Abstract
Understanding the conditions that drive variation in recruitment of key estuarine species can be important for effective conservation and management of their populations. The Olympia oyster (Ostrea lurida) is native to the Pacific coast of North America and has been a target of conservation efforts, though relatively little information on larval recruitment exists across much of its range. This study examined the recruitment of Olympia oysters at biweekly to monthly intervals at four sites in northern San Francisco Bay from 2010 to 2015 (except 2013). Mean monthly temperatures warmed at all sites during the study, while winter (January–April) mean monthly salinity decreased significantly during a wet year (2011), but otherwise remained high as a result of a drought. A recurring peak in oyster recruitment was identified in mid-estuary, in conditions corresponding to a salinity range of 25–30 and >16 °C at the time of settlement (April–November). Higher average salinities and temperatures were positively correlated with greater peak recruitment. Interannual variation in the timing of favorable conditions for recruitment at each site appears to explain geographic and temporal variation in recruitment onset. Higher winter/spring salinities and warmer temperatures at the time of recruitment corresponded with earlier recruitment onset within individual sites. Across all sites, higher winter/spring salinities were also correlated with earlier onset and earlier peak recruitment. Lower winter salinities during 2011 also resulted in a downstream shift in the location of peak recruitment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variation in recruitment is a key factor determining the population dynamics and structure of many coastal marine and estuarine species, including sessile foundation species. This is especially true in rapidly changing and dynamic environments, such as estuaries, where many species of benthic invertebrates retain sufficient larvae within the estuary to maintain self-sustaining populations (e.g., Bousfield 1955; Bennett et al. 2002; Kunze et al. 2013). A greater understanding of factors governing larval recruitment in these systems would aid in modeling population dynamics, predicting responses to abiotic changes including climate change, and assisting managers with conservation and restoration efforts.
Numerous factors have been proposed to explain temporal and spatial variation in recruitment dynamics, including geographic or temporal differences in larval production, delivery, and settlement onshore. Variation in per-capita reproductive output is often controlled by parental exposure to environmental conditions (e.g., Leslie et al. 2005) and cues stimulating reproduction or larval release, such as temperature (e.g., Southward et al. 1995), phytoplankton (Starr et al. 1990), or turbidity (e.g., Gyory et al. 2013). Delivery of larvae to a specific geographic region may be affected by a range of physical oceanographic processes, such as differences in boundary currents around the lee of headlands (Graham and Largier 1997; Wing et al. 2003; Roughan et al. 2005; Morgan et al. 2012). Estuarine species face several challenges in maintaining populations, as some proportion of larvae produced inside an estuary must be retained, and larvae transiting the open ocean must find and return to an estuary for settlement (Ketchum 1954; Gaines and Bertness 1992). Larval behaviors such as vertical migration to take advantage of inflowing or outflowing tidal currents have been demonstrated for a number of estuarine species (Forward and Tankersley 2001), including Olympia oysters (Peteiro and Shanks 2015). Meanwhile, larval settlement from the water column to habitats onshore and subsequent microhabitat selection is stimulated by a variety of cues, which may be physical (tactile stimulus, changes in water flow) or chemical in nature (e.g., conspecific cues often serve as an attractant, while predator cues may repel settlers) (Crisp 1976; Pawlik 1992; Koehl and Hadfield 2010; Prairie et al. 2012).
Estuaries encompass strong environmental gradients that may impose temporal and geographical variation in physiological constraints on reproduction and recruitment of resident species (Remane and Schlieper 1971; Attrill 2002). These strong gradients are good candidates for potential drivers of recruitment peaks or areas where many larvae recruit in aggregate. The geographic location of these peaks and the timing, magnitude, and duration of recruitment are all potentially influenced by environmental gradients.
The Olympia oyster (Ostrea lurida) is a native bivalve inhabiting intertidal and shallow subtidal rocky shores in estuaries along the North American Pacific coast. Oyster beds support a diverse community of species, provide a variety of ecosystem services, and are a focus of conservation and restoration efforts (Kimbro and Grosholz 2006; Wasson et al. 2014). Relatively little is known about abundance patterns for most life stages of O. lurida (Peteiro and Shanks 2015). Recent field surveys and genetic analyses have established a current range for O. lurida as British Columbia–Baja California, where it approaches, but does not overlap, with its southern congener Ostrea conchaphila (Polson and Zacherl 2009; Raith et al. 2016). Reproduction is seasonal, with females brooding larvae for up to 2 weeks in the spring and summer (Strathmann 1987; Baker 1995). Upon release, larvae then spend 1–8 weeks in the water column before settling onto hard substrates; the sources of variation in these estimates remain unclear, but may include temperature, food availability, and latitude (Strathmann 1987; Baker 1995).
Spatial and temporal patterns of settlement are poorly documented across much of O. lurida’s range, especially in relation to environmental conditions (Pritchard et al. 2015). The seasonality and months during which O. lurida is known to settle have been reported for numerous locations throughout its geographic range (reviewed in Pritchard et al. 2015), but relatively few reports include information on physical environmental conditions such as temperature and salinity, and almost none have statistically examined the relationship between major environmental variables and settlement (but see Seale and Zacherl 2009). Using an experimental population of oysters from a non-estuarine outer coast site in southern California, Coe (1931) found that settlement commenced when water temperature reached 16 °C. Bonnot (1937) and Hopkins (1937) reported Olympia oyster settlement along with environmental parameters for Humboldt Bay and southern Puget Sound, respectively. While neither study examined these relationships quantitatively, Bonnot (1937) reported that peak settlement roughly corresponded to peak temperatures of 18–20 °C. Settlement patterns show no clear relationship with salinity at the time of settlement, although examination of Bonnot’s data suggests that lower winter salinity could be linked to delayed and reduced settlement (Bonnot 1937, Fig. 20). The only recent report on settlement in relation to temperature (Seale and Zacherl 2009) concluded that there was no threshold temperature corresponding with initiation or termination of settlement or with any settlement peaks. Meanwhile, although neither Bonnot (1937) nor Hopkins (1937) linked the onset of settlement to a specific temperature, both studies found that the onset of settlement followed tidal cycles to some degree, starting with a run of neap tides, with peak settlement during successive spring tides.
Significant spatial variation in recruitment and fecundity in San Francisco Bay populations of O. lurida was previously documented in several short-term studies, each lasting about 2 years (Wasson et al. 2014). Here, we examine trends in recruitment in 5 years (over a 6-year period) at four sites along the axis of the northern section of San Francisco Bay, which encompasses a substantial gradient in temperature and salinity. Prior to this study, little was known about the spatial or temporal variation in adult or juvenile oyster abundances along this gradient, although physical conditions at these sites spanned a range of values that seemed likely to influence oyster biology based on the literature (reviewed by Pritchard et al. 2015) and previous observations (Grosholz et al. 2007; Wasson et al. 2014). These sites also encompassed significant variation in oyster population density, including one site (China Camp State Park) that at times had both the densest population and densest recruitment in the entire species range (Wasson et al. 2014). We hypothesized that environmental conditions would be correlated with the magnitude and timing of peak recruitment of O. lurida in San Francisco Bay, as well as the onset of recruitment. Specifically, we hypothesized that both temperature and salinity at each site would be both positively correlated with the magnitude of peak recruitment and inversely correlated with the timing of the onset of recruitment and its peak. We modeled the correlations between environmental conditions and recruitment to determine whether there are specific conditions linked with high recruitment rates.
Methods
Recruitment
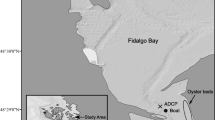
We assessed Olympia oyster recruitment at four sites along the axis of the northern brackish-to-saline region of San Francisco Bay, 2010–2015; the northernmost site (China Camp State Park) is within the San Francisco Bay National Estuarine Research Reserve (SF Bay NERR; Fig. 1; Table 1). A major salinity gradient is consistently present in this region of the estuary, which is sensitive to seasonal and inter-annual changes in weather, such as temperature and precipitation, presenting an ideal area for assessing the responses of the oyster population.
The San Francisco Bay region has a Mediterranean climate, with a dry summer/fall season (June–October) when there is almost no precipitation, and a wet winter/spring season (November–May), with great interannual variation in precipitation (Conomos 1979; Cloern and Jassby 2012). The major sources of freshwater entering the estuary are the Sacramento and San Joaquin Rivers, which join in an inland delta before passing through the Carquinez Strait into San Pablo Bay, which forms the upper portion of San Francisco Bay, and then exit through the Golden Gate. South of the Golden Gate, the South Bay ends in a lagoonal embayment. This geography results in two distinct subregions of the estuary: a river-dominated northern half, and a relatively low-inflow southern half. While the northern half of the estuary can become stratified during higher flow periods in the winters of wet years, the estuary is generally well-mixed in the summer (Conomos 1979). The estuary is highly modified, as previously marshy shorelines have been drained and filled, and over half of the mean annual freshwater flow is diverted for agriculture and drinking water (Conomos et al. 1985; Nichols et al. 1986).
We focused most intensely on the spring-to-fall period, when we expected O. lurida to settle most heavily based on preliminary data, but the duration of collection effort varied yearly due to changing availability of funding and human resources. We targeted significant rocky shoreline or riprap areas that serve as potential oyster habitat. At each site, we deployed two PVC frames consisting of a 1-m crosspiece and two 0.5-m legs staked into the sediment with rebar, so that the crosspiece was at 0 m, mean lower low water (MLLW) parallel to the waterline. On each frame, we attached three white porcelain 110-cm2 tiles to the crosspiece of the frame with the rough, unglazed sides facing down. The unfinished side acted as the collecting surface, because larvae of oysters and other sessile invertebrates preferentially settle on rougher surfaces (Crisp 1976) and O. lurida larvae specifically prefer downward-facing surfaces (Hopkins 1935; Bonnot 1937). We replaced the tiles with new ones ~biweekly 2010–2013 and monthly in 2014 and 2015. We omitted data from 2013 due to infrequent sampling. We counted the number of recruits on each tile using a dissecting microscope and calculated recruitment rates for each sampling period as the number of settlers per square meter per day. Thus, for the purposes of this study, we define recruitment as the settlement and post-settlement survival of oyster larvae during 2- or 4-week deployments.
In 2012 for logistical reasons, we moved the Loch Lomond site approximately 200 m to the south to a location with similar physical characteristics. Later, shoreline construction in early 2014 necessitated an additional move 200 m still further south to a partially sheltered cove.
Environmental Measurements and Data Preparation
We measured temperature every 15 min at each site using loggers (Tidbit and Tidbit v2, Onset Computer, Bourne, Massachusetts) that were attached either to the settlement frames or to an adjacent rebar stake. Loggers, like settlement tiles, were placed at 0 m MLLW. For each site, we processed logger data as daily mean values of air and water temperatures using the SiteParser program (Gilman et al. 2006). We calculated daily mean salinity values for each site using data from nearby continuous water-quality monitoring stations that were maintained by the US Geological Survey (USGS 2015) and the SF Bay NERR (NERR 2015), and compared these daily means with measurements that were taken during biweekly or monthly site visits using a YSI-85 multimeter (YSI, Yellow Springs, Ohio; Online Resource 1). We summarized these mean daily temperature and salinity values as biweekly (or monthly) means during the corresponding settlement tile deployments for each site.
The temperature data record (Table 1) included some gaps due to logger failure. In these cases, we filled in gaps using data from nearby continuous water quality monitoring stations (NERR 2015; USGS 2015). To accurately estimate missing intertidal logger data, we regressed existing logger data against continuous monitoring data from overlapping time periods where data from both sources were available, and we used the resulting model to correct continuous monitoring data for periods corresponding to the missing logger data (see Online Resource 1).
Statistical Analyses
We assessed trends in temperature and salinity across sites and years using linear mixed models. For temperature, we compared mean monthly temperature across sites and years with month specified as a random factor nested within site to account for autocorrelation. For salinity, we compared mean winter/spring (February–May) salinity levels across sites and years, again with month as a random factor nested within site. Analyses were conducted in the R statistical programming environment (R Core Team 2015) using the package lme4 (Bates et al. 2015).
We analyzed the relationship between recruitment and conditions at the time of recruitment using generalized linear mixed models (GLMM) with negative binomial distributions with logit links. The response variable was oyster recruitment rate standardized as number of individuals per square meter per day for a given biweekly or monthly time period. Mean water temperature and salinity for the given biweekly or monthly time period, and their interaction, were considered predictors. We used sampling date as a random factor nested within site to account for repeated measures within each site, and we used year of sampling as a blocking factor. Models were evaluated based on Akaike Information Criteria (AIC). Analyses were conducted in R using the package glmmADMB (Bolker et al. 2013; Skaug et al. 2015), an implementation of the AD Model Builder fitting engine (Fournier et al. 2012).
We examined the relationship between environmental conditions and both the timing of the onset of recruitment and time to peak recruitment rate using ordinary least squares (OLS) regression. When there were two statistically indistinguishable peaks at a site in a given year (e.g., China Camp, 2012: t 2.98 = −0.58, p = 0.60), we used the earlier peak in our analysis. As above, we assessed conditions at the time of recruitment, but because recruitment may be influenced by severe environmental disturbances that cause adult mortality and may suppress reproduction, we also tested the effect of winter/spring low salinity, the most severe such stressor believed to affect San Francisco Bay oysters (Wasson et al. 2014). The response variables were the number of days since 1st January to the retrieval date of tiles marking either recruitment onset or peak recruitment rate. The predictors were mean daily water temperature and salinity for the biweekly or monthly time period corresponding to either onset or peak recruitment at each site, along with their interaction (all at the time of recruitment onset or peak recruitment). In a separate set of models, we evaluated mean daily salinity during the winter/spring season (February–May) as a predictor of the number of days to the onset of recruitment or peak recruitment. For all models, before adding site as a blocking factor, we first considered timing of recruitment across all four sites to examine the relationships between the predictors and recruitment timing within each site. Lastly, we evaluated the relationship of environmental variables to the location of earliest recruitment, and the highest peak recruitment within each year.
Results
Environmental Conditions
Physical conditions varied significantly among sites both within and across years (Figs. 2 and 3; Online Resources 2 and 3). Across all sites, in most months, mean daily water temperature increased over the years from 2010 to 2015 (Table 2; Fig. 3; Online Resource 3). The average observed increase was highest during late spring to early fall (May–October), and lowest in the winter months (December–February). Mean daily winter/spring salinity varied significantly among sites (Table 3) and was consistently lowest at China Camp, which is farthest upstream in the estuary, and highest at Brickyard Park, which is farthest downstream (Fig. 2; Online Resource 2). Mean daily winter/spring salinity also varied among years (Table 3), as did the salinity ranges among sites. The greatest spread in mean daily winter/spring salinity was in 2011, which was a wetter year, and with lower salinities occurring farther downstream than in other years (Fig. 2; Online Resource 2). During a record drought (2013–2015), the salinity gradient shifted upstream, and salinities were more similar among sites (Fig. 2; Online Resource 2).
Boxplots of reconstructed mean daily salinity by month at four study sites in San Francisco Bay, 2010–2015 (except 2013). Box represents interquartile range (25th–75th percentile), horizontal line inside box represents median, whiskers extend to minimum and maximum values, up to 1.5 times the interquartile range, beyond which outliers are marked with circles. See Online Resource 1 for derivation of site-specific salinities from nearby continuous monitoring stations. Analysis of winter–spring (February–May) trends is summarized in Table 3
Boxplots of mean daily temperature by month at four study sites in San Francisco Bay, 2010–2015 (except 2013). Box represents interquartile range (25th–75th percentile), horizontal line inside box represents median, whiskers extend to minimum and maximum values, up to 1.5 times the interquartile range, beyond which outliers are marked with circles. Analysis of trends across years is summarized in Table 2
Magnitude of Recruitment
Within each year, we observed significant geographic variation in the magnitude of recruitment rates, with distinct peaks appearing each year (Fig. 4). Across all years, China Camp generally had the highest recruitment rates, reaching a maximum of 907.8 ind. m−2 day−1 in 2014, but there was some variation in which site had the highest (peak) recruitment rate, with slightly higher rates at Point San Quentin in 2010 and Loch Lomond in 2011 (Table 1; Fig. 4). Brickyard Park consistently had the lowest peak recruitment rates, with a maximum of 54.6 ind. m−2 day−1 in 2014. There was no significant difference in recruitment at Loch Lomond before (2010, 2011, 2012) and after (2014, 2015) the site was moved to a partially sheltered cove (Online Resource 4). The previous winter’s freshwater flow to the estuary did not predict the locations of either the earliest recruitment or the recruitment peak.
The magnitude of peak recruitment was correlated with mean temperature and salinity conditions at each site, with a significant interaction between mean temperature and mean salinity (Tables 4 and 5). Warmer temperatures and higher salinities correlated strongly with higher recruitment rates, regardless of site (Figs. 5 and 6).
Oyster recruitment rate by month as a function of salinity and temperature at four study sites in San Francisco Bay, 2010–2015 (except 2013). Points are located at the mean daily temperature and salinity that occurred at each site in a given month and are sized in proportion to the rate of recruitment observed. Open circles indicate zero recruitment. Absence of points indicates no data available, except for Loch Lomond and China Camp from March and April 2011 (not shown; salinity <<15, zero recruitment)
Oyster recruitment rates as a function of salinity and temperature at the time of recruitment at four sites in San Francisco Bay, 2010–2015 (except 2013). Points are located at the mean daily temperature and salinity that occurred at each site in a given month and are sized in proportion to the rate of recruitment observed. Open circles indicate zero recruitment
Timing of Recruitment
The onset of recruitment occurred earlier each year across all sites, advancing from late June/early July in 2010 to April by 2015 (Fig. 5). When considering all four sites together, first recruitment occurred significantly earlier in years when mean daily winter salinities were higher (F 1, 9 = 9.264, p = 0.014). Peak recruitment across all sites also occurred significantly earlier following higher mean daily salinities during the preceding winter (F 1, 4 = 25.297, p = 0.0007) (Tables 6 and 8). Mean water temperature and salinity conditions during recruitment were not correlated with timing of first recruitment across all sites (F 1, 6 = 0.156, p = 0.707 for temperature; F 1, 6 = 2.762, p = 0.148 for salinity). Nor were conditions during recruitment correlated with timing of peak recruitment (F 1, 2 = 0.407, p = 0.589 for salinity), although there was a positive correlation with mean daily temperature (F 1, 2 = 16.176, p = 0.0566). Recruitment onset was earlier in later years despite the switch from biweekly to monthly tile deployment periods for 2014–2015, which would tend to bias toward later observed onset of recruitment because the longer deployment times result in later retrieval dates.
The timing of the arrival of warmer temperatures (>16 °C) and more saline conditions (>25) that we linked to increased recruitment in the spring and summer varied from year to year at a given site (Fig. 5). In 2010, these conditions began in June, but especially July, which coincided with the onset of recruitment. In 2011, a wetter year, these conditions did not occur until August. In 2014 and 2015, these conditions appear earlier, by April (Fig. 5). Within each site, the onset of recruitment was positively correlated with the arrival of warmer water temperatures (F 1, 8 = 12.255, p = 0.0081), but there was no relationship with salinities at the time of recruitment (F 1, 8 = 2.662, p = 0.141) (Tables 7 and 8; Fig. 7). However, the onset of recruitment was inversely related to the previous winter’s salinity regime (F 1, 16 = 8.749, p = 0.0093), as well as to site (F 3, 16 = 10.552, p = 0.0004) (Tables 7 and 8; Fig. 8). Thus, interannual variation in the timing of favorable conditions for recruitment at each site appears to explain geographic and temporal variation in recruitment onset. In contrast, within each site, none of the environmental variables was significantly correlated with the timing of peak recruitment (F 1, 7 = 0.041, p = 0.845 for temperature at recruitment, F 1, 7 = 1.129, p = 0.323 for salinity at recruitment, F 1, 16 = 2.183, p = 0.159 for previous winter/spring mean daily salinity).
Timing of first recruitment (i.e., onset) at four study sites in San Francisco Bay, 2010–2015 (except 2013) is predicted by average temperature (but not salinity) conditions at the time of recruitment. See Table 7 for linear model results. The last two digits of each year are used to represent the day of first recruitment at each site in that year, and the mean daily salinity (left) or temperature (right) is shown for the corresponding the biweekly or monthly tile deployment when recruitment was first detected. Dashed horizontal lines represent mean daily salinity or temperature ±1 SD. See Online Resource 1 for derivation of site-specific salinities
Timing of first recruitment (i.e., onset) at four study sites in San Francisco Bay, 2010–2015 (except 2013) is predicted by the previous winter/spring (February–May) salinity. The last two digits of each year are used to represent the day of first recruitment at each site in that year, and the corresponding mean daily winter/spring salinity. Mean daily winter/spring salinity levels at each site are inversely related to the number of days to first recruitment in the same calendar year (Table 7). Dashed horizontal lines represent mean daily salinity ±1 SD. See Online Resource 1 for derivation of site-specific salinities
Discussion
Predicting peaks in recruitment is key for conservation and restoration of Olympia oysters because these efforts rely on natural spatset in many areas, either in siting construction of restoration structures or for collection of natural spat used to seed other locations (Wasson et al. 2014; Pritchard et al. 2015). Our results suggest that peak recruitment for Olympia oysters occurs in predictable temperature and salinity conditions each year (>16 °C and salinity 25–30) and that continued monitoring of those conditions would enable forecasts of the timing, location, and magnitude of peak recruitment. In addition, the geographic location of the recruitment peak along the estuarine gradient in northern San Francisco Bay was broadly predicted by the winter/spring salinity regime (i.e., winter/spring freshwater input to the estuary). These results confirmed our hypotheses of positive relationships between temperature and salinity conditions and the magnitude of oyster recruitment at each site. Earlier onset of recruitment was correlated with warmer temperatures and higher mean daily winter/spring salinity. Considering all sites together, higher winter/spring salinity was also correlated with earlier peak recruitment, but contrary to our hypothesis, peak recruitment was not predicted by either temperature or salinity at the time of recruitment.
Because the hard substrate required for larval oyster settlement is in limited supply in many regions, restoration efforts frequently rely on spreading shell or installing artificial structures such as oyster “reef balls” to provide greater substrate area (Wasson et al. 2014). These efforts must be timed so as to capture the settlement of oysters, and if possible, to avoid the preemptive settlement of other species such as barnacles or tubeworms that may compete for space (e.g., Trimble et al. 2009; Wasson et al. 2014; Pritchard et al. 2015). Our results suggest that potential oyster recruitment could be maximized by timing the deployment of restoration structures to occur when conditions reach 16 °C and 25–30 salinity, and by adjusting timing and location of substrate deployment based on winter/spring salinity conditions. These results are a significant advance in understanding the population biology of Olympia oysters and will allow oyster conservation and restoration to take maximum advantage of the natural cycles of resident populations.
Over the 6-year study period, the optimal conditions for recruitment occurred at radically different times—even in different seasons—due to significant interannual variation in environmental conditions linked to larger-scale climate patterns and regional hydrological cycles. By 2015, we observed oyster recruitment several months earlier than in the first 2 years of the study, which was likely a result of record drought conditions that led to generally warmer and drier conditions across western North America (Diffenbaugh et al. 2015; Swain 2015). Meanwhile, during the wetter year (2011), higher freshwater outflow from storms and snowmelt resulted in the persistence of slightly cooler temperatures throughout the spring. However, the key factor in delayed settlement in this year appeared to be salinity levels that were <25 until July 2011 (Fig. 2). The wetter spring also caused significant oyster mortality, especially at China Camp, which previously had the densest oyster population in the entire species range, and five times greater than the next most densely populated site in San Francisco Bay (Wasson et al. 2014). This drastic reduction in adult populations likely resulted in substantially lower larval output, which would reduce available larvae for settlement; if the populations at our sites received larvae from these areas, then the magnitude of subsequent recruitment onshore would be reduced. Peak recruitment in 2011 was approximately half as much as in 2010.
Within years, recruitment fluctuated from tidal cycle to tidal cycle, likely reflecting the ebb and flow of reproductive activity and larval release by adults (Coe 1931; Hopkins 1936; Bonnot 1937). At a monthly scale, these biweekly fluctuations tend to even out, presenting the appearance of a steady increase in recruitment during spring and summer toward a peak in early fall (September–October), followed by a gradual decline in winter (November–December) (Figs. 4 and 5). The finer-scale variations in recruitment visible at the resolution of biweekly tidal cycles suggest an approximately monthly schedule of larval release among adults, consistent with findings by Bonnot (1937) and Hopkins (1937) that the onset of settlement follows tidal cycles to some degree, starting with a run of neap tides and followed by peak settlement during successive runs of spring tides (Bonnot 1937; Hopkins 1937). Further investigation should examine the influence of tidal cycles on settlement relative to the temperature and salinity effects documented here.
In addition to temperature and salinity, a number of other factors may play a role in driving the observed patterns of oyster recruitment, such as physical aggregation of larvae by currents, shifts in reproductive patterns, and variation in cues and habitat availability. Current-driven aggregations of larvae in the lee of headlands are well documented for several marine invertebrate species, leading to higher recruitment on the open coast (Graham and Largier 1997; Roughan et al. 2005; Mace and Morgan 2006; Morgan et al. 2011). In San Francisco Bay, the Loch Lomond and Point San Quentin sites are both in the lee of headlands relative to the prevailing river flow exiting the estuary, whereas China Camp and Brickyard Park are not (Fig. 1), offering only equivocal support for the importance of leeward aggregations to settlement patterns in an estuarine setting. In a previous shorter study encompassing the entire estuary, we observed much greater oyster recruitment on the western than eastern shore of San Francisco Bay. However, the timing and location of greatest recruitment along the axis of the estuary on the eastern shore followed a similar pattern to that documented in this study for the western shore (Chang et al., unpublished data). The prevailing summer winds blow to the southwest, driving surface and subsurface waters against the western shore of the estuary (Conomos 1979), which could deliver more larvae to the western shore.
Water column conditions also affect reproductive output, which can indirectly affect patterns of settlement by influencing larval supply (Carson 2010; Pritchard et al. 2015). In a previous work, we have found that sites exposed to warmer conditions for longer periods of time have a greater proportion of the oyster population reproducing earlier (Chang et al., unpublished data). These patterns, in turn, may influence local settlement if more larvae are retained in close proximity to the source. Habitat availability and settlement cues also influence larval settlement patterns via larval behaviors that affect substrate selection (e.g., Fuchs and Reidenbach 2013 for Crassostrea virginica), though little is known about these factors in O. lurida (Pritchard et al. 2015).
Across broad latitudinal gradients, mussel and barnacle recruitment are related to regional differences in sea surface temperature (Broitman et al. 2008), producing regional peaks in recruitment for several invertebrate species. These peaks span several orders of magnitude that track temporal and spatial shifts in oceanographic conditions spanning >1000 km of the Pacific coast (Broitman et al. 2008). Here, we documented comparable variation in recruitment rate tied to physical oceanographic conditions on a much smaller scale of just 10 km inside northern San Francisco Bay. Larvae can be retained in the upper, middle, or lower estuary depending on the species (Carriker 1951; Newman 1953; Bousfield 1955; Kunze et al. 2013). Physical oceanographic patterns may also influence both reproduction and recruitment more indirectly by producing good food conditions (Leslie et al. 2005), and further work should examine this possibility for Olympia oysters.
Changing climate regimes and water management policies will likely shift the focal points for settlement peaks such as the ones documented here, with numerous implications for populations and communities (Harley et al. 2006; Cloern and Jassby 2012) as well as for conservation and restoration programs. The regional climate in northern California is predicted to shift toward warmer, drier conditions (Cloern et al. 2011; Cloern and Jassby 2012) with modest changes in mean precipitation (Neelin et al. 2013; Seager et al. 2015) and an increase in the frequency of both very dry and very wet years (Das et al. 2013; Berg and Hall 2015; Yoon et al. 2015). The Olympia oyster population’s center of distribution in San Francisco Bay and other estuaries may move upstream as warmer and drier (and consequently more saline) conditions shift settlement peaks upstream and earlier in the year, on average. Conversely, in wetter years, settlement peaks are likely to shift downstream. The modestly wetter winter of 2010–2011 led to near complete mortality of adult populations at China Camp, and the center of recruitment shifted downstream to Loch Lomond; greater shifts would be expected in response to more extreme wet years. The detection and tracking of such peaks is important for effective management and conservation strategies in the face of continuing anthropogenically driven climate change, particularly as such strategies often rely on natural spatset (Wasson et al. 2014), and because large-scale control of hydrological factors is infeasible in larger estuaries. Although warming appears to lengthen the recruitment season, creating the opportunity for greater accumulation of individuals onshore, these effects occurred most strongly at sites furthest upstream (e.g., China Camp) that are also vulnerable to mass die-offs in wetter years (Wasson et al. 2014). The net implications for the population dynamics of O. lurida remain to be explored. Detailed information on occurrence patterns for different life history stages will enable more comprehensive modeling of likely changes to the population dynamics of Olympia oysters and many other estuarine species.
Long-term, standardized sampling programs, such those espoused by the NERR System, including the NERR System Wide Monitoring Program (SWMP) (Buskey et al. 2015), are critical to detecting spatial and temporal environmental and ecological variation that might otherwise appear random. Salinity and temperature are relatively easily assessed by researchers and managers and are monitored continuously by every NERR SWMP water quality station, as well as by similar stations run by USGS and other organizations. A network of such stations enables broad coverage of regions important to conservation and management, but even highly studied regions like San Francisco Bay have limited coverage in some areas, and would benefit from an expansion of existing monitoring networks.
Studies in which detailed, fine-grained measurements of environmental conditions are coupled with measurements of recruitment across a spatial gradient remain relatively rare. With more systematic studies such as this one, we are likely to see greater evidence for predictable recruitment patterns that map across spatial gradients in accordance with environmental conditions. This knowledge, in turn, will enable a more detailed understanding of the impacts of large-scale environmental changes, including water management regimes and global climate.
References
Attrill, M. 2002. A testable linear model for diversity trends in estuaries. Journal of Animal Ecology 71: 262–269.
Baker, P. 1995. Review of ecology and fishery of the Olympia oyster, Ostrea lurida with annotated bibliography. Journal of Shellfish Research 14: 501–518.
Bates, D., Maechler, M., Bolker, B., and S. Walker. 2015. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-8. Available at: http://CRAN.R-project.org/package=lme4>.
Bennett, W.A., W.J. Kimmerer, and J.R. Burau. 2002. Plasticity in vertical migration by native and exotic estuarine fishes in a dynamic low-salinity zone. Limnology and Oceanography 47: 1496–1507.
Berg, N., and A. Hall. 2015. Increased interannual precipitation extremes over California under climate change. Journal of Climate 28: 6324–6334.
Bolker, B.M., B. Gardner, M. Maunder, C.W. Berg, M. Brooks, L. Comita, E. Crone, S. Cubaynes, T. Davies, P. de Valpine, J. Ford, O. Gimenez, M. Kéry, E.J. Kim, C. Lennert-Cody, A. Magnusson, S. Martell, J. Nash, A. Nielsen, J. Regetz, H. Skaug, and E. Zipkin. 2013. Strategies for fitting nonlinear ecological models in R, AD model builder, and BUGS. Methods in Ecology and Evolution 4: 501–512.
Bonnot, P. 1937. Report on the California oyster industry for 1937. California Fish and Game, 42.
Bousfield, E.L. 1955. Ecological control of the occurrence of barnacles in the Miramichi estuary. Bulletin of the National Museum of Canada 137: 1–69.
Broitman, B.R., C.A. Blanchette, B.A. Menge, J. Lubchenco, C. Krenz, M. Foley, P.T. Raimondi, D. Lohse, and S.D. Gaines. 2008. Spatial and temporal patterns of invertebrate recruitment along the west coast of the United States. Ecological Monographs 78: 403–421.
Buskey, E.J., M. Bundy, M. Ferner, D. Porter, W. Reay, E. Smith, and D. Trueblood. 2015. System-wide monitoring program of the National Estuarine Research Reserve system: research and monitoring to address coastal management issues. In Coastal Ocean observing systems: advances and syntheses, ed. Y. Liu, H. Kerkering, and R.H. Weisberg, 392–414. The Netherlands: Elsevier.
Carriker, M.R. 1951. Ecological observations on the distribution of oyster larvae in New Jersey estuaries. Ecological Monographs 21: 19–38.
Carson, H.S. 2010. Population connectivity of the Olympia oyster in southern California. Limnology and Oceanography 55: 134–148.
Cloern, J.E., and A.D. Jassby. 2012. Drivers of change in estuarine-coastal ecosystems: discoveries from four decades of study in San Francisco Bay. Reviews in Geophysics 50: RG4001.
Cloern, J.E., N. Knowles, L.R. Brown, D. Cayan, M.D. Dettinger, T.L. Morgan, D.H. Schoellhamer, M.T. Stacey, M. van der Wegen, R.W. Wagner, and A.D. Jassby. 2011. Projected evolution of California’s San Francisco Bay-Delta-river system in a century of climate change. PloS One 6: e24465.
Coe, W.R. 1931. Development of the organs and the sequence of the sexual phases in the California oyster (Ostrea lurida). Bulletin of Shellfish 3: 119–139.
Conomos, T.J. 1979. Properties and circulation of San Francisco Bay waters. In San Francisco Bay: the urbanized estuary, ed. T.J. Conomos, 47–84. San Francisco, California: Pacific Division of the American Association for the Advancement of Science.
Conomos, T.J., R.E. Smith, and J.W. Gartner. 1985. Environmental setting of San Francisco Bay. Hydrobiologia 129: 1–12.
Crisp, D.J. 1976. Settlement responses in marine organisms. In Adaptation to environment: essays on the physiology of marine animals, ed. R.C. Newell, 83–124. London: Butterworths.
Das, T., E.P. Maurer, D.W. Pierce, M.D. Dettinger, and D.R. Cayan. 2013. Increases in flood magnitudes in California under warming climates. Journal of Hydrology 501: 101–110.
Diffenbaugh, N.S., D.L. Swain, and D. Touma. 2015. Anthropogenic warming has increased drought risk in California. Proceedings of the National Academy of Sciences of the United States of America 112: 3931–3936.
Forward, R.B. Jr., and R.A. Tankersley. 2001. Selective tidal-stream transport of marine animals. In Oceanography and marine biology, ed. R.B. Gibson, M. Barnes, and R.J.A. Atkinson, 305–353. London: Taylor & Francis.
Fournier, D.A., H.J. Skaug, J. Ancheta, J. Ianelli, A. Magnusson, M.N. Maunder, A. Nielsen, and J. Sibert. 2012. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software 27: 233–249.
Fuchs, H.L., and M.A. Reidenbach. 2013. Biophysical constraints on optimal patch lengths for settlement of a reef-building bivalve. PloS One 8: e71506. doi:10.1371/journal.pone.0071506.
Gaines, S.D., and M.D. Bertness. 1992. Dispersal of juveniles and variable recruitment in sessile marine species. Nature 360: 579–580.
Gilman, S.E., C.D. Harley, D.C. Strickland, O. Vanderstraeten, M.J. O'Donnell, and B. Helmuth. 2006. Evaluation of effective shore level as a method of characterizing intertidal wave exposure regimes. Limnology and Oceanography: Methods 4: 448–457.
Graham, W.M., and J. Largier. 1997. Upwelling shadows as nearshore retention sites: the example of northern Monterey Bay. Continental Shelf Research 17: 509–532.
Grosholz, E., Moore, J., Zabin, C., Attoe, S. and Obernolte, R. 2007. Planning for native oyster restoration in San Francisco Bay. Final Report to California Coastal Conservancy. 40 pp.
Gyory, J., J. Pineda, and A. Solow. 2013. Turbidity triggers larval release by the intertidal barnacle Semibalanus balanoides. Marine Ecology Progress Series 476: 141–151.
Harley, C.D., A. Randall Hughes, K.M. Hultgren, B.G. Miner, C.J. Sorte, C.S. Thornber, L.F. Rodriguez, L. Tomanek, and S.L. Williams. 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9: 228–241.
Hopkins, A.E. 1935. Attachment of larvae of the Olympia oyster, Ostrea lurida, to plane surfaces. Ecology 16: 82–87.
Hopkins, A.E. 1936. Ecological observations on spawning and early larval development in the Olympia oyster (Ostrea lurida). Ecology 17: 551–566.
Hopkins, A.E. 1937. Experimental observations on spawning, larval development, and setting in the Olympia oyster Ostrea lurida. Bulletin of the U.S. Bureau of Fisheries 48: 438–503.
Ketchum, B.H. 1954. Relation between circulation and planktonic populations in estuaries. Ecology 35: 191–200.
Kimbro, D.L., and E.D. Grosholz. 2006. Disturbance influences oyster community richness and evenness, but not diversity. Ecology 87: 2378–2388.
Koehl, M.A., and M.G. Hadfield. 2010. Hydrodynamics of larval settlement from a larva's point of view. Integrative and Comparative Biology 50: 539–551.
Kunze, H.B., S.G. Morgan, and K.M.M. Lwiza. 2013. A field test of the behavioral regulation of larval transport. Marine Ecology Progress Series 487: 71–87.
Leslie, H.M., E.N. Breck, F. Chan, J. Lubchenco, and B.A. Menge. 2005. Barnacle reproductive hotspots linked to nearshore ocean conditions. Proceedings of the National Academy of Sciences of the United States of America 102: 10534–10539.
Mace, A.J., and S.G. Morgan. 2006. Larval accumulation in the lee of a small headland: implications for the design of marine reserves. Marine Ecology Progress Series 318: 19–29.
Morgan, S.G., J.L. Fisher, and J.L. Largier. 2011. Larval retention, entrainment, and accumulation in the lee of a small headland: recruitment hotspots along windy coasts. Limnology and Oceanography 56: 161–178.
Morgan, S.G., J.L. Fisher, S.T. McAfee, J.L. Largier, and C.M. Halle. 2012. Limited recruitment during relaxation events: larval advection and behavior in an upwelling system. Limnology and Oceanography 57: 457–470.
National Estuarine Research Reserve System. 2015. System-wide monitoring program. Available at: http://www.nerrsdata.org/. Accessed 1 Dec 2015.
Neelin, J.D., B. Langenbrunner, J.E. Meyerson, A. Hall, and N. Berg. 2013. California winter precipitation change under global warming in the coupled model Intercomparison project phase 5 ensemble. Journal of Climate 26: 6238–6256.
Newman, W.A. 1953. Some ecological considerations on Barnacles of the San Francisco Bay Estuarine System. MA Thesis. University of California, Berkeley, California.
Nichols, F.H., J.E. Cloern, S.N. Luoma, and D.H. Peterson. 1986. The modification of an estuary. Science 231: 567–573.
Pawlik, J.R. 1992. Chemical ecology of the settlement of benthic marine invertebrates. Oceanography and Marine Biology: An Annual Review 30: 273–335.
Peteiro, L., and A. Shanks. 2015. Up and down or how to stay in the bay: retentive strategies of Olympia oyster larvae in a shallow estuary. Marine Ecology Progress Series 530: 103–117.
Polson, M.P., and D.C. Zacherl. 2009. Geographic distribution and intertidal population status for the Olympia oyster, Ostrea lurida carpenter 1864, from Alaska to Baja. Journal of Shellfish Research 28: 69–77.
Prairie, J.C., K.R. Sutherland, K.J. Nickols, and A.M. Kaltenberg. 2012. Biophysical interactions in the plankton: a cross-scale review. Limnology and Oceanography: Fluids and Environments 2: 121–145.
Pritchard, C., A. Shanks, R. Rimler, M. Oates, and S. Rumrill. 2015. The Olympia oyster Ostrea lurida: recent advances in natural history, ecology, and restoration. Journal of Shellfish Research 34: 259–271.
R Core Team. 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available at: http://www.r-project.org/.
Raith, M., D.C. Zacherl, E.M. Pilgrim, and D.J. Eernisse. 2016. Phylogeny and species diversity of gulf of California oysters (Ostreidae) inferred from mitochondrial DNA. American Malacological Bulletin 33: 263–283.
Remane, A., and C. Schlieper. 1971. Biology of brackish water. Stuttgart: Schweizerbart'sche Verlagsbuchhandlung.
Roughan, M., A.J. Mace, J.L. Largier, S.G. Morgan, J.L. Fisher, and M.L. Carter. 2005. Subsurface recirculation and larval retention in the lee of a small headland: a variation on the upwelling shadow theme. Journal of Geophysical Research 110: C10027. doi:10.1029/2005JC002898.
Seager, R., M. Hoerling, S. Schubert, H. Wang, B. Lyon, A. Kumar, J. Nakamura, and N. Henderson. 2015. Causes of the 2011–14 California drought. Journal of Climate 28: 6997–7024.
Seale, E.M., and D.C. Zacherl. 2009. Seasonal settlement of Olympia oyster larvae, Ostrea lurida carpenter 1864 and its relationship to seawater temperature in two southern California estuaries. Journal of Shellfish Research 28: 113–120.
Skaug, H., Fournier, D., Bolker, B., Magnusson, A. and A. Nielsen. 2015. Generalized Linear Mixed Models using AD Model Builder. R package version 0.8.0. Available at: http://CRAN.R-project.org/package=vegan.
Southward, A.J., S.J. Hawkins, and M.T. Burrows. 1995. Seventy years' observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. Journal of Thermal Biology 20: 127–155.
Starr, M., J.H. Himmelman, and J.C. Therriault. 1990. Direct coupling of marine invertebrate spawning with phytoplankton blooms. Science 247: 1071–1074.
Strathmann, M.F. 1987. Reproduction and development of marine invertebrates of the northern Pacific coast: data and methods for the study of eggs, embryos, and larvae. University of Washington Press.
Swain, D.L. 2015. A tale of two California droughts: lessons amidst record warmth and dryness in a region of complex physical and human geography. Geophysical Research Letters 42. doi:10.1002/2015GL066628.
Trimble, A.C., J.L. Ruesink, and B.R. Dumbauld. 2009. Factors preventing the recovery of a historically overexploited shellfish species, Ostrea lurida carpenter 1864. Journal of Shellfish Research 28: 97–106.
United States Geological Survey. 2015. Water quality data for San Francisco Bay. Available at: http://waterdata.usgs.gov/. Accessed 1 Dec 2015.
Wasson, K., Zabin, C., Bible, J., Ceballos, E., Chang, A., Cheng, B., Deck, A., Grosholz, E., Latta, M. and M. Ferner. 2014. A guide to Olympia oyster restoration and conservation: environmental conditions and sites that support sustainable populations in Central California. San Francisco Bay National Estuarine Research Reserve.
Wing, S.R., L. Botsford, L.E. Morgan, J.M. Diehl, and C.J. Lundquist. 2003. Inter-annual variability in larval supply to populations of three invertebrate taxa in the northern California current. Estuarine, Coastal and Shelf Science 57: 859–872.
Yoon, J.H., S.Y. Wang, R.R. Gillies, B. Kravitz, L. Hipps, and P.J. Rasch. 2015. Increasing water cycle extremes in California and in relation to ENSO cycle under global warming. Nature Communications 6: 8657.
Acknowledgments
This work was supported by postdoctoral fellowships to ALC from the CALFED Bay-Delta Authority (R/SF-33) and the Smithsonian Institution, the National Estuarine Research Reserve System Science Collaborative (NOAA grant no. NA09NOS4190153 to MCF), and an award under the Federal Coastal Zone Management Act, administered by the National Oceanic and Atmospheric Administration’s Office for Coastal Management to San Francisco State University for operation of the San Francisco Bay National Estuarine Research Reserve. The authors would like to express sincere gratitude to the Dominican University of California (San Rafael, CA) Invertebrate Zoology (BIO3150, Fall 2011) and Aquatic Ecosystems (HONO3200, Spring 2012, 2013, 2014, and 2016) classes taught by LJS for their devoted counting of oyster recruitment tiles.
Author Contributions
ALC, SGM, and MCF obtained funding; ALC, AKD, SGM, and MCF designed the research; ALC, AKD, LJS, and MCF performed the work; ALC analyzed the data; and ALC, AKD, LJS, SGM, and MCF wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judy Grassle
Electronic Supplementary Material
ESM 1
(DOCX 567 kb)
Rights and permissions
About this article
Cite this article
Chang, A.L., Deck, A.K., Sullivan, L.J. et al. Upstream—Downstream Shifts in Peak Recruitment of the Native Olympia Oyster in San Francisco Bay During Wet and Dry Years. Estuaries and Coasts 41, 65–78 (2018). https://doi.org/10.1007/s12237-016-0182-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0182-1