Abstract
To adequately protect habitat for economically important fishes, the habitats used must first be identified and described. Coastal geomorphology is often overlooked as an influential parameter of fish habitat use in favor of readily available data taken at the time of sampling. We hypothesized that river dynamics (e.g., length, watershed, floodplain connectivity) and mesohabitat categories based on geomorphology (e.g., backwater, river bend) were at least as important as fine-scale microhabitat (e.g., depth, shore type) for describing the distribution and habitat affinities of Common Snook Centropomus undecimalis (hereafter referred to as snook) in coastal rivers of the Atlantic coast and Tampa Bay, Florida. Contrary to previously studied rivers where adult snook abundance increased during a seasonal prey pulse, adult snook abundance in the study rivers differed little between seasons and the rivers were used as nurseries by juveniles. Mesohabitat categories were important for describing snook distribution. For example, the smallest snook (≤ 250 mm total length, TL) strongly selected for backwater habitats while the largest (≥ 851 mm TL) selected river bends. Detailed microhabitat data collected at individual capture locations were helpful in describing the habitat associations of the smallest size group of snook (shallow depths and aquatic macrophytes with various shore types) and for characterizing river bends (high flow, deep water, and lower salinities) but were not strongly associated with other snook sizes or mesohabitat categories. Thus, broader scale habitat features were found to be equally as important as detailed microhabitat and should be considered when informing conservation efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To ensure the protection of habitat for fishes, the habitats used throughout their life history must be appropriately categorized and described. Although defining the quantity and quality of microhabitat is critical to the success of managing fish populations, the spatial distribution of that habitat within the broader environment may be equally as important and is often overlooked (Bradley et al. 2019). Many large-scale programs in the southeastern USA used to monitor the abundance and distribution of fish species (e.g., McMichael Jr. 1991; Winner et al. 2010; Flaherty et al. 2014) include the collection of detailed microhabitat data (e.g., shoreline vegetation and coverage, bottom type, bottom vegetation type and cover) at the time fish are collected as routine parts of sampling. These microhabitat descriptions are frequently analyzed with the goal of associating species with specific habitat types, and the results are often used to protect or restore fish habitat. While critical linkages between fish and habitat have been established with these data, multiple authors (Schlosser 1991; Cooper et al. 1998; D’Ambrosio et al. 2009) have suggested that some of these microhabitat associations may reflect only the day and location where an animal is captured rather than optimal habitat and may show only an indirect link to habitat associations for species with broad distributions or habitat generalists. Thus, these detailed habitat descriptions may not accurately reflect where or why some species reside in an area unless or until combined with habitat descriptions at larger scales (Peterson 2003).
This issue of habitat scale has led to a growing awareness that geomorphological features within a landscape may be driving fish distribution as much as the more commonly assessed habitat descriptions (Thorp et al. 2006; Valesini et al. 2013; Schrandt et al. 2018). In particular, the hierarchical structure of the environment has been discussed in many assessments of rivers and streams; rivers are framed by the landscape through which they flow, and the dynamics (including geology, hydrology, and chemistry) of the river then affect each habitat level below (e.g., reach, segment, pools) down to the more commonly assessed localized habitat (e.g., depth, bottom vegetation, shore type; Vannote et al. 1980; Frissell et al. 1986; Ryder and Kerr 1989; Montgomery et al. 1999; Fausch et al. 2002; Rhoads et al. 2003; Hirzinger et al. 2004; Thorp et al. 2006). While data sets including these types of geomorphological descriptions are becoming more common in estuarine community assessments (Visintainer et al. 2006; Allen et al. 2007; Stevens et al. 2010; Jin et al. 2014; Schrandt et al. 2018), they are regularly included in freshwater studies. This is especially true for intermediate habitat features, those at the scale of about 1 to 100 km, including features such as pools, riffles, reaches, and oxbows within riverine systems and bayous, inlets, and basins within estuaries (Frissell et al. 1986; Fausch et al. 2002). These features are often not as well considered during analysis but can be important at different fish life stages (Frissell et al. 1986; Schlosser 1995; Fausch et al. 2002). In particular, Schlosser (1991, 1995) developed his dynamic landscape model for stream fish that emphasized the importance of the spatial distribution of these intermediate habitats for spawning, feeding, and refugia at various life history stages and then further underscored the role of movement between them. Leaving these features out of descriptions could lead to misinterpretation or oversimplification of distributions when defining the critical habitat for a species.
An ideal candidate species to examine the dynamics of habitat scale and habitat use is the Common Snook Centropomus undecimalis (hereafter referred to as snook), a euryhaline species that occupies a wide range of habitats. Although widely distributed in the tropics, the range in Florida is temperature-limited (lower lethal temperature of about 10 °C; Shafland and Foote 1983) and historically restricted to the southern half of the Florida peninsula. Multiple studies have described their habitat associations and revealed broad descriptions of microhabitat selection. As young-of-the-year (yoy; ≤ 250 mm total length, TL), primary habitat has been described as shallow, slow-moving water bodies with marsh grass and/or mangroves (Fore and Schmidt 1973; McMichael Jr. et al. 1989; Peters et al. 1998; Barbour et al. 2014; Brame et al. 2014). Many of these coastal nursery areas have been lost to development, or are currently under threat, necessitating protection or restoration (Turner et al. 1999; Poulakis et al. 2003; Romañach et al. 2018; Schulz et al. in press). As such, documenting the physical characteristics of nurseries, as well as where they occur within the greater environment, is needed to successfully manage populations (Turner et al. 1999; Romañach et al. 2018). Following their first year, there is a habitat shift with snook becoming more widely distributed (Winner et al. 2010; Barbour et al. 2014; Brame et al. 2014; Cianciotto et al. 2019). As adults (defined in this study as ≥ 251 mm TL, based on the maturity schedule in Taylor et al. 2000), the habitat has been variously described as overhanging shoreline vegetation with the presence of bottom vegetation, mangroves, open sandy beaches, and man-made structures including spillways, bridges, and jetties (Marshall 1958; Volpe 1959; Fore and Schmidt 1973; Gilmore et al. 1983; Taylor et al. 1998; Lowerre-Barbieri et al. 2003; Blewett et al. 2006; Blewett et al. 2009; Stevens et al. 2020). Combined, these studies suggest that, as juveniles, snook are dependent on habitats that may be disappearing and, as adults, can be described as habitat generalists. Thus, determining where snook occur within a broader landscape throughout their lives may be as important as describing what microhabitats they are captured in.
Recent research on snook has focused on the use of freshwater rivers by adult snook, describing seasonal abundance, residency, and movement patterns (Blewett et al. 2009, 2017; Trotter et al. 2012; Boucek and Rehage 2013; Young et al. 2014, 2016). Results have suggested that adult snook move into the freshwater portions of the rivers at times when large floodplains are draining to take advantage of floodplain prey, with seasonal abundances increasing by as much as three times during those periods (Boucek and Rehage 2013; Blewett et al. 2017). Before accepting these descriptions of river use broadly for the species, it is important to note that behavioral contingents and variable habitat use by estuary have been well documented (Winner et al. 2010; Muller et al. 2015; Stevens et al. 2018). Use of the coastal rivers that drain into the Indian River Lagoon on the Atlantic coast or into Tampa Bay on the Gulf of Mexico coast has not been well described. While addressing these data gaps, we sought to contribute to emerging topics related to the effects of scale—micro vs. mesohabitat—in identifying important features that would be useful for snook habitat protection.
This study had three objectives: (1) describe snook seasonal abundance in coastal river draining into the Indian River Lagoon on the Atlantic coast and into Tampa Bay on the Gulf of Mexico coast and directly compare to previously described abundance patterns in southwest Florida rivers, (2) use broad within-river mesohabitats based on geomorphology to examine the distribution of snook in these rivers throughout their life history, and (3) examine specific microhabitat features that characterize either fish size or each mesohabitat. The rivers in this study are small and many have disjointed floodplains and we hypothesize this may influence how snook will use these rivers throughout the year. We also hypothesize that the addition of an intermediate habitat scale (broad within-river mesohabitats) will help better characterize the distribution and habitat affinities of snook at various life history stages in the rivers.
Material and Methods
Study Area
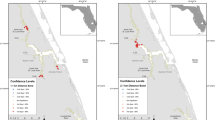
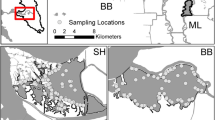
Four rivers were sampled on each coast of Florida. The Loxahatchee, St. Lucie (North and South Forks), and St. Sebastian Rivers drain into the Indian River Lagoon along the Atlantic coast of Florida and were sampled every other month from May 2007 through May 2013 (Table 1, Fig. 1). The Manatee, Little Manatee, Alafia, and Hillsborough, all of which drain into Tampa Bay on the Gulf of Mexico coast, were sampled monthly from January 2014 through December 2017 (Table 1, Fig. 1).
Field Collections
Sampling on both coasts was conducted using boat-mounted electrofishers; each produced between 60 and 1000 V of pulsating direct current at 6–110 amps supplied by a 9-kW generator and controlled by Smith-Root 7.5 or 9.0 GPP controllers (Smith-Root, Inc., Vancouver, Washington). Electrofishing settings were standardized to output about 3000 W of electrical power by accounting for the water conductivity and temperature prior to sampling each transect according to guidance by Burkhardt and Gutreuter (1995). From May 2007 to March 2010 in the Atlantic coast rivers, two electrofishing boats each sampled four to eight 300-m-long transects per river every other month. From May 2010 through March 2013, one boat was used to sample four 300-m-long transects in the same four rivers every other month. Tampa Bay rivers were sampled from January 2014 to December 2017, with four 300-m-long transects sampled monthly per river. Though the time periods on each coast do not overlap, the multiyear approach on both coasts incorporates a wide range of environmental conditions and habitats that helps to broadly characterize the habitat associations of snook.
Transect site selection in all three studies followed a systematic, stratified-random procedure adopted from the FWC’s Fisheries Independent Monitoring (FIM) program as described in Kupschus and Tremain (2001). Locations of transects and direction sampled were randomly selected from a universe of 0.2 km × 0.2 km sampling units in the freshwater and oligohaline portions of the rivers. In all three studies, only a boat equipped with a 9.0 GPP controller was used to sample lower reaches of available habitat due to its ability to achieve the standardized electrical power in water conductivity up to 25,000 μS/cm3. Based on trials prior to these studies and the ineffectiveness of the electrofishing gear in higher salinity waters (Burkhardt and Gutreuter 1995), 15 psu was chosen as the maximum salinity to conduct electrofishing surveys. Preselected, alternate transects were sampled if the salinity at any site was greater than 15 psu.
Each transect was initially traversed in the prescribed direction to ensure navigable conditions and to delineate the boundaries of the transect, and electrofishing was then conducted. Electrofishing generally occurred within 2.5 m of the shoreline while attempting to keep the electrofishing booms directly under and around vegetation, snags, and pilings. Two biologists at the bow of the boat collected all observed snook and placed them in an aerated live well with recirculating water. After completion of the transect, each fish was measured for total length (TL, mm).
Water conditions were measured once near the middle of each transect with a YSI 650 MDS interfaced with a 6-Series 600QS probe (Xylem, Inc., Yellow Springs, Ohio). Parameters measured included surface and bottom measurements of temperature (°C), salinity (psu), and dissolved oxygen (mg/l).
Microhabitat Descriptions
From May 2007 to March 2010 in the Atlantic coast rivers, detailed microhabitat descriptions were collected for each individual fish. In that study, numbered buoys were thrown at the spot each snook first appeared at the surface, allowing detailed habitat descriptions to be associated with individual fish. Five additional buoys were randomly cast along each transect to represent available habitat. A second boat then conducted habitat analysis within a 1.8-m radius of each buoy. This radius was used because it was large enough to account for deviations from where the fish was initially residing to where it was eventually stunned and observed, while small enough to describe microhabitat features that could have influenced the fish’s use of that location. Microhabitat data collected included water depth, shoreline vegetation type and coverage, substrate type, the size and number of submerged structures, aquatic vegetation type and coverage, percent shade, and water velocity (measured with a Marsh-McBirney Flo-mate 2000 portable velocity meter, m/s; Table 1). Structure counts were used to develop a structure score to quantify the amount of size-specific structure within each buoy radius. Structure count was determined by the number of items, both biotic (e.g., leaves, stems, branches) and abiotic (e.g., bridge and dock pilings, pvc), in the water column that occurred within the buoy area and was separated into four size categories consistent with previous studies and based on diameter (i.e., < 2.54, 2.54–9.8, 9.9–25.4, and > 25.4 cm). Counts of the number of items in each size category were made up to 20, after which they were placed into categories (i.e., 21–50, 51–100, or > 100). The structure score (SC) was conceptually similar to indices used by Newbrey et al. (2005) and Dutterer and Allen (2008) and was calculated by
where Size XL is the count of structure > 25.4 cm in diameter, Size L is the count of structure between 9.9 cm and 25.4 cm, Size M is the count of structure between 2.54 cm and 9.9 cm, and Size S is the count of structure < 2.54 cm. The equation was weighted towards larger diameter structures with the assumption that large diameter structures tend to be longer and therefore comprise more surface area than smaller diameter structures. Thus, the count for size XL was multiplied by the group’s minimum size, whereas the counts for sizes L, M, and S were multiplied by the midpoints of their respective structure size group. When a count within a structure size group was recorded as a categorical amount, the midpoint of that count category was used (i.e., counts 21–50 = 35 and counts 51–100 = 75). The exception to this was the maximum count category (> 100) in which the value of 100 was used.
Data Analysis
Geomorphic Mesohabitat Analysis
Using ArcGIS 10.6 (Redland, California), the boundaries of each river were created using a Florida shoreline shapefile digitized at a 40 k:1 scale by USFWS in 1990 and available through FWC’s GIS data download website (http://geodata.myfwc.com/datasets/florida-shoreline-1-to-40000-scale). The shoreline of each river was clipped from the coastline shapefile and individual river boundary shapefiles were made. River boundaries were then reduced to the size of each sampling universe and manually reshaped by tracing over current aerial imagery. The reshaping was necessary to include sections of the rivers that were part of the sampling universe but were not included at the 40 k:1 scale digitizing. The shoreline base layer was intended for illustrative purposes and any measurements derived from this layer are to be considered estimates. The newly created river boundaries were then divided into four mesohabitat zones, determined by the geomorphological qualities of the river (Table 1, Fig. 2). Briefly, the mesohabitat zones are backwater, creek/canal, mainstem, and mainstem bend (Stevens et al. 2010; Table 1, Fig. 2). To determine if a meander was counted as a mainstem bend, a reference circle was placed on the inside of each meander and used as an indicator of the shoreline boundary. A 90° angle was placed on the opposite shoreline and used as an indicator of bend intensity. If the reference circle was positioned completely within the angle, the meander was considered intense enough to be considered a mainstream bend and a polygon was created (Fig. 2). Using the start and end coordinates collected for each transect and the XY2Line tool in ArcMap, rough transect lines were drawn, connecting the start and end points. The transect lines were then manually manipulated to follow the contours of the river boundary layers. Each transect line was joined with all the fisheries’ data attributes, creating a line that contained all associated catch and habitat data.
An example of each mesohabitat zone as determined by the geomorphological qualities of the river (top panel) and the procedure used to determine if a meander was intense enough to be considered a mainstem bend (bottom panel). A reference circle was placed on the inside of each meander and used as an indicator of the shoreline boundary. A 90° angle was placed on the opposite shoreline and used as an indicator of bend intensity. If the reference circle was positioned completely within the angle, the meander was considered intense enough to be considered a mainstream bend
The transect line layers were then overlaid on the river mesohabitat zone layers and analyzed for points of intersection. If a transect line fell completely within a mesohabitat zone, it was associated with that zone. If a transect line intersected multiple mesohabitats, a weighted selection was used to prioritize which mesohabitat zone was associated with the transect. The mainstem zone was weighted the least; if a transect line intersected the mainstem zone and any other zone, the transect was associated with the other zone. This selection process was used to reduce the chance of the mainstem zone overtaking zones of smaller sizes. If a transect line intersected two zones other than the mainstem zone, the zone that contained the majority (≥ 51%) of the transect was selected.
Snook Size Categories
Snook were grouped depending on the analysis. To examine seasonal use of rivers, snook were separated into juveniles (≤ 250 mm TL) and adults (≥ 251 mm TL) based on growth patterns and maturity schedules provided in Taylor et al. (2000). To analyze habitat use at broad within-river mesohabitats, we used four groups based on length frequencies, life-history parameters, and current state of Florida regulations. Juveniles (≤ 250 mm TL) were separated as described above. Adults were further separated into spawning-capable, primarily male snook (251–500 mm TL); spawning-capable snook of both sexes which includes fish of harvestable lengths based on current regulations (501–850 mm TL); and primarily female snook that cannot be harvested based on regulations (≥ 851 mm TL).
Seasonal Catch Rates
A negative binomial regression was used to assess among-season differences in juvenile (≤ 250 mm TL) and adult (≥ 251 mm TL) snook catch rates (i.e., the expected number of snook per transect). During initial model fitting, other distributions were considered (e.g., Poisson), but a residual-based goodness of fit assessment indicated overdispersion and that use of negative binomial distributions were more appropriate. We fit separate regression models for the Atlantic coast and Tampa Bay rivers, resulting in four regression models. In each model, we included season as a fixed-effect categorical variable with four levels: spring (March–May), summer (June–August), fall (September–November), and winter (December–February). To account for potential spatial and temporal dependence, we included year × river combinations (28 on the Atlantic coast and 20 for Tampa Bay) as a random effect associated with the model intercept. The random effects were assumed to be normally distributed with a mean of zero and estimated variance (Gelman and Hill 2007). In each model, we included transect length (300 m) as an offset in the negative binomial model such that estimated catch rates represented the expected number of snook per 100 m of sampled transect. We assessed the precision of parameter estimates by calculating standard errors and 95% confidence intervals, and seasonal differences in catch rates were further assessed by conducting post hoc contrasts (Tukey’s all-pair comparisons with a 95% familywise confidence level) as implemented in the package “emmeans” (Lenth 2019). Lastly, we assessed goodness of fit of all models using a simulation-based, residual analysis approach, implemented in R using ‘DHARMa’ package (Hartig 2019). All analyses were conducted using R version 3.6.0 (R Core Team 2019).
Mesohabitat Selectivity
A habitat selection index (Manly et al. 1993) was used to assess the selection preferences exhibited by each of the four size classes of snook (≤ 250 mm, 251–500 mm, 501–850 mm, and ≥ 851 mm TL) for the four mesohabitat zones (mainstem, mainstem bend, backwater, and creek/canal). The selection index was calculated as the ratio of the observed proportion of individuals using a specific mesohabitat and the observed proportion of available habitats that were classified as that mesohabitat. To express uncertainty in selection ratio estimates, standard errors and 95% confidence intervals were calculated for each selection ratio following Krebs (1999). Lastly, the null selection index was calculated under the assumption that snook of any size exhibited no preference for any of the four mesohabitats by summing the selection indices for each size class across all four mesohabitats and dividing by four (the number of mesohabitats). Individual selection indices above the null indicated a given size class selected a given mesohabitat type, with higher index scores indicated a stronger affinity to the mesohabitat type. Indices below the null indicated avoidance.
Multiple Correspondence Analysis and Microhabitat Associations
Multiple correspondence analysis (MCA) was used to visualize microhabitat associations with both fish size and the geomorphologic mesohabitats using the detailed microhabitat data collected at individual fish catch locations from May 2007 through March 2010 in the Atlantic coast rivers only. MCA was carried out via SAS PROC CORRESP with the MCA option and Greenacre inertia adjustment (Greenacre 1994; SAS Institute, Inc. 2009). In the first MCA comparing microhabitat with fish size, buoys were categorized as random, ≤ 250 mm, 251–500 mm, 501–850 mm, and ≥ 851 mm TL. In the second MCA comparing microhabitat with mesohabitat zone, buoys (those where fish were caught and randomly thrown buoys) were categorized by the mesohabitats. Microhabitat data used in both analyses included water depth, water velocity, salinity, percent shade, bottom type, percent area covered by structure, structure score, and cover type (combined shore type with aquatic vegetation; Table 1). The continuous covariates, water depth, water velocity, salinity, and shade, were treated as categorical variables, separated by the median of their distributions (low corresponded to ≤ the median and high corresponded to > the median). The results of the MCAs were displayed using ordination plots of the first two axes of output.
Results
A total of 6238 and 8689 snook were caught in the Atlantic coast and Tampa Bay rivers, respectively. Total length (TL) ranged from 25 to 1115 mm TL in the Atlantic coast rivers, with 44% of all snook caught considered juveniles (≤ 250 mm TL) and 56% adults (≥ 251 mm TL; Table 2). Though this general pattern was evident in each river, fewer snook of any size were caught in the Loxahatchee River and the most snook were caught in the North Fork of the St. Lucie River. Total length (mm) ranged from 27 to 1024 mm in the Tampa Bay rivers (Fig. 3), where 34% of all snook were juveniles and 66% were adults (Table 2). In those rivers, more snook were caught in the Little Manatee and Alafia (both of which also had higher numbers of juveniles), few snook overall were caught in the Hillsborough, and few juveniles were caught in the Manatee River. Despite these differences and because of the scope of the manuscript, rivers were combined and analyzed by coast with years combined.
Based on the negative binomial regression, catch-per-100-m sampled varied little by season for adult fish (≥ 251 mm TL), but did vary by season for juvenile fish (≤ 250 mm TL) on both coasts (Fig. 4). The simulation-based assessment of residuals from each of the four candidate models indicated that all models provided an adequate fit to the observed data. On the Atlantic coast, mean number of adults caught per 100 m sampled was between 0.9 and 1.2 fish in any season. Despite small decreases in catch in summer and winter, there was no major difference between any seasonal comparisons. The mean number of juveniles ranged from 0.6 and 1.2 fish per 100 m sampled. Summer and winter catches were 1.0 to 2.0 times greater than those in the spring (spring compared to summer, p = 0.008; spring compared to winter, p = 0.0008). In the Tampa Bay rivers, the mean number of adults per 100 m sampled was between 2.0 and 2.9 in any season. In these rivers, more snook were caught in the spring than in the winter (p = 0.04), but there were no other seasonal differences. Fall catches of juveniles (mean = 1.4 fish per 100 m) were higher than any other season and doubled the numbers caught in the spring (mean = 0.7; p = 0.01).
Number of snook (mean ± 95% confidence intervals) collected by electrofishing per 100 m of shoreline in the Atlantic coast rivers (left column) and in Tampa Bay rivers (right column), by season. Snook were separated into juveniles (≤ 250 mm TL; top panel) and adults (≥ 251 mm TL; bottom panel) based on growth patterns and maturity schedules. Y-axis scales are different for each coast. Rivers and years were combined by coast
Available mesohabitat was estimated on each coast, and the majority was categorized as mainstem (66% and 51% in the Atlantic coast and Tampa Bay rivers, respectively; Fig. 5). The mainstem zone overlapped with one of the other mesohabitat zones on 46% of the transects on the Atlantic coast and 28% of the transects in the Tampa Bay rivers, and those transects were classified as the other mesohabitat. In addition, 10% of transects on the Atlantic coast and 2% in the Tampa Bay rivers contained more than one of the smaller mesohabitat zones (mainstem bend, backwater, and creek/canal) and, in those cases, the zone that contained the majority of the transect (≥ 51%) was selected. The amount of backwater mesohabitat was a major difference between the coasts, accounting for 41% of the habitat in the Tampa Bay rivers but only 19% in the Atlantic coast rivers. Canal/creek and mainstem bend habitats represented very little of the available habitat on both coasts (Fig. 5). The results of the mesohabitat selection index indicated snook have strong patterns of distribution between mesohabitat zones based on size, and the patterns were similar on both coasts (Fig. 6). Snook ≤ 250 mm TL were more frequently collected in the backwater mesohabitat; this association was stronger in the Tampa Bay rivers while these small fish also utilized the canals/creeks in the Atlantic coast rivers. Snook spread throughout the other habitats as they grew, decreasing use of the backwaters and increasing use of the creeks/canals, mainstem, and mainstem bends. The largest snook (≥ 851 mm TL) were caught predominately in mainstem bends and, similar to the smallest group, that association was stronger in the Tampa Bay rivers (Fig. 6).
Mesohabitat selection index (mean ± 95% confidence intervals) for Atlantic coast snook (left column) and Tampa Bay snook (right column) in four size categories. Snook were separated into four categories (≤ 250 mm, 251–500 mm, 501–850 mm, and ≥ 851 mm TL) based on length frequencies, life-history parameters, and current state of Florida regulations. Y-axis scales are different for each coast. The dashed line represents the null selection index, calculated under the assumption that snook of any size class did not exhibit a selection for a particular mesohabitat zone. It was calculated by summing the selection indices for each size class across all four mesohabitats and dividing by four. Selection indices above the null indicated a given size selected that mesohabitat type. Selection indices below the null indicated avoidance
As indicated by the MCAs, however, relationships between microhabitat and either snook size or mesohabitat zone were not strong (Fig. 7). With respect to size, there was some separation between the smallest (≤ 250 mm TL) and the largest (≥ 851 mm TL) size groups; however, inertia was low on both axes, indicating that the microhabitat characteristics do not explain those differences well (Fig. 7 top panel). Despite that, snook ≤ 250 mm TL were associated with habitats that included aquatic macrophytes and shallow depth. Snook in the intermediate size groups (251–500 mm TL and 501–850 mm TL) fell close to the random buoys suggesting that these size groups use a wide range of available microhabitat. Microhabitat also did not define mesohabitat zones well, with low inertia values on both axes (Fig. 7 bottom panel). Mainstem, backwater, and canals/creeks all fell close to the center, indicating these mesohabitats can be described by a variety of microhabitats. Mainstem bend, however, was associated with both higher depth and higher velocity and with lower salinity.
Multiple correspondence analysis plots used to illustrate microhabitat associations with snook size categories (top panel) and mesohabitat type (bottom panel). Snook were separated into four categories (≤ 250 mm, 251–500 mm, 501–850 mm, and ≥ 851 mm TL) based on length frequencies, life-history parameters, and current state of Florida regulations. Each mesohabitat type was determined by the geomorphological qualities of the river. MCAs are based on detailed microhabitat descriptions collected in a 1.8-m radius around each individual fish from May 2007 to March 2010 in the Atlantic coast rivers only. Continuous variables were treated categorically as high and low values based on the median of their distributions. R indicates the buoys randomly cast along transects to represent available habitat
Discussion
In Florida, fish habitat associations and distributions are often analyzed on a coast-wise basis (Muller et al. 2015), at the scale of an estuary (Winner et al. 2010), or at a microhabitat level (Stevens et al. 2020). In addition to describing seasonal catch of snook in rivers that have not been well described, we also sought to contribute to the emerging topic of habitat scale as a valuable tool for describing fish habitat use and distribution. In this study, snook partitioned by size within mesohabitats (an intermediate habitat scale based on geomorphology) in the rivers draining into the Indian River Lagoon on the Atlantic coast and into Tampa Bay on the Gulf of Mexico coast. In the Atlantic coast rivers, where detailed microhabitat was available at individual fish capture locations, mesohabitat was as important as the microhabitat for describing river use by snook.
Seasonally, catch per 100 m of shoreline was generally consistent across seasons for adults on both coasts, indicating year-round use of the freshwater portions of the rivers. This sharply contrasts previous results by Boucek and Rehage (2013) and Blewett et al. (2017) in the rivers of Everglades National Park (ENP) and Charlotte Harbor in southwest Florida. Both of those studies described strong seasonal patterns of river use by snook that were tied to the seasonal drawdown of large drainage basins into the rivers and a subsequent influx of floodplain prey; the authors concluded snook moved into the freshwater portions of rivers to take advantage of those resources. We suggest that floodplain connectivity differs among the rivers in Florida and explains the differences in seasonality observed among the systems. In general, the rivers on the Atlantic coast and in Tampa Bay are much smaller in length and drainage basin than those studied in Charlotte Harbor, while similar in length with smaller drainage basins when compared to ENP (182 and 31 km in length with 5959- and 1700-km2 drainage basins, respectively; Blewett et al. 2013; Boucek et al. 2017, 2019; Stevens et al. 2018). Nearly all of the drainage basins to the Atlantic coast rivers have been extensively altered for agriculture and development and most of the rivers have water control structures that modulate the amount and timing of flow and constrain it to localized point sources. Though anglers do report increased catches at water control structures and spillways during high flows (J. Whittington, pers. comm.), the limited duration and localization of flows likely do not allow us to detect any seasonal increases in abundance. Similarly, two of the rivers (Hillsborough and Manatee Rivers) in Tampa Bay are dammed to provide drinking water for the surrounding municipalities, restricting both flow rates and how water flows into the rivers. More importantly, the dams have separated the floodplains from the lower rivers, which likely reduces the amount of floodplain prey available to consumers such as snook. The altered floodplains and controlled flows in our study systems contrast with the intact floodplains and unimpeded flows in the previously studied rivers, and likely explain the lack of seasonal changes in adult snook abundance in our study rivers.
Further, catch rates in the study rivers were comparable to the nearby open estuaries, suggesting snook are using the rivers in these areas as extensions of the estuary. In the Indian River Lagoon, where the Atlantic coast rivers drain, catch per 100 m of shoreline ranged from 0.8 snook in the northern lagoon to 4.7 snook in the southern lagoon (numbers include snook we classified as juveniles; Winner et al. 2010). Catches in the Tampa Bay estuary are about 3.3 snook per 100 m of shoreline (Winner et al. 2010). Both the Indian River Lagoon and Tampa Bay have been highly developed and subject to increasing anthropogenic effects (Greening et al. 2014; Krebs et al. 2014; Adams et al. 2019). Although snook appear to be using the rivers in this study as an extension of the estuary, their use may also increase in the future if they avoid the estuary proper as habitat and water quality continue to degrade.
Seasonal catch rates of yoy and juveniles (≤ 250 mm TL) in the study rivers reflect the known spawning period and recruitment to nursery habitat. Juvenile abundance nearly doubles in the summer and winter in the Atlantic coast rivers and in fall in the Tampa Bay rivers. Snook spawn over a prolonged season extending from April through October (Taylor et al. 1998). In Tampa Bay, peak spawning occurs in June and July with known spawning sites occurring near the mouths of many of our study rivers (Taylor et al. 1998, 2000; Lowerre-Barbieri et al. 2014), and yoy and juveniles increase in abundance in the fall just after peak spawning. Similarly, on the Atlantic coast, primary spawning inlets occur just outside of the study rivers; however, peak spawning occurs slightly later in July and August (Young et al. 2014). The increase in yoy and juvenile catches also occurs slightly later, peaking in winter months. In other estuaries in Florida, spawning sites are either known or thought to occur along the beaches and nearshore shallow reefs rather than near the rivers and nursery habitat consists of coastal ponds and creeks closer to those areas (Peters et al. 1998; Taylor et al. 1998; Stevens et al. 2007; R. Boucek, pers. comm.). Thus, it appears that proximity to a spawning site is likely the biggest indicator of whether a river will be used as nursery habitat.
Mesohabitat was particularly helpful in explaining the distribution of snook by size within the rivers, while detailed microhabitat was less helpful in defining habitat associations. The yoy and juvenile snook (≤ 250 mm TL) strongly selected backwater and, in the Atlantic coast rivers, creeks and canals. This size class was loosely associated (indicated by relatively low inertia in MCA) with shallow depths and aquatic macrophytes combined with a variety of shoreline vegetation (mangroves, non-native trees and shrubs, and snags). This is similar to previous descriptions of nursery habitat: low-energy, shallow streams, canals, creeks, and lagoons with overhanging shoreline vegetation (Fore and Schmidt 1973; Gilmore et al. 1983; McMichael Jr. et al. 1989; Barbour et al. 2014; Brame et al. 2014; Schulz et al. 2020). These backwater habitats likely serve an important function for the small snook. The low-energy environment allows them to invest their energy primarily into growth, feeding on an abundance of small prey in the area (e.g., mosquitofish, sailfin molly; Adams et al. 2009; Stevens et al. 2010), while the shallow waters and aquatic vegetation allow them to hide from larger predators. While the backwater mesohabitat accounts for much of the available habitat in the Tampa Bay rivers (41%), it accounts for only about 19% of the available habitat in the Atlantic coast rivers.
Snook in the two intermediate size classes (251–500 mm TL and 501–850 mm TL) were found in a variety of the mesohabitat zones, decreasing use of the backwater zone and spreading into the mainstem and mainstem bends as they grew. These size classes were also more closely associated with the microhabitat assessed at the random buoys further supporting the idea that adult snook are habitat generalists. The largest snook (≥ 851 mm TL), however, selected the mainstem bend mesohabitat. While the largest snook separated from the other size classes in the microhabitat MCA, they were not associated with any specific microhabitat characteristics. The mainstem bends, however, were associated with high flow and high depth, low salinity, and shorelines consisting of trees and shrubs. These more dynamic habitats likely serve an important function for the largest snook. Adult snook opportunistically feed on prey abundant in their environment (Blewett et al. 2006; Stevens et al. 2010; Dutka-Gianelli et al. 2011; Blewett et al. 2013; Boucek and Rehage 2013); as ambush predators, they often orient themselves to face moving water and wait for prey to be carried down the current. These high flow, deep bends provide an ideal ambush spot for the largest snook to wait for prey but are likely too dynamic and would require too much energy for the smaller conspecifics. Though bends are important to the largest snook, they are rare (representing less than 8% of the available mesohabitat on either coast).
Given the rarity of backwater habitat on the Atlantic coast and bends on both coasts, as well as their importance to the smallest and largest size classes of snook, respectively, these mesohabitats should be conserved or restored. For example, lands adjacent to backwaters and river bends could be prioritized for acquisition by state and nonprofit organizations. The acquisition of land may allow for the natural migration of bends and provide a buffer to preserve shoreline vegetation in both bends and backwaters (Turner et al. 1999; Larsen et al. 2006). Channelization efforts for boating access along the mainstem could affect both mesohabitats, with potential impacts to depth and flow (e.g., sediment accumulation in backwaters). Particular attention should be given to water quality for those smaller creeks and canals leading into backwaters to avoid eutrophication and algal blooms. Degraded habitats could be slated for restoration efforts, focusing on depth, flow, and shoreline habitat characteristics to provide quality backwater nurseries (Cicchetti and Greening 2011).
There were a few limitations with this study that could have affected the results. In this study, each 300-m-long transect was categorized by mesohabitat zones. Although a transect often did intersect more than one mesohabitat type (56% of all transects in the Atlantic coast rivers and 30% of transects in the Tampa Bay rivers), the transect and all snook caught on it were associated with the predominate mesohabitat type. Microhabitat data were collected within a 1.8-m radius of buoys thrown randomly along the transect and at locations where individual snook were first sighted. Although very specific characteristics were therefore obtained for each individual, unfortunately, the exact latitude and longitude were not recorded for each buoy and buoys were characterized by mesohabitat zone the same as transects were. Thus, for example, a small snook or a buoy with specific microhabitat characteristics could have come from a backwater mesohabitat but become associated with another mesohabitat zone because of where the majority of the transect occurred. Despite this limitation, mesohabitat zone was useful in describing the distribution of snook at various sizes and the smallest and largest size classes clearly selected specific mesohabitat zones. Microhabitat, however, was not as useful with respect to either fish size or mesohabitat zone. Based on the results of the MCA and the low inertia values, only a few characteristics were loosely associated with the smallest size snook and with mainstem bends. If this study is to be repeated elsewhere, the authors would suggest shorter transects that do not overlap mesohabitat zones and/or unique latitude and longitudes for each individual. Associations between mesohabitat zone and fish size, and between microhabitat and both fish size and mesohabitat, may be stronger without the issue created by our transect length.
There is increasing awareness that scale is an important consideration when describing fish habitat use (Thorp et al. 2006; Valesini et al. 2013; Schrandt et al. 2018). While standard assessments are adequate for describing the habitat in the immediate location that a fish is captured, they often leave out the broader environment, which may play an important role in fish distribution and habitat use. This is especially important for a generalist species such as snook. In this study, we used river dynamics and an intermediate scale of broad within-river mesohabitats based on river geomorphology to better describe the seasonal abundance and distribution of snook in small coastal rivers. Further, we attempted to stress the importance of including these additional habitat descriptors which may better reveal where and how fish partition themselves within their environment. In this study, within-river mesohabitats were a good indicator of where different size classes of fish would reside. Regardless of the microhabitat characteristics within them, these mesohabitats provide important functions with respect to feeding and refugia, particularly for snook at the smallest and largest sizes. While the scales used in this study worked well in these rivers and with this study species, the type and scale of habitat descriptors may vary with the species, research objectives, and management goals.
References
Adams, A.J., R.K. Wolfe, and C.A. Layman. 2009. Preliminary examination of how human-driven freshwater flow alteration affects trophic ecology of juvenile snook (Centropomus undecimalis) in estuarine creeks. Estuaries and Coasts 32 (4): 819–828. https://doi.org/10.1007/s12237-009-9156-x.
Adams, D.H., D.M. Tremain, R. Paperno, and C. Sonne. 2019. Florida lagoon at risk of ecosystem collapse. Science 365 (6457): 991–992. https://doi.org/10.1126/science.aaz0175.
Allen, D.M., S.S. Haertel-Borer, B.J. Milan, D. Bushek, and R.F. Dame. 2007. Geomorphological determinants of nekton use of intertidal salt marsh creek. Marine Ecology Progress Series 329: 57–71. https://doi.org/10.3354/meps329057.
Barbour, A.B., A.J. Adams, and K. Lorenzen. 2014. Size-based, seasonal, and multidirectional movements of an estuarine fish species in a habitat mosaic. Marine Ecology Progress Series 507: 263–276. https://doi.org/10.3354/meps10837.
Blewett, D.A., R.A. Hensley, and P.W. Stevens. 2006. Feeding habits of common snook, Centropomus undecimalis, in Charlotte Harbor, Florida. Gulf and Caribbean Research 18 (1): 1–14. https://doi.org/10.18785/gcr.1801.01.
Blewett, D.A., P.W. Stevens, T.R. Champeau, and R.G. Taylor. 2009. Use of rivers by common snook Centropomus undecimalis in Southwest Florida: A first step in addressing the overwintering paradigm. Florida Scientist 72 (4): 310–324.
Blewett, D.A., P.W. Stevens, and M.E. Call. 2013. Comparative ecology of euryhaline and freshwater predators in a subtropical floodplain river. Florida Scientist 76 (2): 166–190.
Blewett, D.A., P.W. Stevens, and J. Carter. 2017. Ecological effects of river flooding on abundance and body condition of a large, euryhaline fish. Marine Ecology Progress Series 563: 211–218. https://doi.org/10.3354/meps11960.
Boucek, R.E., and J.S. Rehage. 2013. No free lunch: Displaced marsh consumers regulate a prey subsidy to an estuarine consumer. Oikos 122 (10): 1453–1464. https://doi.org/10.1111/j.1600-0706.2013.20994.x.
Boucek, R.E., M.R. Heithaus, R. Santos, P.W. Stevens, and J.S. Rehage. 2017. Can animal habitat use patterns influence their vulnerability to extreme climate events? An estuarine sportfish case study. Global Change Biology 23 (10): 4045–4057. https://doi.org/10.1111/gcb.13761.
Boucek, R.E., A.A. Trotter, D.A. Blewett, J.L. Ritch, R. Santos, P.W. Stevens, J.A. Massie, and J.S. Rehage. 2019. Contrasting river migrations of common snook between two Florida rivers using acoustic telemetry. Fisheries Research 213: 219–225. https://doi.org/10.1016/j.fishres.2018.12.017.
Bradley, M., R. Baker, I. Nagelkerken, and M. Sheaves. 2019. Context is more important than habitat type in determining use by juvenile fish. Landscape Ecology 34 (2): 427–442. https://doi.org/10.1007/s10980-019-00781-3.
Brame, A.B., C.C. McIvor, E.B. Peebles, and D.J. Hollander. 2014. Site fidelity and condition metrics suggest sequential habitat use by juvenile common snook. Marine Ecology Progress Series 509: 255–269. https://doi.org/10.3354/meps10902.
Burkhardt, R.W., and S. Gutreuter. 1995. Improving electrofishing catch consistency by standardizing power. North American Journal of Fisheries Management 15 (2): 375–381. https://doi.org/10.1577/1548-8675(1995)015%3C0375:IECCBS%3E2.3.CO;2.
Cianciotto, A.C., J.M. Shenker, A.J. Adams, J.J. Rennert, and D. Heuberger. 2019. Modifying mosquito impoundment management to enhance nursery habitat value for juvenile common snook (Centropomus undecimalis) and Atlantic tarpon (Megalops atlanticus). Environmental Biology of Fishes 102 (2): 403–416. https://doi.org/10.1007/s10641-018-0838-8.
Cicchetti, G., and H. Greening. 2011. Estuarine biotope mosaics and habitat management goals: An application in Tampa Bay, FL, USA. Estuaries and Coasts 34 (6): 1278–1292. https://doi.org/10.1007/s12237-011-9408-4.
Cooper, S.D., S. Diehl, K. Kratz, and O. Sarnelle. 1998. Implications of scale for patterns and processes in stream ecology. Australian Journal of Ecology 23 (1): 27–40. https://doi.org/10.1111/j.1442-9993.1998.tb00703.x.
D’Ambrosio, J.L., J.L. Williams, J.D. Witter, and A. Ward. 2009. Effects of geomorphology, habitat, and spatial location on fish assemblages in a watershed in Ohio, USA. Environmental Monitoring and Assessment 148 (1-4): 325–341. https://doi.org/10.1007/s10661-008-0163-3.
Dutka-Gianelli, J., R. Taylor, E. Nagid, J. Whittington, and K. Johnson. 2011. Habitat utilization and resources partitioning of apex predators in coastal rivers of Southeast Florida. Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, In House Report: IHR F2771-07-11-F.
Dutterer, A.C., and M.S. Allen. 2008. Spotted sunfish habitat selection at three Florida rivers and implications for minimum flows. Transactions of the American Fisheries Society 137 (2): 454–466. https://doi.org/10.1577/T07-039.1.
Fausch, K.D., C.E. Torgersen, C.V. Baxter, and H.W. Li. 2002. Landscapes to riverscapes: Bridging the gap between research and conservation of stream fishes. BioScience 52 (6): 483–498. https://doi.org/10.1641/0006-3568(2002)052[0483:LTRBTG]2.0.CO;2.
Flaherty, K.E., T.S. Switzer, B.L. Winner, and S.F. Keenan. 2014. Regional correspondence in habitat occupancy by gray snapper (Lutjanus griseus) in estuaries of the southeastern United States. Estuaries and Coasts 37 (1): 206–228. https://doi.org/10.1007/s12237-013-9652-x.
Fore, P.L., and T.W. Schmidt. 1973. Biology of juvenile and adult snook, Centropomus undecimalis, in the Ten Thousand Islands, Florida. US Environmental Protection Agency, Surveillance and Analysis Division. Publication number EPA 904: 9–74.
Frissell, C.A., W.J. Liss, C.E. Warren, and M.D. Hurley. 1986. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environmental Management 10 (2): 199–214. https://doi.org/10.1007/BF01867358.
Gelman, A., and J. Hill. 2007. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press.
Gilmore, R.G., C.J. Donohoe, and D.W. Cooke. 1983. Observations on the distribution and biology of east-Central Florida populations of the common snook, Centropomus undecimalis (Bloch). Florida Scientist 46 (3/4): 313–336.
Greenacre, M.J. 1994. Multiple and joint correspondence analysis. In Correspondence analysis in the social sciences: Recent developments and applications, ed. M.L. Greenacre and J. Blasius, 141–161. London: Academic Press.
Greening, H., A. Janicki, E. Sherwood, R. Pribble, and J.O.R. Johansson. 2014. Ecosystem responses to long-term nutrient management in an urban estuary: Tampa Bay, Florida, U.S.A. Estuarine, Coastal and Shelf Science 151 (A): 1–16. https://doi.org/10.1016/j.ecss.2014.10.003.
Hartig, F. 2019. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.4. https://CRAN.R-project.org/package=DHARMa
Hirzinger, V., H. Keckeis, H.L. Nemeschkal, and F. Schiemer. 2004. The importance of inshore areas for adult fish distribution along a free-flowing section of the Danube, Austria. River Research and Applications 20 (2): 137–149. https://doi.org/10.1002/rra.739.
Jin, B., W. Xu, L. Guo, J. Chen, and C. Fu. 2014. The impact of geomorphology of marsh creeks on fish assemblage in Changjiang River estuary. Chinese Journal of Oceanology and Limnology 32 (2): 469–479. https://doi.org/10.1007/s00343-014-3002-0.
Krebs, C.J. 1999. Ecological methodology. New York: Benjamin/Cummings.
Krebs, J.M., S.S. Bell, and C.C. McIvor. 2014. Assessing the link between coastal urbanization and the quality of nekton habitat in mangrove tidal tributaries. Estuaries and Coasts 37 (4): 832–846. https://doi.org/10.1007/s12237-013-9724-y.
Kupschus, S., and D. Tremain. 2001. Associations between fish assemblages and environmental factors in nearshore habitats of a subtropical estuary. Journal of Fish Biology 58 (5): 1383–1403. https://doi.org/10.1111/j.1095-8649.2001.tb02294.x.
Larsen, E.W., E.H. Girvetz, and A.K. Fremier. 2006. Assessing the effects of alternative setback channel constraint scenarios employing a river meander migration model. Environmental Management 37 (6): 880–897. https://doi.org/10.1007/s00267-004-0220-9.
Lenth, R. 2019. Emmeans: Estimated marginal means, aka least-squares means. R package version 1 (4): 3.01 https://CRAN.R-project.org/package=emmeans.
Lowerre-Barbieri, S.K., F.E. Vose, and J.A. Whittington. 2003. Catch-and-release fishing on a spawning aggregation of common snook: Does it affect reproductive output? Transactions of the American Fisheries Society 132 (5): 940–952. https://doi.org/10.1577/T02-001.
Lowerre-Barbieri, S., D. Villegas-Rios, S. Walters, J. Bickford, W. Cooper, R. Muller, and A. Trotter. 2014. Spawning site selection and contingent behavior in common snook, Centropomus undecimalis. PLoS One 9 (7): e101809. https://doi.org/10.1371/journal.pone.0101809.
Manly, B.F.J., L.L. MacDonald, and D.L. Thomas. 1993. Resource selection by animals: Statistical design and analysis for field studies. Chapman and Hall Press, New York. https://doi.org/10.1007/0-306-48151-0.
Marshall, A.R. 1958. A survey of the snook fishery of Florida, with studies of the biology of the principal species, Centropomus undecimalis (Bloch). Florida State Board of Conservation No. 22: 39p.
McMichael, R.H., Jr., K.M. Peters, and G.R. Parsons. 1989. Early life history of the snook, Centropomus undecimalis, in Tampa Bay, Florida. Northeast Gulf Science 10 (2): 113–126. https://doi.org/10.18785/negs.1002.05.
McMichael, R.H., Jr. 1991. Florida’s marine fisheries-independent monitoring program. In Proceedings, Tampa Bay area scientific information symposium 2 (BASIS), ed. S.F. Treat and P.A. Clark, 255–261. Tampa: Tampa Bay Regional Planning.
Montgomery, D.R., E.M. Beamer, G.R. Pess, and T.P. Quinn. 1999. Channel type and salmonid spawning distribution and abundance. Canadian Journal of Fisheries and Aquatic Sciences 56 (3): 377–387. https://doi.org/10.1139/f98-181.
Muller, R.G., A.A. Trotter, and P.W. Stevens. 2015. The 2015 stock assessment update of common snook, Centropomus undecimalis. Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, In House Report: IHR: 2015–2004.
Newbrey, M.G., M.A. Bozek, M.J. Jennings, and J.E. Cook. 2005. Branching complexity and morphological characteristics of coarse woody structure as lacustrine fish habitat. Canadian Journal of Fisheries and Aquatic Sciences 62 (9): 2110–2123. https://doi.org/10.1139/f05-125.
Peters, K.M., R.E. Matheson Jr., and R.G. Taylor. 1998. Reproduction and early life history of common snook, Centropomus undecimalis (Bloch), in Florida. Bulletin of Marine Sciences 62: 509–529. https://doi.org/10.15517/rbt.v0i0.3131.
Peterson, M.S. 2003. A conceptual view of environmental-habitat-production linkages in tidal river estuaries. Reviews in Fisheries Sciences 11 (4): 291–313. https://doi.org/10.1080/10641260390255844.
Poulakis, G.R., D.A. Blewett, and M.E. Mitchell. 2003. The effects of season and proximity to fringing mangroves on seagrass-associated fish communities in Charlotte Harbor, Florida. Gulf of Mexico Science 21 (2): 171–184. https://doi.org/10.18785/goms.2102.03.
Core Team, R. 2019. R: A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing https://www.R-project.org/.
Rhoads, B.L., J.S. Schwartz, and S. Porter. 2003. Stream geomorphology, bank vegetation, and three-dimensional habitat hydraulics for fish in midwestern agricultural streams. Water Resources Research 39 (8). https://doi.org/10.1029/2003WR002294.
Romañach, S.S., D.L. DeAngelis, H.L. Koh, Y. Li, S.Y. The, R.S. Raja Barizan, and L. Zhai. 2018. Conservation and restoration of mangroves: Global status, perspectives, and prognosis. Ocean and Coastal Management 154: 72–82. https://doi.org/10.1016/j.ocecoaman.2018.01.009.
Ryder, R.A., and S.R. Kerr. 1989. Environmental priorities: Placing habitat in hierarchic perspective. Canadian Special Publication of Fisheries and Aquatic Sciences 105: 2–12.
SAS Institute, Inc. 2009. The CORRESP procedure. (https://support.sas.com/documentation/cdl/en/statug/59654/HTML/default/corresp_toc.htm Accessed 18 November 2019).
Schlosser, I.J. 1991. Stream fish ecology: A landscape perspective. BioScience 41 (10): 704–712. https://doi.org/10.2307/1311765.
Schlosser, I.J. 1995. Critical landscape attributes that influence fish population dynamics in headwater streams. Hydrobiologia 303 (1-3): 71–81. https://doi.org/10.1007/BF00034045.
Schrandt, M.N., T.S. Switzer, C.J. Stafford, K.E. Flaherty-Walia, R. Paperno, and R.E. Matheson. 2018. Similar habitats, different communities: Eastern Gulf of Mexico deep polyhaline seagrass fish assemblages relate more to estuary morphology than latitude. Estuarine, Coastal, and Shelf Science 213: 217–229. https://doi.org/10.1016/j.ecss.2018.08.022.
Schulz, K., P.W. Stevens, J.E. Hill, A.A. Trotter, J.L. Ritch, K.L. Williams, J.T. Patterson, and Q.M. Tuckett. 2020. In press. Coastal wetland restoration improves habitat for juvenile sportfish in Tampa Bay, Florida. USA. Restoration Ecology. https://doi.org/10.1111/rec.13215.
Shafland, P.L., and K.J. Foote. 1983. A lower lethal temperature for fingerling snook, Centropomus undecimalis. Northeast Gulf Science 6 (2): 175–177. https://doi.org/10.18785/negs.0602.12.
Stevens, P.W., D.A. Blewett, and G.R. Poulakis. 2007. Variable habitat use by juvenile common snook, Centropomus undecimalis (Pisces: Centropomidae): Applying a life-history model in a southwest Florida estuary. Bulletin of Marine Science 80 (1): 93–108.
Stevens, P.W., M.F. Greenwood, C.F. Idelberger, and D.A. Blewett. 2010. Mainstem and backwater fish assemblages in the tidal Caloosahatchee River: Implications for freshwater inflow studies. Estuaries and Coasts 33 (5): 1216–1224. https://doi.org/10.1007/s12237-010-9318-x.
Stevens, P.W., R.E. Boucek, A.A. Trotter, J.L. Ritch, E.R. Johnson, C.P. Shea, D.A. Blewett, and J.S. Rehage. 2018. Illustrating the value of cross-site comparisons: Habitat use by a large, euryhaline fish differs along a latitudinal gradient. Fisheries Research 208: 42–48. https://doi.org/10.1016/j.fishres.2018.07.005.
Stevens, P.W., J. Dutka-Gianelli, E.J. Nagid, A.A. Trotter, K.G. Johnson, T. Tuten, and K.A. Whittington. 2020. Niche partitioning among snook (Pisces: Centropomidae) in river of southeastern Florida and implications for species range limits. Estuaries and Coasts 42 (1): 1–13. https://doi.org/10.1007/s12237-019-00650-x.
Taylor, R.G., H.J. Grier, and J.A. Whittington. 1998. Spawning rhythms of common snook in Florida. Journal of Fish Biology 53 (3): 502–520. https://doi.org/10.1111/j.1095-8649.1998.tb00998.x.
Taylor, R.G., J.A. Whittington, H.J. Grier H. J., and R.E. Crabtree. 2000. Age, growth, maturation, and protandric sex reversal in common snook, Centropomus undecimalis, from the east and west coasts of South Florida. Fishery Bulletin 98 (3): 612–612.
Thorp, J.H., M.C. Thoms, and M.D. Delong. 2006. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Research and Applications 22 (2): 123–147. https://doi.org/10.1002/rra.901.
Trotter, A.A., D.A. Blewett, R.G. Taylor, and P.W. Stevens. 2012. Migrations of common snook from a tidal river with implications for skipped spawning. Transactions of the American Fisheries Society 141 (4): 1016–1025. https://doi.org/10.1080/00028487.2012.675903.
Turner, S.J., S.F. Thrush, J.E. Hewitt, V.J. Cummings, and G. Funnell. 1999. Fishing impacts and the degradation or loss of habitat structure. Fisheries Management and Ecology 6 (5): 401–420. https://doi.org/10.1046/j.1365-2400.1999.00167.x.
USGS National Hydrography Dataset and Watershed Boundary Dataset. n.d. (https://viewer.nationalmap.gov/basic/?basemap=b1&category=nhd&title=NHD%20View Accessed 11 February 2019).
Valesini, F.J., J.R. Tweedley, K.R. Clarke, and I.C. Potter. 2013. The importance of regional, system-wide and local spatial scales in structuring temperate estuarine fish communities. Estuaries and Coasts 37 (3): 525–547. https://doi.org/10.1007/s12237-013-9720-2.
Vannote, R.L., G.W. Minshall, K.W. Cummins, J.R. Sedell, and C.E. Cushing. 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37 (1): 130–137. https://doi.org/10.1139/f80-017.
Visintainer, T.A., S.M. Bollens, and C.S. Simenstad. 2006. Community composition and diet of fishes as a function of tidal channel geomorphology. Marine Ecology Progress Series 321: 227–243. https://doi.org/10.3354/meps321227.
Volpe, A.V. 1959. Aspects of the biology of the common snook, Centropomus undecimalis (Bloch), of southwest Florida. Florida State Board of Conservation 31: 1–37.
Winner, B.L., D.A. Blewett, R.H. McMichael Jr., and C.B. Guenther. 2010. Relative abundance and distribution of common snook along shoreline habitats of Florida estuaries. Transactions of the American Fisheries Society 139 (1): 62–79. https://doi.org/10.1577/T08-215.1.
Young, J.M., B.G. Yeiser, and J.A. Whittington. 2014. Spatiotemporal dynamics of spawning aggregations of common snook on the east coast of Florida. Marine Ecology Progress Series 505: 227–240. https://doi.org/10.3354/meps10774.
Young, J.M., B.G. Yeiser, E.R. Ault, J.A. Whittington, and J. Dutka-Gianelli. 2016. Spawning site fidelity, catchment, and dispersal of common snook along the east coast of Florida. Transactions of the American Fisheries Society 145 (2): 400–415. https://doi.org/10.1080/00028487.2015.1131741.
Acknowledgments
The authors thank R. Taylor, K. Johnson, W. Strong, T. Tuten, J. Kerns, S. Marsh, J. Young, A. Berry, B. Yeiser, K. Nault, N. Trippel, A. Dutterer, L. Bivens, J. Estes, D. Krause, J. Colvocoresses, J. Carroll, K. Cook, K. Rynerson, D. Westmark, and A. Brett, as well as countless volunteers, for the field support and R. Kiltie and C. Shea for the statistical analysis.
Funding
Funding for field sampling and data analysis was supported by the State of Florida Saltwater Fishing License sales, US Department of the Interior, US Fish and Wildlife Service, Federal Aid for Sport Fish Restoration, provided to the Florida Fish and Wildlife Commission and through US Fish and Wildlife Service Grant No F-127-R-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark S. Peterson
Rights and permissions
About this article
Cite this article
Trotter, A.A., Ritch, J.L., Nagid, E. et al. Using Geomorphology to Better Define Habitat Associations of a Large-Bodied Fish, Common Snook Centropomus undecimalis, in Coastal Rivers of Florida. Estuaries and Coasts 44, 627–642 (2021). https://doi.org/10.1007/s12237-020-00801-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00801-5