Tilman and Kareiva 1997). Furthermore, spatial processes are one of the key ways organisms respond to environmental variation, including rapid human-induced environmental change, making the spatial ecology of animals (i.e., their movements, space, and/or habitat use and distributions), a key issue in conservation, restoration, and resource management efforts (Sih et al. 2011; Allen and Singh 2016). In fisheries, quantifying the spatial ecology of fish and their habitats is a key–yet undervalued–component of fishery assessment and management (Ciannelli et al. 2008; Cooke et al. 2016). For instance, accounting for fish movement in stock assessments can substantially alter estimates of stock size, fishing mortality, and recruitment, yet is done infrequently (Goethel et al. 2011; Crossin et al. 2017).
Critical to the greater incorporation of spatial ecology into fisheries is gaining an understanding of the drivers of space use. Nathan et al. (2008) in the formulation of the movement ecology paradigm point to external or environmental factors as one of the fundamental drivers of animal movement and distribution. Understanding the influence of environmental drivers on the spatial ecology of fishes is key to predicting their spatiotemporal occurrence and abundance and informing the design of biological assessments (Cooke et al. 2016). For fishes, relevant environmental factors include physical (e.g., temperature, currents, and habitat structure) and chemical gradients (e.g., oxygen, nutrients, and salinity levels), and the biotic conditions that govern prey resource levels, and predation regimes.
Among these drivers, previous research has shown that freshwater flows can have a major influence on the ecology of fish, including their space use, movements, distributions, and survival (Robins et al. 2005; Poff and Zimmerman 2010; Gillson 2011; Pierce et al. 2021). Variation in flow regime components such as magnitude, frequency, duration, timing, and rate-of-change (Poff et al. 1997) should drive the spatial ecology of fishes. Of particular, interest is the effect of freshwater inflows on economically-valuable coastal fisheries that are estuarine-dependent (Loneragan and Bunn 1999; Robins et al. 2005; Gillson 2011). These effects may result via alterations to salinity regimes, nutrient fluxes, and other important physicochemical regimes (e.g., oxygen), as well as via changes to habitat quality and quantity, and/or influences on primary and secondary production (reviewed by Gillson 2011). For example, in Australia, fishery yields of Barramundi (Lates calcarifer), an economically important recreational and aquaculture species in the Indo-West Pacific, are strongly related to freshwater inflows, benefiting from the higher productivity of freshwater (Roberts et al. 2019), and expected to decline under reduced flow scenarios associated with climate change and increased human demands (Robins et al. 2005; Tanimoto et al. 2012).
While linkages between freshwater inflows and coastal fisheries production have been well-established in other systems (e.g., Australia, Robins et al. 2005; Gillson 2011; Taylor et al. 2014; Williams et al. 2017), we lack this understanding for the subtropics/tropics in the western hemisphere, particularly for economically-valuable recreational fisheries. Importantly, the role and directionality of operating mechanisms underlying the effects of altered freshwater inflow remain poorly understood. Understanding these are critical to adaptive management strategies, especially in the face of projected changes in freshwater availability with climate change (Rodell et al. 2018), and increasing anthropogenic water demands (Davis et al. 2015). Furthermore, recent research points to the importance of establishing flow-ecology relationships for avoiding crossing thresholds in water management that may lead to ecological collapse and for understanding socioecological tradeoffs (Rosenfeld 2017; Poff 2018).
An economically valuable species in the subtropic/tropics of the western hemisphere that is sensitive to freshwater inflows is the common snook (Centropomus undecimalis). Common snook (hereafter snook) inhabit riverine systems of the western Atlantic, the Gulf of Mexico, and the Caribbean Sea; they are protandric hermaphrodites (born males and change sex to females) and obligate marine spawners (Taylor et al. 2000; Young et al. 2020). Previous research suggests that their ecology is closely tied to freshwater inputs (Winner et al. 2010; Boucek and Rehage 2013; Lowerre-Barbieri et al. 2014; Boucek et al. 2016; Blewett et al. 2017). In Florida and the Everglades region, snook are a popular tropical species that support a substantial largely catch-and-release recreational fishery (average of 8.3 million fish caught per year, with 95–99% of them released annually; Muller et al. 2015; Munyandorero et al. 2020), yet the exact nature of their interactions with freshwater inflows is only beginning to be fully understood (Blewett et al. 2017; Stevens et al. 2018).
Our previous research has indicated that the foraging behaviors of snook are closely tied to seasonal fluctuations in freshwater flow in the ecotonal headwaters of coastal Everglades rivers. At low flows during the dry season, this headwaters region receives a pulse of prey from drying marshes upstream, which readily subsidizes higher-order consumers, including snook (Boucek and Rehage 2013; Boucek et al. 2016; Rezek et al. 2020), bull sharks (Matich and Heithaus 2014), and likely juvenile tarpon (Griffin et al. 2018). Both isotope and stomach content data show that during the dry season, snook that spend more time at the headwaters rely on freshwater prey that emigrate as marshes dry at the peak of the dry season (typically between March–May, Boucek and Rehage 2013; Rezek et al. 2020). This results in a seasonal trophic coupling of the marsh and mangrove food webs, which is mediated by low stages, and the associated seasonal redistribution of consumers (Boucek and Rehage 2013; Matich and Heithaus 2014; Boucek et al. 2017; Rezek et al. 2020).
This study asks how does variation in freshwater inflows affects the distribution and movement of snook in the coastal Everglades. We used a combination of electrofishing sampling and acoustic telemetry to track fine-scale distributional and movement patterns of snook in the Shark River in relation to river stage height. We hypothesized that variation in stage should cause important habitat shifts and changes in the movement behavior of snook, with implications for the angling catchability of the species and overall productivity of the fishery. More specifically, we expected that during the dry season, at low river stage levels, electrofishing catches would locally increase due to aggregation of snook in response to increased foraging opportunities, and thus create snook aggregations in specific areas of the hydroscape, namely the upstream most reaches of the river (Boucek and Rehage 2013; Boucek et al. 2016; Rezek et al. 2020). Similarly, we expected that the movement of individuals would increase at low flow levels, due to an increase in foraging activity.
Methods
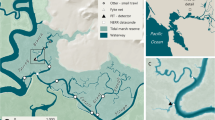
We tracked the distribution and movement of snook in the upper Shark River Estuary, located in the southwestern region of Everglades National Park, FL, USA (Fig. 1). The Shark River Estuary is a tidal river system 30 km in length and is one of the main drainages of the coastal Everglades, delivering freshwater from upstream marshes of the Shark River Slough downstream to the Gulf of Mexico, and is the subject of the Florida Coastal Everglades Long Term Ecological Research (FCE LTER) program. The FCE LTER provides long-term datasets on relevant hydrological and ecological variables (http://fcelter.fiu.edu/; Childers 2006). The system effectively functions as an upside-down estuary with marine inputs supplying limiting nutrients landward (Childers et al. 2006). In this region as elsewhere in the Everglades, freshwater inputs are a principal driver of ecological processes, affecting spatiotemporal patterns in productivity, biogeochemical processes, community structure, species’ distributional patterns, and recreational fisheries productivity (Chen and Twilley 1999; Davis et al. 2005; Ewe et al. 2006; Rosenblatt and Heithaus 2011; Boucek and Rehage 2013, 2015). In particular, the marked seasonality in rainfall, with a wet season during the warmer months (June to October) and a dry season (November to May) in winter and spring, is a dominant feature of the ecosystem (Price et al. 2008).
Map and an aerial image of the upper Shark River Estuary in the western region of Everglades National Park, FL, USA. White diamonds denote acoustic stations, while black circles denote electrofishing stations. The grey square in the bottom map shows the location of the closest hydrological station. Green denotes red mangroves along river shorelines, while brown denotes freshwater graminoid marshes
Electrofishing Sampling
To quantify whether changes in river stage resulted in habitat shifts in snook, we conducted standardized electrofishing between January 2006 and April 2017 along five fixed sites located at the upstream most reaches of the river (for additional sampling details see Boucek and Rehage 2013; Boucek et al. 2016; Fig. 1). Sites encompassed three first-order creeks and two sites located along the main stem of the river (mean depth 2006–2017 = 1.26 m), with an average salinity of 1.1 PSU across the 12 years of sampling (range = 0.2–13.6 PSU).
Sites were sampled using a boat-mounted, generator-powered electrofisher (two-anode, one-cathode Smith-Root 9.0 unit, Smith-Root, Vancouver, WA, USA). Sampling was conducted three times per year: November–December corresponding to the wet season (high inflows), February–March corresponding to the early dry season (medium inflows), and April–May corresponding to the late dry season (low inflows, Fig. 2). Previous research with a more frequent sampling approach (monthly sampling events) found that the November to June period used in this study adequately captured seasonal changes in snook abundance (Boucek et al. 2016). Three replicate electrofishing transects were conducted at each of the 5 sites (3 transects × 5 sites × 3 seasons × 12 years = 540 expected samples). The final sample size was 520 samples due to a small number of missing samples and the fact that in year 12 of the study, sampling did not occur in the wet season.
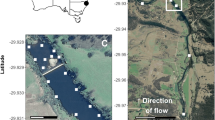
An example of a snook’s acoustic detection record over from tagging (June 2012 to the last detection in August 2015, plotted in relation to river stage levels (m NADV88). Black symbols are daily detections for tag # 51318, while white symbols denote stage at the Bottle Creek hydrostation (see Fig. 1). The photo shows the snook when tagged. The seasonal variation depicted with high stages in the wet and marsh drying in the dry season shown across the 3 years is characteristic of the region and duration of the study
For each transect, the boat was run at idle speed at a randomly selected creek shoreline and 5 min of pedal time was applied (Rehage and Loftus 2007). Power output was standardized to 1500 W, given temperature and conductance conditions measured at the beginning of each sample (Burkhardt and Gutreuter 1995). We recorded the distance traveled during each sample using a GPS. Electrofishing catch per unit effort (CPUE) was standardized for distance and is reported as the number of snook caught or shocked per 100 m of shoreline ((CPUE/distance traveled) × 100 m, Boucek and Rehage 2013). All snook caught were counted and released after a brief recovery at the site of collection. Electrofishing sampling was reviewed and approved by Florida International University’s Institutional Animal Care and Use Committee (IACUC # 15–046).
Acoustic Telemetry
Along with the electrofishing sampling, we tracked the distribution and movements of individual snook in the upper Shark River Estuary using acoustic telemetry (Boucek et al. 2017; Matich et al. 2017; Massie et al. 2020; Rezek et al. 2020). The acoustic array consists of autonomous VR2W (Innovasea Systems Inc.) listening receivers, spaced approximately 1–3 km apart, extending from the upper reaches down to the coastal regions of the Shark and Harney Rivers, at the opening to the Gulf of Mexico in Ponce de Leon Bay. For this study, we focused on the five most upstream acoustic stations, located between river km 20 and 28 (Fig. 1). If a tagged snook passes a listening station, the autonomous device records the unique tag ID, along with a date and time stamp. Our previous tracking research has shown that the receiver coverage is adequate to account for the distribution of snook and other higher-order taxa within the river, with a detection range of > 500 m (Rosenblatt and Heithaus 2011; Boucek et al. 2017; Matich et al. 2017, Massie et al. 2020).
We tracked the space use of 76 acoustically-tagged snook across these five receivers between May 1, 2012 and April 19, 2016. Snook were caught via electrofishing in the upper half of the river and tagged with an acoustic VEMCO V16 (n = 40) or V13 (n = 36) transmitter (mean interpulse delay = 120 s). Following standardized surgical procedures for these species, each individual was surgically implanted with a tag within 2–3 min of capture and immediately release post-surgery (see Adams et al. 2009; Trotter et al. 2012; Lowerre-Barbieri et al. 2014; Boucek et al. 2017 for additional details). The standard length of all tagged fish ranged from 45–86 cm. Acoustic tagging of snook was reviewed and approved by FIU’s Institutional Animal Care and Use Committee (IACUC # 15–013).
We used snook acoustic detections in two analyses. First, to complement our electrofishing surveys, we calculated the daily proportion of tracked snook that were detected in the upper portion of the Shark River Estuary across four water years when tracking data were available for snook (May 2012 to April 2016). For the proportion of detections, we first quantified the daily proportion of detections at the upper river, relative to detections mid-river (Tarpon Bay, Fig. 1) and downriver (downstream of Tarpon Bay, Matich et al. 2017; Boucek et al. 2017). Following Boucek et al. (2017), we standardized the proportions of detections across the three river zones using the equation:
where the standardized daily proportion of snook within zone i is a function of the difference between daily observed proportion in zone i and the average daily proportion for that habitat during the entire time series, divided by the daily proportion for that zone during the entire time series.
Second, to obtain a movement rate of snook (kilometers moved/day), we calculated the daily distance moved for all fish detected. We conducted this analysis for a randomly selected subset of the snook tagged for approximately three hydrological cycles (45 fish detected between November 1, 2012 and September 30, 2015). We first assigned river distances to all receivers using Google Earth™ following Trotter et al. (2012), and for each fish, we obtained the total distance moved in a day by summing the differences among receiver distances. For example, if a receiver was placed at river km 30 and a second receiver was placed at river km 35, and a snook was detected going back and forth three times, the total distance moved was calculated to be 30 km/day. We repeated this analysis for all 45 fish when were detected by focal receivers, resulting in 14,017 daily movement rates, which we averaged across fish to obtain a mean movement rate per day (n = 1064 days).
Relating Snook Variables to River Stage
We used linear regressions to examine the relationships between electrofishing CPUE (# of snook/100 m river shoreline; ln(y + 1) transformed), the proportion of snook detected at the headwaters, snook movement rate (kilometers/day), and river stage. CPUE values were aggregated across the five sites (and the three transects within each site) by taking the mean value and regressing those seasonal sample means (n = 35 seasonal estimates of snook abundance), as our interest was on the temporal variation in snook distribution. The standardized daily proportion of fish detected and the daily movement rate (averaged across individuals) were smoothed by binning these across 11-day bins (Boucek et al. 2017). The 11-day binning was chosen based on the previous estuarine fish acoustic tracking showing that the 11-day temporal window is an adequate period to reduce autocorrelation among observations with a minimal loss of information (Walsh et al. 2013). River stage data, a measure of river water elevation relative to NADV88, was obtained from the closest hydrological station to our sites, Bottle Creek (US Geological Survey, https://sofia.usgs.gov/exchange/sfl_hydro_data/; Fig. 1). River stage data were averaged over the temporal resolution of the snook data (i.e., the 11-day bins for the detection and movement rate data and the days of sampling for the electrofishing data). Regressions were performed in R v3.2.5 (R Core Team 2017).
Results
We found evidence that stage height explained a substantial proportion of headwater electrofishing catch per unit effort, the proportion of detections by headwater acoustic receivers, and movement rates of snook in the Everglades (all adjusted R2 values > 0.40). Overall, we found a negative relationship of river stage on the three snook variables measured for the upper Shark River Estuary snook. Snook abundance was the highest, more snook were detected, and their movement rates were faster at lower stages. Across 12 years of electrofishing samples, we caught an average of 2.33 snook per 100 m of river shoreline, but these snook catches varied significantly with river stage. Snook CPUE was negative related to river stage, with the highest CPUE recorded at the lowest stages (β = −2.579, SE = 0.403, F1,33 = 40.93, p < 0.0001, adjusted R2 = 0.540, Fig. 3a). Snook catches averaged 0.77 fish/100 m in high stages and peaked at 4.47 fish/100 m in low stage low flow conditions.
Average snook CPUE in electrofishing samples (log-transformed) (a) and proportion of fish detected in acoustic telemetry plotted as a function of river stage (m NADV88) (b) Stage data come from Bottle Creek hydrostation (see Fig. 1), and shaded areas represent 95% confidence intervals. Data points in a are 35 seasonal estimates of CPUEs across 12 years of electrofishing. Data points in b are 11-day bins of standardized daily proportion of fish detected at the headwaters (Fig. 1) across the 4 years of tracking data
Similarly, for the acoustic data, the proportion of tagged snook detected was also negatively related to river stage (β = −0.476, SE = 0.047, F1,135 = 100.9, p < 0.0001, adjusted R2 = 0.423). At low stage, up to 70% of the tagged snook were detected in the upper reaches, while less than 30% were detected at peak river stages (Fig. 3b). Last, the rate at which snook moved also varied negatively as a function of stage (β = −2.322, SE = 0.280, F1, 95 = 68.83, p < 0.0001, adjusted R2 = 0.414). Snook moved faster, approaching 2 km/day at the lowest stages of the peak of the dry season, but moved less than 0.5 km/day at the highest stages of the wet seasons (Fig. 4).
Average snook movement rate (km/day) as a function of river stage (m NADV88) for snook detected in acoustic telemetry samples. Stage data come from Bottle Creek hydrostation (see Fig. 1), and shaded areas represent 95% confidence intervals. Data points are 11-day bins of daily movement rates (averaged across individuals) for the 4 years of tracking data
Discussion
Ecological processes vary in space, and at the coast, spatial heterogeneity often results from variation in freshwater flows (e.g., Taylor et al. 2014). Throughout coastal systems, freshwater inflows can act as a master variable influencing ecosystem function, structure, and services (Alber 2002). Furthermore, flow variation has been shown to influence the movement patterns of estuarine fish (Crook et al. 2010; Sakabe and Lyle 2010; Williams et al. 2017; Roberts et al. 2019) and affect fisheries production (Robins et al. 2005; Gillson 2011). In our study, we examined the effects of variability in stage on the distribution and movement of snook in the coastal Everglades, an ecosystem with a highly impacted hydrology. The impetus behind this study was to obtain a more detailed understanding of the factors driving the spatial ecology of economically-valuable recreational fisheries along Florida’s coasts. Freshwater flows were the focal driver examined because of their critical role in driving the ecology of estuarine ecosystems, and their vulnerability to both current and future anthropogenic threats (e.g., competing freshwater demands and climate change; Davis et al. 2015). Both our electrofishing sampling and acoustic telemetry showed strong negative relationships to freshwater inflows in the upper Shark River Estuary.
Electrofishing effectiveness (e.g., the production of an electrical field that is of sufficient size and intensity to induce a capture-prone response by fish) is known to be reduced in high conductivity waters (Lieschke et al. 2019). In our study, high-conductivity conditions were experienced in the dry season, yet catches of snook were higher in the dry season, suggesting no or minimal variation in electrofishing sampling effectiveness between the wet and dry season. Our results show the agreement of the standardized electrofishing sampling with the acoustic tracking data, corroborating the pattern of higher snook numbers at the headwaters in the dry season, and increasing confidence that tagging studies with small samples sizes (10’s of individuals) can capture patterns of abundance documented for larger population sizes and over longer periods (e.g., 654 snook caught over 12 years in this study).
These findings contribute additional resolution to the effects of hydrological variation on consumer dynamics in the Shark River Estuary. Both top and mesoconsumers show a high reliance on freshwater marsh prey sources that pulse into the upper river at low seasonal stages, as upstream marshes dry and large numbers of prey and other consumers are displaced (Boucek and Rehage 2013; Matich and Heithaus 2014). Snook and Bull Sharks move to the Shark River Estuary headwaters (upper river zone) in the dry season to take advantage of this subsidy, creating temporally variable trophic linkages between mangrove and freshwater food webs (Matich et al. 2017). Results from this study confirm that increases in snook numbers and detections occur locally at low flow conditions, matching the timing when marsh prey numbers have been documented to be the highest (largely freshwater sunfishes, Lepomis spp; Boucek and Rehage 2013; Boucek et al. 2016; Rezek et al. 2020). These local, dry seasons aggregations of snook upstream may also increase their vulnerability to extreme cold events (Boucek et al. 2017), known to cause major declines in snook populations and their fishery (Stevens et al. 2016; Santos et al. 2016). In sum, these findings reveal an aggregation of snook at the upstream reaches of the river during the dry season, originating from elsewhere in the system and in response to improved foraging opportunities. This is not to say that snook prefer low flows nor that low flows result in high numbers of snook. This is merely a concentration effect driven by low stages, which act to concentrate prey and locally increase prey availability and vulnerability. As previous work has shown, these prey are produced by high stages in the wet season (Jardine et al. 2012; Botson et al. 2016; Rezek et al. unpubl. data), and thus the production of snook and other fisheries relies on high flows. Thus, our findings emphasize the importance of naturally variable hydrological regimes to maintaining species populations, energy flow pathways, and ecosystem processes in aquatic systems (Poff et al. 1997; Lytle and Poff 2004; Poff and Zimmerman 2010). For management, this translates into managing for historical variability, as well as for resilience, such that flows sustain socially-valuable ecological components (e.g., snook) while relying on an adaptive management framework (Poff 2018).
Aggregations of snook in response to seasonal drawdowns in water level and improved foraging opportunities in the upper Shark River Estuary concur with studies on the importance of flow to large, riverine consumers for tropical floodplain systems. In these systems, flows affect the extent of floodplain inundation, and thus both prey production and availability (Junk et al. 1989; Winemiller and Jepsen 1998). At low flows, seasonal drying makes large volumes of vertebrate (small fishes) and invertebrate prey (e.g., crayfishes), produced high in floodplains during the wet season, available to mobile consumers in riverine channels (Winemiller and Jepsen 1998; Hoeinghaus et al. 2006; Robins et al. 2006). Thus, these low stages concentrate prey and create foraging opportunities (Boucek and Rehage 2013). Recent research on snook in the Peace River shows that both snook abundance and body condition (the ratio of weight to length, and an indicator of overall health) increase from summer to fall as water levels decrease (Blewett et al. 2017). The study also shows that over an 8-year time series, both annual abundance and condition were positively related to mean annual river flow.
In combination, Blewett et al. (2017) and our study highlight two key points about the effects of inflows on snook. First, the effect of freshwater flow for snook appears to be mainly mediated via a trophic pathway (i.e., affecting prey abundance and availability), and not by effects of physicochemical conditions, such as salinity or oxygen nor other factors (e.g., avoidance of predators). Second, as shown by previous floodplain research (e.g., Winemiller and Jepsen 1998; Hoeinghaus et al. 2006; Botson et al. 2016), the effect of flow on prey is two-faceted: high flows are required for production, and low flows are needed for creating prey concentrations and increasing prey vulnerability to consumers. These finding parallel predator–prey-hydrology relationships documented for Everglades wading birds. Wading birds are dependent on both long periods of inundation that drive prey production, and on water recession rates that concentrate prey, in order to maximize foraging success (Gawlik 2002; Beerens et al. 2011; Botson et al. 2016). These relationships are thought to be the main factor limiting reproductive success, and the recovery of wading bird populations, a key measure of ecosystem restoration success for the Everglades ecosystem (Gawlik 2002; Frederick et al. 2009).
While low flows may be beneficial to snook because of prey concentration effects, extreme low flows are not. During a year of severe drought, with minor floodplain inundation, Blewett et al. (2017) showed no increase in abundance nor body condition, and a diet comprised mostly of small-bodied species (Palaemonid shrimp). Similarly in the Shark River Estuary, our previous research has shown that droughts can sever this trophic linkage between marsh prey production and estuarine consumers (Boucek et al. 2016). Post-drought, the prey subsidy to snook decreased by 75% in biomass, and the diet composition of the snook switched to lower quality prey (e.g., invertebrates with lower caloric content than fishes). This finding underscores the importance of understanding the nonlinearities in flow-ecology relationships (Rosenfeld 2017) for key ecosystem service providers, such as valuable recreational fisheries species. In a recent meta-analysis of ecological flow responses to altered flow regimes, Poff and Zimmerman (2010) noted that the abundance, diversity, and demographic rates of fish consistently declined in response to both elevated and reduced flow magnitudes.
We hypothesize that the increases in the movement rate of snook at low flows reflect an increase in foraging activity at the high dry-season prey concentrations. Organisms are typically expected to increase prey search behavior, which has costs, as prey profitability increases (e.g., optimal foraging theory, Stephens et al. 2007). In a series of meta-analyses examining the effects of flow magnitude on movement, Taylor and Cooke (2012) reported a positive effect of flow on non-migratory movements and upstream migratory movements, but no effect of flows on swimming activity (analogous to our movement rate). They suggest that the effects of river flow on activity are likely the result of complex foraging decisions, reflecting trade-offs between swimming costs, prey availability and accessibility, and internal energy state, but very few studies have examined movement at this scale. Novel technologies that combine acoustic telemetry with biosensors (e.g., tracking jaw-motion events or acceleration data loggers, Hussey et al. 2015; Lear et al. 2019) can improve the ability to disentangle foraging behavior from movement patterns. Furthermore, future work should also examine the sensory ecology of consumers and the role of cues in driving their movements, such as the cues that drive the movement of snook upstream in response to dry season prey concentrations.
Information on the links between recreational fisheries and key environmental drivers is often lacking. This information is particularly important in light of research showing that similar to commercial fisheries, recreational fisheries can be prone to population collapse and stock depletion, yet are often data-poor, which severely limits our ability to sustainably manage them (Post et al. 2002; Post 2013). Arlinghaus and Cooke (2009) estimate that across countries with reliable statistics, about 10% of the adult population participates in recreational angling. In Florida, one in five anglers fished in the Everglades, generating US $1.2 billion in economic activity in the region (Fedler 2009). Yet, despite this high socioeconomic value, the degree to which recreational fisheries may be unsustainably impacted by altered freshwater flows, future climate change, and associated coastal degradation remains poorly understood. In his review, Gillson (2011) suggests that protecting natural flow regimes should be an effective management strategy to maintain the production of coastal fisheries. Our study contributes to evidence establishing the magnitude and directionality of the dependency of the snook on freshwater inflows. Future research should extend these analyses to the relationships between flows and the dynamics of the snook fishery (both catch and effort) in the Everglades and identify how variations in seasonal hydrological regimes influence the production of freshwater marsh prey to characterize the underlying mechanisms that mediate interannual variation in the productivity of the snook fishery. These empirical relationships are essential to avoiding tipping points and collapse in the provisions of ecosystem services (Rosenfeld 2017). Importantly, these relationships are key to evaluating tradeoffs in water allocation among multiple demand nodes (e.g., Mirchi et al. 2018), particularly when it concerns large ecosystem restoration efforts and decreasing water availability scenarios, such as the case of the Everglades (Obeysekera et al. 2011).
References
Adams, A., R.K. Wolfe, N. Barkowski, and D. Overcash. 2009. Fidelity to spawning grounds by a catadromous fish Centropomus undecimalis. Marine Ecology Progress Series 389: 213–222.
Alber, M. 2002. A conceptual model of estuarine freshwater inflow management. Estuaries 25: 1246–1261.
Allen, A.M., and N.J. Singh. 2016. Linking movement ecology with wildlife management and conservation. Frontiers in Ecology and Evolution 3: 155.
Arlinghaus, R., and S.J. Cooke. 2009. Recreational fisheries: Socioeconomic importance conservation issues and management challenges. In Recreational Hunting Conservation and Rural Livelihoods, ed. B. Dickson, J. Hutton, and W.M. Adams, 39–58. Oxford: Wiley-Blackwell.
Beerens, J.M., D.E. Gawlik, G. Herring, and M.I. Cook. 2011. Dynamic habitat selection by two wading bird species with divergent foraging strategies in a seasonally fluctuating wetland. The Auk 128: 651–662.
Blewett, D.A., P.W. Stevens, and J. Carter. 2017. Ecological effects of river flooding on abundance and body condition of a large euryhaline fish. Marine Ecology Progress Series 563: 211–218.
Botson, B.A., D.E. Gawlik, and J.C. Trexler. 2016. Mechanisms that generate resource pulses in a fluctuating wetland. PLOS ONE 11: e0158864.
Boucek, R.E., and J.S. Rehage. 2013. No free lunch: Displaced marsh consumers regulate a prey subsidy to an estuarine consumer. Oikos 122: 1453–1464.
Boucek, R.E., and J.S. Rehage. 2015. A tale of two fishes: Using recreational angler records to examine the link between fish catches and floodplain connections in a subtropical coastal river. Estuaries and Coasts 38: 124–135.
Boucek, R.E., M. Soula, F. Tamayo, and J.S. Rehage. 2016. A once in 10 year drought alters the magnitude and quality of a floodplain prey subsidy to coastal river fishes. Canadian Journal of Fisheries and Aquatic Sciences 73: 1672–1678.
Boucek, R.E., M.R. Heithaus, R. Santos, P. Stevens, and J.S. Rehage. 2017. Can animal habitat use patterns influence their vulnerability to extreme climate events? An estuarine sportfish case study. Global Change Biology 23: 4045–4057.
Burkhardt, R.W., and S. Gutreuter. 1995. Improving electrofishing catch consistency by standardizing power. North American Journal of Fisheries Management 15: 375–381.
Chen, R., and R.R. Twilley. 1999. Patterns of mangrove forest structure and soil nutrient dynamics along the Shark River Estuary Florida. Estuaries and Coasts 22: 955–970.
Childers, D.L. 2006. A synthesis of long-term research by the Florida Coastal Everglades LTER Program. Hydrobiologia 569: 531–544.
Childers, D.L., J.N. Boyer, S.E. Davis, C.J. Madden, D.T. Rudnick, and F.H. Sklar. 2006. Relating precipitation and water management to nutrient concentrations in the oligotrophic “upside-down” estuaries of the Florida Everglades. Limnology and Oceanography 51: 602–616.
Ciannelli, L., P. Fauchald, K.-S. Chan, V.N. Agostini, and G.E. Dingsør. 2008. Spatial fisheries ecology: recent progress and future prospects. Journal of Marine Systems 71: 223—236.
Cooke, S. J., E. G. Martins, D. P. Struthers, L. F. Gutowsky, M. Power, S. E. Doka, J, M. Dettmers, D. A. Crook, M. C. Lucas, C. M. Holbrook, and C. C. Krueger. 2016. A moving target-incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-016-5228-0.
Crook, D.A., W.M. Koster, J.I. Macdonald, S.J. Nicol, C.A. Belcher, D.R. Dawson, D.J. O’mahony, D. Lovett, A. Walker, and L. Bannam. 2010. Catadromous migrations by female tupong (Pseudaphritis urvillii) in coastal streams in Victoria Australia. Marine and Freshwater Research 61: 474–483.
Crossin, G.T., M.R. Heupel, C.M. Holbrook, N.E. Hussey, S.K. Lowerre-Barbieri, V.M. Nguyen, G.D. Raby, et al. 2017. Acoustic telemetry and fisheries management. Ecological Applications 27: 1031–1049.
Davis, J., A.P. O’Grady, A. Dale, A.H. Arthington, P.A. Gell, P.D. Driver, N. Bond, et al. 2015. When trends intersect: The challenge of protecting freshwater ecosystems under multiple land use and hydrological intensification scenarios. Science of the Total Environment 534: 65–78.
Davis, S.M., D.L. Childers, J.J. Lorenz, H.R. Wanless, and T.E. Hopkins. 2005. A conceptual model of ecological interactions in the mangrove estuaries of the Florida Everglades. Wetlands 25: 832–842.
Ewe, S.M., E.E. Gaiser, D.L. Childers, D. Iwaniec, V.H. Rivera-Monroy, and R.R. Twilley. 2006. Spatial and temporal patterns of aboveground net primary productivity (ANPP) along two freshwater-estuarine transects in the Florida Coastal Everglades. Hydrobiologia 569: 459–474.
Fedler, T. 2009. The economic impact of recreational fishing in the Everglades region. Miami: Bonefish and Tarpon Trust.
Frederick, P., D.E. Gawlik, J.C. Ogden, M.I. Cook, and M. Lusk. 2009. The White Ibis and Wood Stork as indicators for restoration of the everglades ecosystem. Ecological Indicators 9: S83–S95.
Gawlik, D.E. 2002. The effects of prey availability on the numerical response of wading birds. Ecological Monographs 72: 329–346.
Gillson, J. 2011. Freshwater flow and fisheries production in estuarine and coastal systems: Where a drop of rain is not lost. Reviews in Fisheries Science 19: 168–186.
Goethel, D.R., T.J. Quinn, and S.X. Cadrin. 2011. Incorporating spatial structure in stock assessment: Movement modeling in marine fish population dynamics. Reviews in Fisheries Science 19: 119–136.
Griffin, L.P., J.W. Brownscombe, A.J. Adams, R.E. Boucek, J.T. Finn, M.R. Heithaus, J.S. Rehage, S.J. Cooke, and A.J. Danylchuk. 2018. Keeping up with the Silver King: Using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon (Megalops atlanticus) in the coastal waters of the southeastern United States. Fisheries Research 205: 65–76.
Hoeinghaus, D., K. Winemiller, C. Layman, D. Arrington, and D. Jepsen. 2006. Effects of seasonality and migratory prey on body condition of Cichla species in a tropical floodplain river. Ecology of Freshwater Fish 15: 398–407.
Hussey, N.E., S.T. Kessel, K. Aarestrup, S.J. Cooke, P.D. Cowley, A.T. Fisk, R.G. Harcourt, et al. 2015. Aquatic animal telemetry: A panoramic window into the underwater world. Science 348: 1255642.
Jardine, T.D., B.J. Pusey, S.K. Hamilton, N.E. Pettit, P.M. Davies, M.M. Douglas, V. Sinnamon, I.A. Halliday, and S.E. Bunn. 2012. Fish mediate high food web connectivity in the lower reaches of a tropical floodplain river. Oecologia 168: 829–838.
Junk, W.J., P.B. Bayley, and R.E. Sparks. 1989. The flood pulse concept in river-floodplain systems. In Proceedings of the International Large River Symposium, ed. D.P. Dodge, 110–127. Ottawa: Canadian Special Publication of Fisheries and Aquatic Sciences.
Lear, K.O., G.R. Poulakis, R.M. Scharer, A.C. Gleiss, and N.M. Whitney. 2019. Fine-scale behavior and habitat use of the endangered smalltooth sawfish (Pristis pectinata): Insights from accelerometry. Fishery Bulletin 117: 348–359.
Legendre, P., and M.J. Fortin. 1989. Spatial pattern and ecological analysis. Vegetatio 80: 107–138.
Lieschke, J.A., J.C. Dean, and A. Pickworth. 2019. Extending the effectiveness of electrofishing to estuarine habitats: Laboratory and field assessments. Transactions of the American Fisheries Society 148: 584–591.
Levin, S. 1994. Patchiness in marine and terrestrial systems: From individuals to populations. Philosophical Transactions of the Royal Society of London B 343: 99–103.
Loneragan, N., and S. Bunn. 1999. River flow and estuarine food-webs: Implications for the production of coastal fisheries with an example from the Logan River southeast Queensland. Australian Journal of Ecology 24: 431–440.
Lowerre-Barbieri, S., D. Villegas-Ríos, S. Walters, J. Bickford, W. Cooper, R. Muller, and A. Trotter. 2014. Spawning site selection and contingent behavior in common snook. Centropomus undecimalis. PLOS One 9: e101809.
Lytle, D.A., and N.L. Poff. 2004. Adaptation to natural flow regimes. Trends in Ecology & Evolution 19: 94–100.
Massie, J.A., B.A. Strickland, R.O. Santos, J. Hernandez, N. Viadero, R.E. Boucek, H. Willoughby, M.R. Heithaus, and J.S. Rehage. 2020. Going downriver: Patterns and cues in hurricane-driven movements of common snook in a subtropical coastal river. Estuaries and Coasts 43: 1158–1173.
Matich, P., and M.R. Heithaus. 2014. Multi-tissue stable isotope analysis and acoustic telemetry reveal seasonal variability in the trophic interactions of juvenile bull sharks in a coastal estuary. Journal of Animal Ecology 83: 199–213.
Matich, P., J.S. Ault, R.E. Boucek, D.R. Bryan, K.R. Gastrich, C.L. Harvey, M.R. Heithaus, et al. 2017. Ecological niche partitioning within a large predator guild in a nutrient-limited estuary. Limnology and Oceanography 62: 934–953.
Mirchi, A., D.W. Watkins, V. Engel, M.C. Sukop, J. Czajkowski, M. Bhat, J. Rehage, D. Letson, Y. Takatsuka, and R. Weisskoff. 2018. A hydro-economic model of South Florida water resources system. Science of the Total Environment 628–629: 1531–1541.
Munyandorero, J., A.A. Trotter, P.W. Stevens, and R.G. Muller. 2020. The 2020 stock assessment of common snook Centropomus undecimalis. St. Petersburg: Florida Fish and Wildlife Research Institute In House Report: IHR 2020–004.
Muller, R.G., A.A. Trotter, and P.W. Stevens. 2015. The 2015 stock assessment update of Common Snook Centropomus undecimalis. St. Petersburg: Florida Fish and Wildlife Conservation Commission IHR 2015-004.
Nathan, R., W.M. Getz, E. Revilla, M. Holyoak, R. Kadmon, D. Saltz, and P.E. Smouse. 2008. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences 105: 19052–19059.
Obeysekera, J., M. Irizarry, J. Park, J. Barnes, and T. Dessalegne. 2011. Climate change and its implications for water resources management in south Florida. Stochastic Environmental Research and Risk Assessment 25: 495–516.
Pierce, J.L., M.V. Lauretta, R.J. Rezek, and J.S. Rehage. 2021. Survival of Florida Largemouth Bass in a coastal refuge habitat across years of varying drying severity. Transactions of the American Fisheries Society 150 (4): 435–451. https://doi.org/10.1002/tafs.10274.
Poff, N., J.D. Allan, M.B. Bain, J.R. Karr, K.L. Prestegaard, B.D. Richter, R.E. Sparks, et al. 1997. The natural flow regime: A paradigm for river conservation and restoration. BioScience 47: 769–784.
Poff, N.L. 2018. Beyond the natural flow regime? Broadening the hydro-ecological foundation to meet environmental flows challenges in a non-stationary world. Freshwater Biology 63: 1011–1021.
Poff, N.L., and J.K. Zimmerman. 2010. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshwater Biology 55: 194–205.
Post, J. 2013. Resilient recreational fisheries or prone to collapse? A decade of research on the science and management of recreational fisheries. Fisheries Management and Ecology 20: 99–110.
Post, J.R., M. Sullivan, S. Cox, N.P. Lester, C.J. Walters, E.A. Parkinson, A.J. Paul, et al. 2002. Canada’s recreational fisheries: The invisible collapse? Fisheries 27: 6–17.
Price, R.M., P.K. Swart, and H.E. Willoughby. 2008. Seasonal and spatial variation in the stable isotopic composition (δ18O and δD) of precipitation in south Florida. Journal of Hydrology 358: 193–205.
R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rehage, J.S., and W.F. Loftus. 2007. Seasonal fish community variation in headwater mangrove creeks in the southwestern Everglades: An examination of their role as dry-down refuges. Bulletin of Marine Science 80: 625–645.
Rezek, R.J., J.A. Massie, J.A. Nelson, R.O. Santos, N.M. Viadero, R.E. Boucek, and J.S. Rehage. 2020. Individual consumer movement mediates food web coupling across a coastal ecosystem. Ecosphere 11: e03305.
Roberts, Brien H., John R. Morrongiello, Alison J. King, David L. Morgan, Thor M. Saunders, Jon Woodhead, and David A. Crook. 2019. Migration to freshwater increases growth rates in a facultatively catadromous tropical fish. Oecologia 191: 253–260.
Robins, J., D. Mayer, J. Staunton-Smith, I. Halliday, B. Sawynok, and M. Sellin. 2006. Variable growth rates of the tropical estuarine fish barramundi Lates calcarifer (Bloch) under different freshwater flow conditions. Journal of Fish Biology 69: 379–391.
Robins, J.B., I.A. Halliday, J. Staunton-Smith, D.G. Mayer, and M.J. Sellin. 2005. Freshwater-flow requirements of estuarine fisheries in tropical Australia: A review of the state of knowledge and application of a suggested approach. Marine and Freshwater Research 56: 343–360.
Rodell, M., J.S. Famiglietti, D.N. Wiese, J.T. Reager, H.K. Beaudoing, F.W. Landerer, and M.-H. Lo. 2018. Emerging trends in global freshwater availability. Nature 557: 651–659.
Rosenblatt, A.E., and M.R. Heithaus. 2011. Does variation in movement tactics and trophic interactions among American alligators create habitat linkages? Journal of Animal Ecology 80: 786–798.
Rosenfeld, J.S. 2017. Developing flow–ecology relationships: Implications of nonlinear biological responses for water management. Freshwater Biology 62: 1305–1324.
Sakabe, R., and J.M. Lyle. 2010. The influence of tidal cycles and freshwater inflow on the distribution and movement of an estuarine resident fish Acanthopagrus butcheri. Journal of Fish Biology 77: 643–660.
Santos, R., J.S. Rehage, R. Boucek, and J. Osborne. 2016. Shift in recreational fishing catches as a function of an extreme cold event. Ecosphere 7: e01335.
Sih, A., M.C. Ferrari, and D.J. Harris. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evolutionary Applications 4: 367–387.
Stephens, D.W., J.S. Brown, and R.C. Ydenberg. 2007. Foraging: Behavior and ecology. Chicago: University of Chicago Press.
Stevens, P., D. Blewett, R.E. Boucek, J.S. Rehage, B. Winner, J. Young, J. Whittington, et al. 2016. Resilience of a tropical sport fish population to a severe cold event varies across five estuaries in southern Florida. Ecosphere 7: e01400.
Stevens, P.W., R.E. Boucek, A.A. Trotter, J.L. Ritch, E.R. Johnson, C.P. Shea, D.A. Blewett, and J.S. Rehage. 2018. Illustrating the value of cross-site comparisons: Habitat use by a large, euryhaline fish differs along a latitudinal gradient. Fisheries Research 208: 42–48.
Tanimoto, M., J. Robins, M. O’Neill, I. Halliday, and A. Campbell. 2012. Quantifying the effects of climate change and water abstraction on a population of barramundi (Lates calcarifer) a diadromous estuarine finfish. Marine and Freshwater Research 63: 715–726.
Taylor, M.D., D.E. van der Meulen, M.C. Ives, C.T. Walsh, I.V. Reinfelds, and C.A. Gray. 2014. Shock stress or signal? Implications of freshwater flows for a top-level estuarine predator. PLOS One 9: e95680.
Taylor, M.K., and S.J. Cooke. 2012. Meta-analyses of the effects of river flow on fish movement and activity. Environmental Reviews 20: 211–219.
Taylor, R.G., J.A. Whittington, H.J. Grier, and R.E. Crabtree. 2000. Age, growth, maturation, and protandric sex reversal in common snook Centropomus undecimalis from the east and west coasts of South Florida. Fishery Bulletin 98: 612–612.
Tilman, D., and P.M. Kareiva. 1997. Spatial ecology: The role of space in population dynamics and interspecific interactions, vol. 30. Princeton: Princeton University Press.
Trotter, A.A., D.A. Blewett, R.G. Taylor, and P.W. Stevens. 2012. Migrations of common snook from a tidal river with implications for skipped spawning. Transactions of the American Fisheries Society 141: 1016–1025.
Walsh, C., I. Reinfelds, M. Ives, C.A. Gray, R.J. West, and D.E. van der Meulen. 2013. Environmental influences on the spatial ecology and spawning behaviour of an estuarine-resident fish Macquaria colonorum. Estuarine Coastal and Shelf Science 118: 60–71.
Williams, J., J.S. Hindell, G.P. Jenkins, S. Tracey, K. Hartmann, and S.E. Swearer. 2017. The influence of freshwater flows on two estuarine resident fish species show differential sensitivity to the impacts of drought flood and climate change. Environmental Biology of Fishes 100: 1121–1137.
Winemiller, K.O., and D.B. Jepsen. 1998. Effects of seasonality and fish movement on tropical river food webs. Journal of Fish Biology 53: 267–296.
Winner, B.L., D.A. Blewett, R.H. McMichael Jr., and C.B. Guenther. 2010. Relative abundance and distribution of common snook along shoreline habitats of Florida estuaries. Transactions of the American Fisheries Society 139: 62–79.
Young, J.M., B.G. Yeiser, J.A. Whittington, and J. Dutka-Gianelli. 2020. Maturation of female common snook Centropomus undecimalis: Implications for managing protandrous fishes. Journal of Fish Biology 97: 1317–1331.
Funding
This project was funded by the National Science Foundation (NSF) Water, Sustainability, and Climate (WSC) program NSF EAR-1204762, and by the Monitoring and Assessment Plan of the Comprehensive Everglades Restoration Plan (CERP) through the US Army Corps of Engineers. The project was developed with the support from the Florida Coastal Everglades (FCE) Long Term Ecological Research (LTER) program (NSF DEB-1237517) and Everglades National Park.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Henrique Cabral
Rights and permissions
About this article
Cite this article
Rehage, J.S., Boucek, R.E., Santos, R.O. et al. Untangling Flow-Ecology Relationships: Effects of Seasonal Stage Variation on Common Snook Aggregation and Movement Rates in the Everglades. Estuaries and Coasts 45, 2059–2069 (2022). https://doi.org/10.1007/s12237-022-01065-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01065-x