Abstract
Low freshwater inflow estuaries are common worldwide, yet our understanding of phytoplankton dynamics in these systems is limited compared to river-influenced estuaries. Combining 3 years of monthly sampling data with analysis of a lower frequency, multidecadal dataset, we examined nutrient-phytoplankton dynamics in Baffin Bay-Upper Laguna Madre, TX (USA), a low-inflow lagoonal estuary. During a low rainfall, high salinity period (corresponding with non-El Niño conditions), phytoplankton community biovolume was high and consisted of a near monoculture of the harmful “brown tide” organism, Aureoumbra lagunensis. With the onset of El Niño conditions, rainfall increased and salinity decreased. Other phytoplankton groups became more prevalent, namely diatoms and the mixotrophic ciliate, Mesodinium sp., while prevalence of A. lagunensis declined. Although El Niño conditions corresponded with increased inorganic nitrogen concentrations, this did not lead to a near-term (weeks-months) increase in phytoplankton biovolume, indicating that the stimulatory effects of nutrient pulses may have been countered by the decreased residence times associated with increased inflow. Overall, results demonstrate that low freshwater inflows, as well as high residence times and salinities, can lead to increased phytoplankton biomass and decreased phytoplankton diversity, despite relatively low external nutrient loadings. With future expansion of arid/semiarid regions and/or increasing human freshwater demands, Baffin Bay and similar systems may experience lower inflows, more pronounced hypersalinity, and presumably less diverse phytoplankton communities, potentially dominated by harmful taxa as seen here.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuarine phytoplankton population dynamics are complex, due in part to the interactive effects of physical drivers such as freshwater inflow variability, tides, and winds (Paerl et al. 2006; Cloern et al. 2014; Dijkstra et al. 2019). While our understanding of phytoplankton dynamics in “classical” or river-dominated estuaries has increased greatly over the past three decades (e.g., Cloern 1996; Pinckney et al. 1999; Smayda and Borkman 2008; Rothenberger et al. 2009), research on phytoplankton dynamics has lagged in many low-inflow estuaries despite their broad distribution worldwide (reviewed in Largier 2010). Low-inflow estuaries are systems that experience minimal freshwater inflows for prolonged periods, ranging from seasonal to multiannual timescales (Largier 2010), and are thus likely distinct from an ecological standpoint compared to river-dominated estuaries that have a constant freshwater inflow. For example, hypersaline conditions that are stressful to many estuarine organisms occasionally occur in low-inflow estuaries due to a lack of freshwater source (Largier 2010; Tweedley et al. 2019). A prolonged lack of riverine-derived nutrient loads could lead to nutrient limitation of phytoplankton growth and biomass accumulation (Wetz et al. 2011; Phlips et al. 2020), but intense nutrient recycling under the long residence time conditions that would be expected in many low-inflow estuaries may alleviate nutrient limitation (Cloern 2001; Pinckney et al. 2001). In fact, several studies have demonstrated that poor flushing, along with ample regenerated nutrient stocks, leads to extended periods of elevated phytoplankton biomass in these systems (Knoppers et al. 1991; Hemraj et al. 2017; Barroso et al. 2018). These factors suggest that low-inflow, long-residence time estuaries could conceivably be more susceptible to the harmful effects of anthropogenic nutrient loading than river-dominated systems (Bricker et al. 2008; Hemraj et al. 2017; Warwick et al. 2018).
Aside from being susceptible to nutrient pressures, research suggests that low-inflow estuaries in some regions may also experience decreases in inflow due to both climate change and human uses. For example, models project decreasing precipitation with climate change in many subtropical regions worldwide (e.g., Trenberth 2011) and even point to expansion of semi-arid/arid climates in the future (Chan and Wu 2015). In many cases, freshwater supplies in these regions are already stressed by human demands. Growing populations are expected to put additional stress on freshwater resources while also exacerbating nutrient loads (Flemer and Champ 2006; Hallett et al. 2018).
Recognizing that low-inflow estuaries may be sensitive to large-scale environmental changes and stressors, and that some are at the forefront of climate-induced precipitation changes (decreases), it is important to develop management strategies to address these challenges. To do this, however, it is necessary to first understand the ecological processes in them, especially those associated with sensitive indicators of environmental change. Phytoplankton biomass (chlorophyll) is a long-held indicator of nutrient pressures, while phytoplankton taxonomic composition has been proposed as an indicator of freshwater inflow extremes and nutrient pressures in estuaries (e.g., Paerl et al. 2010; Paerl et al. 2014; Lemley et al. 2016; Hemraj et al. 2017).
Baffin Bay is a low-inflow, lagoonal estuary that has been experiencing symptoms of eutrophication over the past three decades, namely high and increasing nutrient levels and recurring blooms of the “brown tide” alga, Aureoumbra lagunensis (Wetz et al. 2017; Cira and Wetz 2019). Blooms of this organism have been associated with ecosystem disruptive effects such as loss of seagrass due to shading, reductions in zooplankton biomass, and decreased diversity of benthic invertebrates (Buskey and Hyatt 1995; Onuf 1996; Ward et al. 2000). Efforts are now underway to seek solutions to the excessive nutrient loads that have affected Baffin Bay, namely by quantifying nutrient load sources and by establishing nutrient load reduction targets. In order to gauge the effectiveness of nutrient load reductions, it is important to understand and account for the role of natural variability in indicators such as phytoplankton biomass. Likewise, for these efforts to be effective from an ecosystem health standpoint, it is necessary for ecosystem/nutrient management efforts to account for conditions favoring blooms of harmful taxa including A. lagunensis as opposed to ecologically “healthy” taxa such as diatoms. In this study, we combined 3 years of monthly monitoring data with data from a multidecadal quarterly sampling program to quantify nutrient-phytoplankton dynamics in the bay, and to put those relationships in the context of large-scale climate variability.
Methods
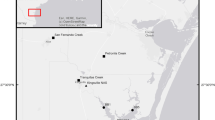
Baffin Bay, TX
Baffin Bay is a shallow (< 1–3 m average depth), microtidal (0.5 m tidal range) low-inflow estuary on the South Texas coast (Diener 1975). The Baffin Bay watershed is dominated by agriculture (41% of surface area as of 2010; NOAA 2019) and the climate is semi-arid (Breier et al. 2010). Due to low freshwater inflow and high rates of evaporation, mean residence time in Baffin Bay is estimated to be > 1 year on average and the system can be hypersaline at times (Breier et al. 2010). Vertical stratification in the system is rare, and mixing is primarily wind-driven (Orlando Jr. et al. 1993; Tunnell Jr 2002).
Analysis of Historic Data, 1993–2015
Broadscale patterns in salinity, total Kjeldahl nitrogen (TKN), and chlorophyll in Baffin Bay and adjacent Upper Laguna Madre were quantified using data from the Texas Commission on Environmental Quality’s (TCEQ) quarterly water quality monitoring program. Data were assessed for five sites from 1993 to 2015 (BB3, BB6, LM1, LM2, LM3; Fig. 1). Relationships with El Niño-Southern Oscillation (ENSO) conditions were evaluated by classifying the water quality data according to associated El Niño conditions (EN; ONI ≥ 0.5) or non-El Niño conditions (non-EN; ONI < 0.5), as determined using the Ocean Niño Index (ONI, a rolling 3-month mean sea surface temperature anomaly in the east-central tropical Pacific, NOAA Climate Prediction Center). Descriptive statistics (mean ± standard deviation) were used to assess spatial trends and to compare data between EN and non-EN conditions. TCEQ data that were reported as less than the reporting limit were considered to be one half of the reporting limit for analysis. This occurred in <1% of TKN samples and ~20% of chlorophyll samples.
Monthly Field Sampling Program, 2013–2016
Five sites in Baffin Bay were visited monthly for a 3-year period, from May 2013 to April 2016 (BB1–BB5; Fig. 1). At each site, vertical profiles of temperature and salinity were taken with a calibrated YSI ProPlus sonde. Salinity stratification was calculated by subtracting surface salinity from near bottom salinity. Surface water was collected in acid-washed 1 L (for biogeochemical analyses) or 500 mL (for plankton analyses) amber polycarbonate bottles and stored in the dark (the 1-L bottles were also stored on ice) for transport to the laboratory for analysis. Near bottom samples were not collected for phytoplankton or nutrients because of the shallow water column and pervasive well-mixed conditions.
Upon return to the laboratory, samples from the 1 L bottles were analyzed for inorganic nutrients (silicate, ammonium [NH4+], nitrate + nitrite [N+N], orthophosphate), total dissolved nitrogen (TDN), and dissolved organic carbon (DOC). Bottles were gently inverted and ~50-mL subsamples were filtered through combusted 25 mm GF/F filters. The filtrate was stored frozen (−20 °C) until analysis. For inorganic nutrients, subsamples were thawed to room temperature and analyzed on a Seal QuAAtro autoanalyzer following methods described in Wetz et al. (2017). Additional subsamples were thawed to room temperature and analyzed for DOC and TDN on a Shimadzu TOC-Vs analyzer with nitrogen module (see methods in Wetz et al. 2017). Dissolved inorganic nitrogen (DIN) was calculated as the sum of NH4+ and N+N. Dissolved organic nitrogen (DON) was calculated by subtracting DIN from TDN.
A. lagunensis and picocyanobacteria were enumerated with flow cytometry. To prepare samples for enumeration, 500-mL bottles were gently inverted and 4-mL subsamples were preserved with 1% glutaraldehyde and stored frozen (−80 °C) until analysis with flow cytometry. Samples were thawed under subdued lighting, then filtered through 20-μm mesh. Subsamples used to enumerate A. lagunensis were incubated under subdued lighting with a primary antibody specific to A. lagunensis (custom polyclonal antibody from ThermoFisher), followed by a fluorescently labeled secondary antibody (fluorescein isothiocyanate, FITC). Subsamples used to enumerate picocyanobacteria were not fluorescently labeled. Subsamples for A. lagunensis and picocyanobacterial abundance were analyzed on an Accuri C6 flow cytometer equipped with an optical filter for detection of FITC (A. lagunensis), chlorophyll a,b (picocyanobacteria), and side scatter. Accuracy of the cytometer was monitored daily prior to use, and the detection limit for A. lagunensis was calculated following Koch et al. (2014) and Cira and Wetz (2019).
Other phytoplankton groups and ciliates were enumerated with light microscopy. Upon return to the laboratory, 500-mL bottles were gently inverted and 60-mL subsamples were preserved with 3% acid Lugol’s solution and stored at room temperature in the dark until analysis. To prepare samples for enumeration, bottles were gently inverted and 5-mL subsamples were poured into Utermöhl chambers and allowed to settle overnight. The subsample volume and settling time were adjusted as needed (i.e., during bloom events), allowing > 1 h settling time per mL settled. Phytoplankton and ciliate taxa were enumerated and measured at × 100–× 600 on an Olympus IX71 inverted microscope. Biovolume was estimated using geometric shapes for each enumerated taxa (Hillebrand et al. 1999; Sun and Liu 2003). Due to inherent difficulty in differentiating some taxa with light microscopy and acid Lugol’s preserved cells, taxa were grouped as dinoflagellates, diatoms, cryptophytes, chlorophytes, unidentified flagellates, picocyanobacteria, A. lagunensis, and the ciliate Mesodinium sp. (a mixotrophic ciliate) for analysis. Diatoms were further categorized as planktonic (e.g., Skeletonema, Chaetoceros, Pseudo-nitzschia) or benthic forms (e.g., naviculoids, Cylindrotheca) following categories described in Shaffer and Sullivan (1988) and MacIntyre et al. (2011).
Spatiotemporal differences in water quality were determined using principal components analysis (PCA) on loge-transformed data. Spatiotemporal differences in planktonic community composition were characterized using non-metric multi-dimensional scaling (nMDS) on loge(x+1) biovolume data. PCA and nMDS analyses were conducted using PRIMER 7 software (Clarke et al. 2014).
Data from the Texas Water Development Board was used to estimate the residence time (monthly freshwater input based on rainfall data/bay volume) of Baffin Bay during 2013–2016. To do this, residence time was first estimated as the proportion of monthly modeled freshwater inflow to bay volume using historic data (1977–2010). Then, the relationship between residence time and monthly rainfall during that timeframe was estimated. Finally, this relationship was applied to rainfall data from 2013–2016 to estimate residence times during that period (more information provided in the Electronic Supplementary Material; ESM 1, ESM 2).
Results
Spatial-Temporal Variability in Water Quality Conditions Related to ENSO from Long-Term Time Series, 1993–2015
Across all sites, salinity was lower during EN conditions than non-EN conditions (Fig. 2a). During EN conditions, salinities were highest at the mouth of Baffin Bay (LM1, 39.2 ± 7.6) and lower in both Baffin Bay (BB3, 31.7 ± 12.4; BB6, 33.7 ± 10.5) and Upper Laguna Madre (LM2, 33.1 ± 7.9; LM3, 31.0 ± 6.0; Fig. 2a). During non-EN conditions, salinities were high and varied little from upper Baffin Bay to the mouth (BB3, 39.0 ± 14.4; LM1, 39.9 ± 8.6). Slightly lower salinities were observed in Upper Laguna Madre (LM3, 35.6 ± 6.6; Fig. 2a).
TKN concentrations ranged from a low of 66.7 ± 29.3 μM at LM3 to a high of 124.4 ± 45.4 μM at BB3. Concentrations were higher and more variable in Baffin Bay than Upper Laguna Madre (Fig. 2b). At BB3, BB6, and LM1, TKN concentrations were higher by an average of 17.6 μM during EN conditions than non-EN conditions (Fig. 2b). In contrast, TKN concentrations were lower by an average of 5.7 μM during EN conditions compared to non-EN conditions at LM2 and LM3 (Fig. 2b).
Chlorophyll concentrations ranged from 8.4 ± 9.1 μg L−1 at LM3 to a high of 26.1 ± 27.5 μg L−1 at BB3 (Fig. 2c), though maximum concentrations reached 139.0 μg L−1 at BB3 during non-EN conditions. Chlorophyll concentrations were higher within and at the mouth of Baffin Bay (BB3, BB6, and LM1) than in Upper Laguna Madre (LM2 and LM3) under both EN and non-EN conditions. This difference was more apparent under EN conditions when concentrations in Baffin Bay (BB3, 25.2 ± 33.4 μg L−1; BB6, 22.4 ± 20.2 μg L−1; LM1, 22.5 ± 22.1 μg L−1) were > twofold higher than in Upper Laguna Madre (LM2, 6.6 ± 7.6 μg L−1; LM3, 6.6 ± 5.2 μg L−1; Fig. 2c). Within and at the mouth of Baffin Bay, chlorophyll concentrations were similar between EN and non-EN conditions (average concentrations varied by < 2.0 μg L−1, or < 10%; Fig. 2c). In Upper Laguna Madre, chlorophyll concentrations were higher in non-EN conditions by 2.3 μg L−1 at LM3, and by 6.5 μg L−1 at LM2 (which reflect ~30% and ~100% increase compared with EN conditions, respectively; Fig. 2c).
Monthly Environmental Conditions from 2013 to 2016
The first 2 years of sampling were characterized by drought (May 2013–March 2015), with non-EN conditions in place for most of this time (May 2013–November 2014). The last year of sampling occurred during EN conditions and was characterized by high rainfall beginning in March 2015. Non-EN conditions corresponded with higher salinity and DOC concentrations at all sites in the monthly field-sampling program (BB1–BB5; Figs. 3 and 4). Sites BB1 and BB2, at the heads of Cayo del Grullo and Laguna Salada, generally had higher DON and orthophosphate concentrations, and lower DIN to dissolved inorganic phosphorus (DIP) ratios than sites BB3–BB5 (Fig. 4).
From April to June 2015, salinity stratification was observed (ESM 3a) but was generally low on other dates. Temperatures peaked in summer months and were lowest in winter months (ESM 3b). The lowest average winter temperatures progressively increased each year (2013/2014, 9.9 ± 0.4 °C; 2014/2015, 14.0 ± 0.5 °C; 2015/2016, 17.7 ± 0.9 °C; ESM 3b). Average NH4+ and N+N concentrations were almost twice as high from April 2015 onward under low salinity conditions (NH4+, 5.6 ± 6.9 μM; N+N, 2.9 ± 7.2 μM) compared to prior high salinity conditions (NH4+, 3.0 ± 3.6 μM; N+N, 1.5 ± 4.4 μM; ESM 3c,d). Likewise, orthophosphate concentrations were higher under low salinity conditions (low salinity, 1.2 ± 2.4 μM; high salinity, 0.7 ± 1.5 μM) and had distinctive peaks during the spring of 2015 (ESM 3e). Silicate concentrations were lower under low salinity conditions (54.5 ± 48.1 μM) compared to high salinity conditions (78.3 ± 16.3 μM; ESM 3f), corresponding with increased biovolume of diatoms during low salinities (see the “Phytoplankton Dynamics from 2013 to 2016” section). Lowest DON concentrations occurred during the spring of 2015, reaching a minimum average of 41 μM in May 2015 (ESM 3g). DON was consistently higher than concurrent DIN concentrations throughout the sampling period (ESM 3c,d,g). Overall, there was no clear temporal trend in DON concentrations (ESM 3g). Variability in ciliate biovolume was not aligned with seasonal cycles or interannual shifts in salinity conditions (ESM 3h). Ciliate biovolume was typically higher in 2015 than the other years (ESM 3h).
From May 2013 to February 2015, residence time was estimated to be > 1 year for all but three months. Residence time was estimated to be < 1 year from March 2015 through June 2015, reaching < 1 month in May 2015. After June 2015, residence time increased again and was estimated to be > 1 year for all but one month from July 2015 to April 2016 (ESM 2).
Phytoplankton Dynamics from 2013 to 2016
Chlorophyll concentrations were lower on average under low (April 2015–April 2016) compared with high (May 2013–March 2015) salinity conditions (low salinity, 16.2 ± 14.3 μg L−1; high salinity, 25.7 ± 11.7 μg L−1; ESM 4). During high salinity conditions, chlorophyll concentrations peaked in summer months, but the seasonal pattern was less pronounced after the onset of low salinity conditions in 2015 (ESM 4). The seasonal trends in chlorophyll, as well as the overall decrease in chlorophyll associated with the shift from high to low salinity conditions, were reflected by trends in phytoplankton biovolume (Fig. 5).
Biovolume of phytoplankton groups at five monitoring stations in Baffin Bay, (a) BB1, (b) BB2, (c) BB3, (d) BB4, and (e) BB5, from May 2013 to April 2016. When A. lagunensis was BDL, biovolume was calculated using 40,000 cells mL−1, 0.5 of the detection limit for the fluorometric enumeration method. Salinity for each site is included as a dashed gray line
nMDS analysis showed that the phytoplankton community was different in EN and non-EN periods (Fig. 6). During high salinity conditions from May 2013 to March 2015, the phytoplankton community was dominated by A. lagunensis, which accounted for an average of 87% of community biovolume during this period (Fig. 5). The second and third most prevalent groups were dinoflagellates and diatoms, only accounting for an average of 9% and 3% of community biovolume, respectively (Fig. 5). A. lagunensis biovolume peaked in summer months (Fig. 5), as did the proportion of A. lagunensis in relation to other groups (Fig. 5). In contrast, dinoflagellate biovolume peaked in winter months, and diatom biovolume peaked in spring months (Fig. 5).
After the onset of low salinity conditions in spring 2015, phytoplankton biovolume decreased by over 40% on average and community composition changed (Fig. 5). A. lagunensis biovolume decreased sharply across all sites and remained low at most sites in the bay (Fig. 5). The exception was BB2, where A. lagunensis biovolume rebounded by summer 2015, despite the low (< 30) salinity measured during this time (Fig. 5b). Average dinoflagellate biovolume also decreased from the high to low salinity period (Fig. 5). Biovolume of diatoms was higher in low salinity conditions, accounting for an average of 29% of community biovolume (Fig. 5). Most diatom biovolume in the high salinity period consisted of benthic taxa (89%), while during the low salinity period planktonic taxa dominated (64%; ESM 5). Benthic taxa were primarily pennate forms such as naviculoids and Cylindrotheca spp. Planktonic diatoms were dominated by Rhizosolenia spp., though Thalassionema spp. and Thalassiosira spp. were also commonly observed. Mesodinium sp. was also more common during the low salinity period, when it was present in 80% of samples (compared with 15% during the high salinity period; Fig. 5). Highest Mesodinium sp. biovolume tended to occur in winter (Fig. 5). For example, in the winter of 2015/2016, its presence was widespread throughout the bay and it was the primary component of phytoplankton biovolume at all sites except BB2 (Fig. 5).
Other phytoplankton groups, including cryptophytes, chlorophytes, picocyanobacteria, and unidentified flagellates, were a minor component of phytoplankton community biovolume. Among these groups, a clear temporal trend was only identified for picocyanobacteria, the biovolume of which mirrored that of A. lagunensis and peaked in summer months (ESM 6). Picocyanobacteria had higher biovolume during the high salinity conditions compared to the low salinity conditions, but consistently accounted for < 1% of phytoplankton biovolume overall (Fig. 5).
Discussion
In this study, we examined nutrient-phytoplankton dynamics in Baffin Bay-Upper Laguna Madre, TX. Baffin Bay-Upper Laguna Madre experiences a range of salinities from brackish to hypersaline, as captured in this study. This is common in semiarid systems where extended drought can be punctuated by episodic high rainfall (e.g., Norwine 1978; Barroso et al. 2016). By augmenting an intensive 3-year monthly monitoring program in Baffin Bay with analysis of a decades-long dataset of lower frequency sampling, we were able to examine nutrient-phytoplankton dynamics across a range of salinity conditions and relate those dynamics to large-scale climate patterns. Results from this study increase our understanding of phytoplankton dynamics in low-inflow estuaries and show that while nutrient concentrations and phytoplankton composition behaved similar to river influenced estuaries in response to rainfall/inflow variability, the phytoplankton biomass response was more complex and requires consideration of multiple environmental drivers. We elaborate below on the spatial and temporal distribution of nutrients and phytoplankton in the system, as well as environmental and ecological factors that contributed to these similarities and differences with river-influenced estuaries.
Texas estuaries have notable interannual variability in freshwater inflow, due in large part to the strong influence of El Niño-Southern Oscillation (ENSO) on regional precipitation patterns (Tolan 2007). In the long-term dataset, salinities were found to be lower during El Niño (EN) compared to non-EN conditions due to higher rainfall during the former (see also Tolan 2007). In the 3-year monitoring dataset, there was a lag from when ENSO conditions shifted in fall 2014 to when salinity changes were observed in Baffin Bay in spring 2015, similar to the ~ 5-month lag described for the Texas coast by Tolan (2007). Salinity variability was more pronounced in Baffin Bay than in neighboring Upper Laguna Madre, which would be expected given that Upper Laguna Madre is closer to the Gulf of Mexico and would experience water exchange that modulates its salinities (Smith 1975, 1988).
The effects of ENSO extended beyond the physical conditions in Baffin Bay. During EN conditions, TKN was higher in Baffin Bay and at the mouth of Baffin Bay compared with non-EN conditions. Similarly, in the monthly sampling program, total dissolved nitrogen (TDN) increased by 11 μM from January 2015 (just prior to EN-related salinity decrease) to April 2016 (during EN). The net increase in nitrogen (as TKN or TDN) during EN conditions in both the long-term and 3-year dataset, as well the observed increase in NH4+ and N+N concentrations concurrent with high rainfall and a decrease in salinity in the 3-year dataset, indicates a watershed source. These findings are consistent with observations of increased nitrogen loadings during high rainfall periods from other estuaries with agriculturally dominated watersheds (Caffrey et al. 2007; Peierls et al. 2012). Based on spatial patterns in the historical data, it appears that watershed-derived TKN is diluted by tidal exchange and mixing as water masses move out of Baffin Bay into Upper Laguna Madre. Further work is needed to understand exchange of nutrients between Baffin Bay and Upper Laguna Madre, as the Upper Laguna Madre contains important seagrass beds that are sensitive to nutrient enrichment (Street et al. 1997; TPWD 1999).
For phytoplankton, there is typically a trade-off between the stimulatory effects of nutrient pulses and the increased flushing associated with inflow events (Peierls et al. 2012; Azevedo et al. 2014). Increased nutrient availability under high inflow conditions may stimulate primary production and has been shown to lead to blooms of fast-growing phytoplankton taxa (e.g., chlorophytes, diatoms; Cloern and Dufford 2005; Paerl et al. 2014; Carstensen et al. 2015). However, extremely high inflows, such as due to hurricane passage, can lead to reductions in phytoplankton growth and biomass due to light limitation and/or washout effects (Mitrovic et al. 2011; Paerl et al. 2014). Under low-inflow conditions, nutrient limitation is possible (e.g., Wetz et al. 2011; Phlips et al. 2020). However, in shallow lagoonal systems, internal nutrient recycling and sources can often sustain phytoplankton growth for some time and allow for biomass accumulation in the absence of significant external inputs (Pinckney et al. 2001; Glibert et al. 2010; Geyer et al. 2018). This appears to be the case in Baffin Bay-Upper Laguna Madre, where chlorophyll/phytoplankton biovolume was generally higher during non-EN conditions despite lower apparent nutrient concentrations. A recent study in Baffin Bay found very high NH4+ concentrations in sediment porewater and argued that fluxes of sediment-derived nutrients can be significant (Lopez et al. 2018). Another recent study found that DON was susceptible to high rates of photoammonification in Baffin Bay (Felix and Campbell 2019), which would be a ready source of nitrogen for phytoplankton during low-inflow conditions given the persistently high ambient DON concentrations (Wetz et al. 2017). In addition, studies have concluded that the dominant phytoplankton taxa during drought/non-EN conditions (A. lagunensis) has mixotrophic capabilities, implying that it is able to grow on some fraction of the DON in this system (Buskey et al. 1998; Agostoni and Erdner 2011). Thus, while external nutrient loadings affect nutrient stocks in this system, on timescales relevant to phytoplankton growth, external loadings may be less important for phytoplankton population dynamics than other factors.
In this study, A. lagunensis accounted for ~90% of phytoplankton community biovolume during non-EN, high salinity conditions (May 2013–March 2015). This is consistent with previous work showing the prevalence of A. lagunensis blooms in the system during low rainfall, low inorganic nutrient conditions (e.g., Buskey et al. 2001; Cira and Wetz 2019). A strong temporal coherence between picocyanobacteria and A. lagunensis was observed, consistent with prior studies (Kang et al. 2015; Hall et al. 2018). However, picocyanobacteria biovolume was relatively low and inconsequential, which was somewhat surprising given that conditions were conducive to picocyanobacteria (i.e., generally warm waters, low freshwater inflows, low inorganic nutrient concentrations; Murrell and Lores 2004). The minor relative importance of picocyanobacteria in Baffin Bay may partially be explained by an allelopathic effect that A. lagunensis has been shown to have on competitors, including picocyanobacteria (Kang and Gobler 2018). Additionally, picocyanobacteria have been shown to be more affected by “top-down” controls than A. lagunensis (Buskey et al. 2003). The hypersaline conditions often found in Baffin Bay may intensify preferential grazing on picocyanobacteria because high salinities are associated with increased secretion of a grazer-deterring mucus by A. lagunensis (Liu and Buskey 2000), shifting grazing pressure to the picocyanobacteria. Dinoflagellates were also not a major component of the phytoplankton community overall, but did occasionally have relatively high biovolume in the winter months during non-EN conditions at select sites. Seasonal peaks of dinoflagellate biovolume in winter months are a common feature in other coastal systems (Rothenberger et al. 2009; Lee 2017) and have been explained in part by mixotrophy, which allows dinoflagellates to thrive under low light conditions (Millette et al. 2017). Unfortunately, we were unable to resolve mixotrophic from non-mixotrophic taxa because of difficulty associated with enumerating Lugol’s-preserved samples (e.g., Rothenberger et al. 2009), so the ecological role and drivers of the winter dinoflagellate peaks remain enigmatic at this point.
As rainfall increased and salinity decreased in spring 2015 after the onset of EN conditions, A. lagunensis biovolume in Baffin Bay dropped sharply while diatoms and other taxa (e.g., the mixotrophic ciliate Mesodinium sp.) became more prevalent. This reflects a shift in phytoplankton community composition and an increase in diversity that could be due to several factors, including (1) release of competition for nutrients because of the increased nutrient loads and concentrations associated with inflow (e.g., Dorado et al. 2015), (2) increased flushing that favored fast growing taxa, and/or (3) release from the stress of harsh salinities (Cornelius 1984; Schapira et al. 2010). The decline in dominance of A. lagunensis during rain events has previously been attributed to less optimal salinities (Buskey et al. 1998). In laboratory studies, A. lagunensis growth rates peak at ~ 0.6 day−1 in salinities of 30–50 but decrease at salinities < 30 (Buskey et al. 1998). Although salinity plays an important role in A. lagunensis blooms, Hall et al. (2018) pointed to a significant role for residence time in the population dynamics of an A. lagunensis bloom in Guantánamo Bay. This is an important distinction because residence time changes concurrently with salinity changes and thus the effects of one can be attributed to changes in the other. During this 3-year monitoring program, estimates of residence time were > 1 year for most of the study but decreased to < 30 days in May 2015. This coincided with a bay-wide decrease in A. lagunensis biovolume. A residence time of < 30 days would account for a dilution loss of > 3% (day−1) within Baffin Bay, which could be significant for A. lagunensis. However, as seen at BB2 in summer 2015, A. lagunensis biovolume rebounded, even with salinities < 30. Interestingly, this occurred during a period of increasing residence times. Cira and Wetz (2019) found that A. lagunensis abundances were persistently high at BB2 compared with the rest of Baffin Bay, and proposed BB2 is an “incubator” for A. lagunensis within the bay. BB2 has many conditions favorable to A. lagunensis including high DON, shallowness that would facilitate benthic-pelagic coupling of nutrients, and it is thought to be less well flushed than other parts of the bay. Other studies have also shown that similar small, poorly flushed tributaries can serve as incubators for slow growing harmful phytoplankton taxa (e.g., Phlips et al. 1999; Qin and Shen 2019). In this case, the unique nature of BB2 from a nutrient standpoint and as a potential incubator for A. lagunensis seed stock offers an opportunity to focus watershed restoration efforts to maximize reduction in the future frequency of blooms.
The decrease in A. lagunensis biovolume during the high rainfall, low salinity period of spring 2015 corresponded with an increase in biovolume of diatoms and Mesodinum sp. Prior to 2015, benthic forms of diatoms (primarily naviculoids) were most prevalent, whereas in 2015, planktonic forms (primarily Rhizosolenia spp., Thalassionema spp.) became more prevalent. Vertical stratification is rare in Baffin Bay; thus, benthic diatom prevalence during the high salinity period likely reflects resuspension due to wind-induced water column mixing (Shaffer and Sullivan 1988; Orlando Jr. et al. 1993). The dominance of both planktonic diatoms and Mesodinium sp. during the low salinity period can be attributed to higher freshwater inflow and accompanying nutrient pulses. Many planktonic diatom taxa are high-nutrient adapted and have high growth rates (~1–2 d−1; e.g., Shikata et al. 2008; Dorado et al. 2015; Barroso et al. 2016). For example, blooms of Rhizosolenia often occur after freshets, suggesting an important role for riverine-derived nutrients and/or lower salinity conditions (e.g., Malej et al. 1995; Buskey et al. 2001; Rothenberger et al. 2009). The increase in biovolume of Mesodinium sp. is consistent with studies on its relationship with freshwater inflow and salinity (e.g., Johnson et al. 2013). Even though Mesodinium sp. is a mixotroph, nitrate can be its primary nitrogen source and the organism may require relatively high nutrient concentrations (Smith Jr. and Barber 1979; Lindholm 1985). Highest Mesodinium sp. biovolume was observed in winter, consistent with findings from other systems showing seasonal peaks in cooler months (Sanders 1995; Johnson et al. 2013). This has been proposed to be related to a combination of factors such as high nutrient concentrations, low salinities, and low light levels, as well as decreased predation, which create seasonal windows of opportunity for Mesodinium sp. (Sanders 1995; Johnson et al. 2013). Taylor et al. (1971) reported that maximum Mesodinium sp. abundances occurred at temperatures just above 15 °C, which would limit Mesodinium sp. to the cooler months in Baffin Bay. The combination of lower salinities, greater nutrient availability, and optimal temperatures may explain why highest Mesodinium sp. biovolume was found in the winter of 2015/2016.
Conclusions
This study highlights the substantial interannual variability in nutrients and phytoplankton biomass/composition that occurs in Baffin Bay-Upper Laguna Madre, a low-inflow South Texas estuary. Results show similarities to river-dominated estuaries in terms of inorganic nutrient concentrations, which tended to be higher under higher inflows, and in terms of phytoplankton composition, which favored slow-growing taxa during lower inflows and faster growing taxa during higher inflows (Olli et al. 2019). Interestingly during low inflow, long residence time conditions, phytoplankton biomass was high despite very low inorganic nutrient concentrations, pointing to the importance of regenerated nutrients or DON uptake. The high phytoplankton biomass was composed of a near monoalgal bloom of the harmful “brown tide” organism A. lagunensis. These findings are consistent with emerging evidence showing that in some estuaries, decreases in freshwater inflows and associated increases in residence times and salinities can still facilitate high phytoplankton biomass and decreased phytoplankton diversity (Schapira et al. 2010; Barroso et al. 2016; Hemraj et al. 2017). If broadly applicable, the implication of this is that with future expansion of arid/semiarid regions and/or increasing human freshwater demands, susceptible estuaries will experience lower inflows, more pronounced hypersalinity and presumably less diverse phytoplankton communities. The question is will these communities be dominated by harmful taxa such as seen here, or non-harmful taxa? It is possible that Baffin Bay represents an extreme case, where a combination of nutrient pollution, high organic nutrient levels, and low inflows has pushed the system to dominance by harmful taxa (Wetz et al. 2017). Additional data is needed from other similar systems to determine if findings presented here are broadly applicable to low-inflow estuaries.
References
Agostoni, M., and D.L. Erdner. 2011. Analysis of ammonium transporter and urease gene expression in Aureoumbra lagunensis. Harmful Algae 10 (5): 549–556.
Azevedo, I.C., A.A. Bordalo, and P. Duarte. 2014. Influence of freshwater inflow variability on the Douro estuary primary productivity: a modelling study. Ecological Modelling 272: 1–15.
Barroso, H.S., H. Becker, and V.M.M. Melo. 2016. Influence of river discharge on phytoplankton structure and nutrient concentrations in four tropical semiarid estuaries. Brazilian Journal of Oceanography 64 (1): 37–48.
Barroso, H.S., T. Tavares, M. Soares, T. Garcia, B. Rozendo, A. Vieira, P. Viana, T. Pontes, T. Ferreira, J.P. Filho, C. Schettini, and S. Santaella. 2018. Intra-annual variability of phytoplankton biomass and nutrients in a tropical estuary during severe drought. Estuarine, Coastal and Shelf Science 213: 283–293.
Breier, J.A., C.F. Breier, and H.N. Edmonds. 2010. Seasonal dynamics of dissolved Ra isotopes in the semi-arid bays of south Texas. Marine Chemistry 122 (1-4): 39–50.
Bricker, S.B., B. Longstaff, W. Dennison, A. Jones, K. Boicourt, C. Wicks, and J. Woerner. 2008. Effects of nutrient enrichment in the nation’s estuaries: a decade of change. Harmful Algae 8 (1): 21–32.
Buskey, E.J., and C.J. Hyatt. 1995. Effects of the Texas (USA) ‘brown tide’ alga on planktonic grazers. Marine Ecology Progress Series 126: 285–292.
Buskey, E.J., B. Wysor, and C. Hyatt. 1998. The role of hypersalinity in the persistence of the Texas “brown tide” in the Laguna Madre. Journal of Plankton Research 20 (8): 1553–1565.
Buskey, E.J., H. Liu, C. Collumb, and J.G.F. Bersano. 2001. The decline and recovery of a persistent Texas brown tide algal bloom in the Laguna Madre (Texas, USA). Estuaries 24 (3): 337–346.
Buskey, E.J., H. Deyoe, F.J. Jochem, and T.A. Villareal. 2003. Effects of mesozooplankton removal and ammonium addition on planktonic trophic structure during a bloom of the Texas ‘brown tide’: a mesocosm study. Journal of Plankton Research 25 (2): 215–228.
Caffrey, J.M., T.P. Chapin, H.W. Jannasch, and J.C. Haskins. 2007. High nutrient pulses, tidal mixing and biological response in a small California estuary: variability in nutrient concentrations from decadal to hourly time scales. Estuarine, Coastal and Shelf Science 71 (3-4): 368–380.
Carstensen, J., R. Klais, and J.E. Cloern. 2015. Phytoplankton blooms in estuarine and coastal waters: seasonal patterns and key species. Estuarine, Coastal and Shelf Science 162: 98–109.
Chan, D., and Q. Wu. 2015. Significant anthropogenic-induced changes of climate classes since 1950. Scientific Reports 5 (1): 13487.
Cira, E.K., and M.S. Wetz. 2019. Spatial-temporal distribution of Aureoumbra lagunensis (“brown tide”) in Baffin Bay, Texas. Harmful Algae 89: 101669.
Clarke, K.R., R.N. Gorley, P.J. Somerfield, and R.M. Warwick. 2014. Primer v7: User Manual/ Tutorial. Plymouth, UK: Primer-E.
Cloern, J.E. 1996. Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics 34 (2): 127–168.
Cloern, J.E. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Cloern, J.E., and R. Dufford. 2005. Phytoplankton community ecology: principles applied in San Francisco Bay. Marine Ecology Progress Series 285: 11–28.
Cloern, J.E., S.Q. Foster, and A.E. Kleckner. 2014. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11 (9): 2477–2501.
Cornelius, S.E. 1984. An ecological survey of Alazan Bay, Texas. Kingsville: Caesar Kleburg Wildlife Research Institute.
Diener, R.A. 1975. Cooperative Gulf of Mexico estuarine inventory and study - Texas: area description. Seattle: National Oceanic and Atmospheric Administration.
Dijkstra, Y.M., R.J. Chant, and J.R. Reinfelder. 2019. Factors controlling seasonal phytoplankton dynamics in the Delaware River Estuary: an idealized model study. Estuaries and Coasts 42 (7): 1839–1857.
Dorado, S., T. Booe, J. Steichen, A.S. McInnes, R. Windham, A. Shepard, A.E.B. Lucchese, H. Preischel, J.L. Pinckney, S.E. Davis, D.L. Roelke, and A. Quigg. 2015. Towards an understanding of the interactions between freshwater inflows and phytoplankton communities in a subtropical estuary in the Gulf of Mexico. PLos ONE 10 (7): e0130931.
Felix, J.D., and J. Campbell. 2019. Investigating reactive nitrogen sources that stimulate algal blooms in Baffin Bay. Corpus Christi: Coastal Bend Bays and Estuaries Program.
Flemer, D.A., and M.A. Champ. 2006. What is the future fate of estuaries given nutrient over-enrichment, freshwater diversion and low flows? Marine Pollution Bulletin 52 (3): 247–258.
Geyer, N.L., M. Huettel, and M.S. Wetz. 2018. Phytoplankton spatial variability in the river-dominated estuary, Apalachicola Bay, Florida. Estuaries and Coasts 41 (7): 2024–2038.
Glibert, P.M., J.N. Boyer, C.A. Heil, C.J. Madden, B. Sturgis, and C.S. Wazniak. 2010. Blooms in lagoons: different from those of river-dominated estuaries. In Coastal Lagoons: Critical Habitats of Environmental Change, ed. M.J. Kennish and H.W. Paerl, 91–113. Boca Raton: CRC Press.
Hall, N.S., R.W. Litaker, W.J. Kenworthy, M.W. Vandersea, W.G. Sunda, J.P. Reid, D.H. Slone, and S. Butler. 2018. Consortial brown tide − picocyanobacteria blooms in Guantánamo Bay, Cuba. Harmful Algae 73: 30–43.
Hallett, C.S., A.J. Hobday, J.R. Tweedley, P.A. Thompson, K. McMahon, and F.J. Valesini. 2018. Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Regional Environmental Change 18 (5): 1357–1373.
Hemraj, D.A., M.A. Hossain, Q. Ye, J.G. Qin, and S.C. Leterme. 2017. Plankton bioindicators of environmental conditions in coastal lagoons. Estuarine, Coastal and Shelf Science 184: 102–114.
Hillebrand, H., C. Dürselen, D. Kirschtel, U. Pollingher, and T. Zohary. 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35 (2): 403–424.
Johnson, M.D., D.K. Stoecker, and H.G. Marshall. 2013. Seasonal dynamics of Mesodinium rubrum in Chesapeake Bay. Journal of Plankton Research 35 (4): 877–893.
Kang, Y., and C.J. Gobler. 2018. The brown tide algae, Aureococcus anophagefferens and Aureoumbra lagunensis (Pelagophyceae), allelopathically inhibit the growth of competing microalgae during harmful algal blooms. Limnology and Oceanography 63 (2): 985–1003.
Kang, Y., F. Koch, and C.J. Gobler. 2015. The interactive roles of nutrient loading and zooplankton grazing in facilitating the expansion of harmful algal blooms caused by the pelagophyte, Aureoumbra lagunensis, to the Indian River Lagoon, FL, USA. Harmful Algae 49: 162–173.
Knoppers, B., B. Kjerfve, and J. Carmouze. 1991. Trophic state and water turn-over time in six choked coastal lagoons in Brazil. Biogeochemistry 14: 149–166.
Koch, F., Y. Kang, T.A. Villareal, D.M. Anderson, and C.J. Gobler. 2014. A novel immunofluorescence flow cytometry technique detects the expansion of brown tides caused by Aureoumbra lagunensis to the Caribbean Sea. Applied and Environmental Microbiology 80 (16): 4947–4957.
Largier, J. 2010. Low-inflow estuaries: hypersaline, inverse, and thermal scenarios. In Contemporary Issues in Estuarine Physics, ed. A. Valle-Levinson, 247–272. Cambridge: Cambridge University Press.
Lee, H. A. 2017. Effects of physical disturbance on phytoplankton diversity and community composition in Galveston Bay, TX, during an extreme flooding event. M.S. Thesis, Texas A&M University-College Station.
Lemley, D.A., J.B. Adams, and G.C. Bate. 2016. A review of microalgae as indicators in South African estuaries. South African Journal of Botany 107: 12–20.
Lindholm, T. 1985. Mesodinium rubrum – a unique photosynthetic ciliate. Advances in Aquatic Microbiology 3: 1–48.
Liu, H., and E.J. Buskey. 2000. The exopolymer secretions (EPS) layer surrounding Aureoumbra lagunensis cells affects growth, grazing, and behavior of protozoa. Limnology and Oceanography 45 (5): 1187–1191.
Lopez, C.V., D. Murgulet, A. Douglas, and V. Murgulet. 2018. Impacts of temporal and spatial variation of submarine groundwater discharge on nutrient fluxes to Texas coastal embayments, Phase III (Baffin Bay). Corpus Christi: Texas General Land Office.
MacIntyre, H.L., A.L. Stutes, W.L. Smith, C.P. Dorsey, A. Abraham, and R.W. Dickey. 2011. Environmental correlates of community composition and toxicity during a bloom of Pseudo-nitzschia spp. in the northern Gulf of Mexico. Journal of Plankton Research 33 (2): 273–295.
Malej, A., P. Mozetic, V. Malacic, S. Terzic, and M. Ahel. 1995. Phytoplankton responses to freshwater inputs in a small semi-enclosed gulf (Gulf of Trieste, Adriatic Sea). Marine Ecology Progress Series 120: 111–122.
Millette, N.C., J.J. Pierson, A. Aceves, and D.K. Stoecker. 2017. Mixotrophy in Heterocapsa rotundata: a mechanism for dominating the winter phytoplankton. Limnology and Oceanography 62 (2): 836–845.
Mitrovic, S.M., L. Hardwick, and F. Dorani. 2011. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. Journal of Plankton Research 33 (2): 229–241.
Murrell, M.C., and E.M. Lores. 2004. Phytoplankton and zooplankton seasonal dynamics in a subtropical estuary: importance of cyanobacteria. Journal of Plankton Research 26 (3): 371–382.
NOAA, 2019. Land Cover: 2010. Coastal Change Analysis Program (C-CAP) Regional Land Cover. NOAA Office for Coastal Management. www.coast.noaa.gov. Accessed 2 November 2019.
Norwine, J. 1978. Twentieth-century semi-arid climates and climatic fluctuations in Texas and northeastern Mexico. Journal of Arid Environments 1 (4): 313–325.
Olli, K., R. Ptacnik, R. Klais, and T. Tamminen. 2019. Phytoplankton species richness along coastal and estuarine salinity continua. The American Naturalist 194 (2): E41–E51.
Onuf, C.P. 1996. Seagrass responses to long-term light reduction by brown tide in upper Laguna Madre, Texas: distribution and biomass patterns. Marine Ecology Progress Series 138: 219–231.
Orlando, S.P., Jr., L.P. Rozas, G.H. Ward, and C.J. Klein. 1993. Salinity characteristics of Gulf of Mexico Estuaries. Silver Spring: National Oceanic and Atmospheric Administration, Office of Ocean Resources Conservation and Assessment.
Paerl, H.W., L.M. Valdes, B.L. Peierls, J.E. Adolf, and L.W. Harding Jr. 2006. Anthropogenic and climatic influences on the eutrophication of large estuarine ecosystems. Limnology and Oceanography 51 (1part2): 448–462.
Paerl, H.W., K.L. Rossignol, S.N. Hall, B.J. Peierls, and M.S. Wetz. 2010. Phytoplankton community indicators of short- and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuaries and Coasts 33 (2): 485–497.
Paerl, H.W., N.S. Hall, B.L. Peierls, K.L. Rossignol, and A.R. Joyner. 2014. Hydrologic variability and its control of phytoplankton community structure and function in two shallow, coastal, lagoonal ecosystems: The Neuse and New River Estuaries, North Carolina, USA. Estuaries and Coasts 37 (S1): 31–45.
Peierls, B.L., N.S. Hall, and H.W. Paerl. 2012. Non-monotonic responses of phytoplankton biomass accumulation to hydrologic variability: a comparison of two coastal plain North Carolina estuaries. Estuaries and Coasts 35 (6): 1376–1392.
Phlips, E.J., S. Badylak, and T.C. Lynch. 1999. Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a subtropical inner-shelf lagoon. Limnology and Oceanography 44 (4): 1166–1175.
Phlips, E.J., S. Badylak, N.G. Nelson, and K.E. Havens. 2020. Hurricanes, El Niño and harmful algal blooms in two sub-tropical Florida estuaries: direct and indirect impacts. Scientific Reports 10 (1): 1910.
Pinckney, J.L., H.W. Paerl, and M.B. Harrington. 1999. Responses of the phytoplankton community growth rate to nutrient pulses in variable estuarine environments. Journal of Phycology 35 (6): 1455–1463.
Pinckney, J.L., H.W. Paerl, P. Tester, and T.L. Richardson. 2001. The role of nutrient loading and eutrophication in estuarine ecology. Environmental Health Perspectives 109: 699–706.
Qin, Q., and J. Shen. 2019. Physical transport processes affect the origins of harmful algal blooms in estuaries. Harmful Algae 84: 210–221.
Rothenberger, M.B., J.M. Burkholder, and T.R. Wentworth. 2009. Use of long-term data and multivariate ordination techniques to identify environmental factors governing estuarine phytoplankton species dynamics. Limnology and Oceanography 54 (6): 2107–2127.
Sanders, R.W. 1995. Seasonal distributions of the photosynthesizing ciliates Laboea strobila and Myrionecta rubra (= Mesodinium rubrum) in an estuary of the Gulf of Maine. Aquatic Microbial Ecology 9: 237–242.
Schapira, M., M.J. Buscot, T. Pollet, S.C. Leterme, and L. Seuront. 2010. Distribution of picophytoplankton communities from brackish to hypersaline waters in a South Australian coastal lagoon. Saline Systems 6 (1): 2.
Shaffer, G.P., and M.J. Sullivan. 1988. Water column productivity attributable to displaced benthic diatoms in well-mixed shallow estuaries. Journal of Phycology 24 (2): 132–140.
Shikata, T., S. Nagasoe, S. Oh, T. Matsubara, Y. Yamasaki, Y. Shimasaki, Y. Oshima, and T. Honjo. 2008. Effects of down- and up- shocks from rapid changes in salinity on survival and growth of estuarine phytoplankters. Journal of the Faculty of Agriculture, Kyushu University 53: 81–87.
Smayda, T.J., and D.G. Borkman. 2008. Nutrient and plankton dynamics in Narragansett Bay. In Science for Ecosystem-based Management, ed. A. Desbonnet and B.A. Costa-Pierce, 431–484. New York: Springer.
Smith, N. 1975. A tide and circulation study of Upper Laguna Madre: May 1, 1974 to April 30, 1975. Port Aransas: University of Texas Marine Science Institute.
Smith, N. 1988. The Laguna Madre of Texas: hydrography of a hypersaline lagoon. In Hydrodynamics of estuaries Vol. II: estuarine case studies, ed. B. Kjerfve, 31–40. Boca Raton: CRC Press.
Smith, W.O., Jr., and R.T. Barber. 1979. A carbon budget for the autotrophic ciliate Mesodinium rubrum. Journal of Phycology 15 (1): 27–33.
Street, G.T., P.A. Montagna, and P.L. Parker. 1997. Incorporation of brown tide into an estuarine food web. Marine Ecology Progress Series 152: 67–78.
Sun, J., and D. Liu. 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25 (11): 1331–1346.
Taylor, F.J.R., D.J. Blackbourn, and J. Blackbourn. 1971. The red-water ciliate Mesodinium rubrum and its “incomplete symbionts”: a review including new ultrastructural observations. Journal of the Fisheries Research Board of Canada 28 (3): 391–407.
Tolan, J.M. 2007. El Nino-Southern Oscillation impacts translated to the watershed scale: estuarine salinity patterns along the Texas Gulf Coast, 1982 to 2004. Estuarine, Coastal and Shelf Science 72 (1-2): 247–260.
TPWD. 1999. Seagrass conservation plan for Texas. Austin: Texas Parks & Wildlife Department, Resource Protection Division.
Trenberth, K.E. 2011. Changes in precipitation with climate change. Climate Research 47 (1): 123–138.
Tunnell, J.W., Jr. 2002. Geography, climate and hydrography. In The Laguna Madre of Texas and Tamaulipas, ed. J.W. Tunnell Jr. and F.W. Judd, 7–27. College Station: Texas A&M University Press.
Tweedley, J.R., S.R. Dittmann, A.K. Whitfield, K. Withers, S.D. Hoeksema, and I.C. Potter. 2019. Hypersalinity: global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In Coasts and Estuaries: the future, ed. E. Wolanski, J.W. Day, M. Elliott, and R. Ramachandran, 523–546. Cambridge: Elsevier.
Ward, L.A., P.A. Montagna, R.D. Kalke, and E.J. Buskey. 2000. Sublethal effects of Texas brown tide on Streblospio benedicti (Polychaeta) larvae. Journal of Experimental Marine Biology and Ecology 248 (1): 121–129.
Warwick, R.M., J.R. Tweedley, and I.C. Potter. 2018. Microtidal estuaries warrant special management measures that recognize their critical vulnerability to pollution and climate change. Marine Pollution Bulletin 135: 41–46.
Wetz, M.S., E.A. Hutchinson, R.S. Lunetta, H.W. Paerl, and J.C. Taylor. 2011. Severe droughts reduce estuarine primary productivity with cascading effects on higher trophic levels. Limnology and Oceanography 56 (2): 627–638.
Wetz, M.S., E.K. Cira, B. Sterba-boatwright, P.A. Montagna, T.A. Palmer, and K.C. Hayes. 2017. Exceptionally high organic nitrogen concentrations in a semi-arid South Texas estuary susceptible to brown tide blooms. Estuarine, Coastal and Shelf Science 188: 27–37.
Acknowledgements
We thank the dedicated citizen scientist volunteers who were integral in the Baffin Bay data collections, including the following: Jim Atkins, Frank Baumann, Jerry Bjork, Jan Cannamore, Al Gordon, Michael Henry, Carl Hooker, Thomas Mullenix, Scott Murray, David Rowsey, Ron Ryon, Jim Scoggins, Charles Sellers, Diane Sellers, John Sutton, and Paul Wimberly. We thank the following individuals for assistance with sample processing: Victor Batres, Kalman Bugica, Tiffany Chen, Kelsey Fisher, Anne-Marie Gavlas, Ken Hayes, Elizabeth Obst, Cory Staryk, Jessica Tolan, Sarah Tominack, Lily Walker, and Hongjie Wang. We thank Dr. Joseph D. Felix, Dr. Christopher J. Gobler, Dr. Longzhuang Li, and Dr. Blair Sterba-Boatwright for their constructive suggestions on this manuscript. We also thank two anonymous reviewers for their constructive suggestions on the manuscript.
Funding
This work was supported in part by an Institutional Grant (award no. NA14OAR4170102) to the Texas Sea Grant College Program from the National Sea Grant Office, by grants from the Texas Coastal Management Program approved by the Texas Land Commissioner pursuant to National Oceanic and Atmospheric Administration (award numbers NA14NOS4190139 and NA17NOS4190139), and with funding from the Celanese Corporation, Kleberg County, Coastal Conservation Association, Saltwater Fisheries Enhancement Association, and Coastal Bend Bays & Estuaries Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by James L. Pinckney
Supplementary Information
ESM 1
(DOCX 414 kb)
Rights and permissions
About this article
Cite this article
Cira, E.K., Palmer, T.A. & Wetz, M.S. Phytoplankton Dynamics in a Low-Inflow Estuary (Baffin Bay, TX) During Drought and High-Rainfall Conditions Associated with an El Niño Event. Estuaries and Coasts 44, 1752–1764 (2021). https://doi.org/10.1007/s12237-021-00904-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-00904-7