Abstract
Due to the unpredictable nature of intense storms and logistical constraints of sampling during storms, little is known about their immediate and long-term impacts on water quality in adjacent aquatic ecosystems. By combining targeted experiments with routine monitoring, we evaluated immediate impacts of two successive storm events on water quality and phytoplankton community response in the tidal James River and compared these findings to a non-storm year. The James River is a subestuary of the Chesapeake Bay and sampling was conducted before, during, and after Hurricane Irene and Tropical Storm (TS) Lee in 2011 and during the same time period (late summer/early fall) in 2012 when there were no storms. We collected and compiled data on nutrient and chlorophyll a concentrations, phytoplankton abundance, nitrogen uptake, primary productivity rates, and surface salinity, temperature, and turbidity in the meso- and polyhaline segments of the James River. Hurricane Irene introduced significant amounts of freshwater over the entire James River and Chesapeake Bay watersheds, while rainfall from TS Lee fell primarily on the tidal fresh region of the James River and headwaters of the Chesapeake Bay. Dinoflagellates dominated the algal community in the meso- and polyhaline segments prior to the storms in 2011, and a mixed diatom community emerged after the storms. In the mesohaline river segment, cyanobacteria abundance increased after TS Lee when salinities were depressed, likely due to washout from the oligohaline and tidal fresh regions of the river. In 2012, dinoflagellates dominated the community in both segments of the river during late summer but diatoms were also abundant and their biomass fluctuated throughout the summer and fall. Cyanobacteria were not present in either segment. Overall, we observed that the high-intensity rainfall from Hurricane Irene combined with high flushing in the headwaters as a result of TS Lee likely reduced primary productivity and altered community composition in the mesohaline segment but not the more estuarine-influenced polyhaline segment. Understanding the influence of high freshwater flow with a short residence time associated with storms is key to the planning and management of estuarine restoration as such disturbances are projected to increase as a result of climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Storms exert tremendous impacts on estuaries and coastal ecosystems through the introduction of freshwater and nutrients associated with runoff and wet atmospheric deposition (Paerl et al. 2001; Howarth et al. 2006; Dwight et al. 2011). In coastal regions, intense rainfall causes increased flow through the watershed as well as more localized impacts from runoff due to inundation and coastal flooding (Lin et al. 2010). Winds associated with storms can also exert substantial impacts through the resuspension of organic-rich, nutrient-laden sediments in shallow systems such as the Chesapeake Bay estuarine complex. Along the east coast of the USA, environmental impacts from large tropical and extra-tropical storms on tidal estuaries vary depending on the amount of rainfall, wind speed and direction, water depth, storm surge, storm intensity, ground-saturation, and adjacent land use (e.g., urban vs. rural) (Greening et al. 2006; Mallin and Corbett 2006). Impacts of these storms on ecological systems are difficult to compare across landscapes because extra-tropical and tropical storms (including hurricanes) are sporadic, and they do not make landfall repeatedly in the same region along the same path, or with the same force (Greening et al. 2006). Further, the state of an ecological system and the adjacent landscape at the point in time when a disturbance occurs, the residence time of the system, and the subsequent meteorological conditions can influence the magnitude and duration of the ecosystem effects of storms. Because of this extreme variability, impacts of storms can range from nearly none, no increase in nutrient delivery to a system and no observable change in ecosystem function, to profound, with instantaneous or prolonged increases in nutrient concentrations, nuisance or harmful algal blooms and significant changes in community structure to affected ecosystems (Mallin and Corbett 2006).
The coastal mid-Atlantic hurricanes Fran (1996) and Bonnie (1998) had direct and acute impacts on the entire Cape Fear River watershed (NC, USA); decreasing chlorophyll a (Chl a) concentrations to almost zero and increasing total nitrogen (TN) and total phosphorus (TP) loads by a factor of 2 to 3. In contrast, hurricanes Floyd (September 1999) and Irene (October 1999) did not affect nutrient loading even though they introduced significant amounts of freshwater and Chl a concentrations decreased initially (Mallin et al. 2002; Mallin and Corbett 2006). Just north of the Cape Fear River watershed, in Pamlico Sound, three major hurricanes in 1999 (Dennis, Floyd, and Irene) resulted in a 2- to 3-fold increase in N loading that contributed to the initiation and persistence of a large algal bloom with high Chl a concentrations persisting for nearly 8 months (Peierls et al. 2003). In addition to higher nutrient loads and algal biomass, these three hurricanes caused an increase in stratification due to the large freshwater input, and subsequent increases in bottom water hypoxia (Paerl et al. 2001). Because of the long residence time of this estuary, nutrient enrichment from large storms affected phytoplankton community diversity and composition over time through storage, recycling, and resuspension of these nutrients in the sediments (Paerl et al. 2006). The impacts of three other storms on the Neuse River estuary in North Carolina were storm-specific. Differences were related to the timing of the storms relative to conditions in the ecosystem (Wetz and Paerl 2008). Because the Neuse River was N-limited when tropical storm Helene (2000) made landfall, the delivery of N to the system stimulated an increase in phytoplankton biomass after the storm (Wetz and Paerl 2008). In contrast, phytoplankton biomass did not increase system-wide after Hurricanes Isabel (2003) and Alex (2004) because the system was already well-mixed and not N-depleted (Wetz and Paerl 2008).
The mid-Atlantic coast of the USA, including the southern Chesapeake Bay region, is impacted by many large storms each year. Coastal storms, including tropical and extra-tropical (northeasters) systems, can exert direct effects on ecosystems when they make landfall delivering extensive rainfall and high winds. Alternatively, many storms take an inland path and deliver precipitation and freshwater through the watershed (Dolan et al. 1988; Blake et al. 2011). Observations of how storms impact the southern Chesapeake Bay region have been sporadic (see Middleton 1984; Sellner 2004). While it is generally accepted that increased nutrient loading through the watershed adversely impacts water quality by contributing to eutrophication, the direct links between storms, nutrient loading, chlorophyll production, phytoplankton community composition, and in the development of monospecific algal blooms is complicated in estuaries (Heisler et al. 2008). Physical factors such as freshwater input, surface heating, and wind and tidally driven mixing can all modulate nutrient inputs, their cycling, and the development of associated water quality impacts (Lucas et al. 1999). In the James River estuary, the third largest tributary of the Chesapeake Bay, local meteorology and associated rainfall have been identified as factors contributing to the initiation of algal blooms that are then transported to other parts of the estuary through estuarine circulation (Morse et al. 2011; Morse et al. 2013). Precipitation associated with summer storms has been implicated in the initiation of Cochlodinium polykrikoides blooms in the summer when water temperatures are above 26 °C (Mulholland et al. 2009; Morse et al. 2011; Morse et al. 2013). Ephemeral blooms of cryptophytes, diatoms, cyanobacteria, and other dinoflagellates have also been related to storm activity and rainfall in the tidal James River estuary (Egerton et al. 2014; Morse et al. 2014).
The James is a partially-mixed, tidally influenced tributary, flowing from its headwaters in the Shenandoah Valley, through the fall line at Richmond, VA (368 km) to its confluence with the lower Chesapeake Bay in the Hampton Roads region of Virginia. The Lower James River, from Richmond, VA to Hampton Roads, VA (177 km) is tidally influenced. The James River, considered America’s “founding river,” is the third largest tributary in the Chesapeake Bay watershed, contributing ~16 % of the streamflow, 12 % of TN load, and 20 % of the TP load to the Chesapeake Bay (U.S. Environmental Protection Agency 2010). It is home to diverse fisheries resources including the iconic Chesapeake Bay blue crab. Unlike the long residence time of the Pamlico/Neuse River complex, the residence time of the James River ranges between 35 days during high flow conditions (typical between November and April) and 95 days during mean flow conditions (Shen and Lin 2006; Bukaveckas and Isenberg 2013).
While the James River is used for recreation, its fisheries resources, and for commerce, the tidal portion in particular is plagued with poor water quality and excessive algae growth (eutrophication) due to high nutrient and sediment loads (State of the James River, 2015). The James River and many of its tributaries are monitored monthly as part of the Virginia Department of Environmental Quality (VA DEQ) Chesapeake Bay Program monitoring program (CBMP), designed to monitor long-term status and trends in the estuary as part of its restoration. However, short-term variability that arises from storms and other meteorological disturbances lasting hours to days are poorly characterized by this monitoring design. The goal of this work was to compare and contrast water quality and phytoplankton community composition and response to storms and relate those to estuarine conditions in the absence of storms. We hypothesized that large storm events affecting the James River estuary would increase nutrient concentrations and alter phytoplankton community composition in the short-term (days to weeks) but, because of its shorter residence time, the James River would recover more rapidly than the Neuse/Pamlico estuarine complex. On-going monitoring programs were leveraged and augmented with pre- and post-storm surface water sampling and underway surface water mapping of water quality parameters (salinity, temperature, chlorophyll fluorescence, dissolved oxygen, pH, and turbidity) to compare water quality conditions and community composition in late summer/early fall during years with and without major storms.
Materials and Methods
Study Site and Ancillary Data
To determine the effects of large storms on water quality and estuarine productivity in the lower James River, we mounted field campaigns in the meso- and polyhaline James River immediately before, during, and after two major storms, Hurricane Irene and Tropical Storm (TS) Lee, in late summer, 2011. We compared these to weekly measurements made during the same time period in 2012, when there were no storms. Hurricane Irene made landfall in southeastern Virginia as a Category 1 storm on 26 August 2011, inundating the lower James River watershed as it moved landward. TS Lee made landfall in the Gulf of Mexico and then moved north, over the James River basin 6 through 8 September 2011, with the bulk of the precipitation delivered to the upper James River watershed. Streamflow data for the James River were obtained from the United States Geological Survey (USGS) streamflow gauge at Richmond, VA (Fig. 1), above the fall line and tidally influenced part of the estuary. Monitoring cruises in the meso- and polyhaline designated segments of the estuary (see below) were conducted weekly between March and October, 2011 and 2012, by the Hampton Roads Sanitation District (HRSD) using an underway data collection system (DATAFLOW) equipped with a YSI 6600 multiparameter Data Sonde that measured fluorescence, temperature, salinity, pH, dissolved oxygen (DO), and turbidity in surface water; data were recorded at 0.25 Hz. Quality assured and controlled data is posted and made publicly available at the VA Estuarine and Coastal Observing System (VECOS) website (http://www3.vims.edu/vecos/). Weekly surface salinity, temperature, and turbidity were averaged for each section in 2011 and 2012 based on DATAFLOW measurements. Regional daily precipitation data were obtained from the National Weather Service (NWS) Advanced Hydrologic Prediction Service and summed for the period from 26 to 28 August 2011 for Hurricane Irene and 6–9 September 2011 for TS Lee. The track for Hurricane Irene was overlain on the precipitation map. Hourly wind speed and direction data was obtained from a station maintained by the NOAA National Ocean Service (NOS) Center for Operational Oceanographic Products (CO-OPS) and at the Chesapeake Bay Bridge Tunnel (CBBT) at the mouth of the Chesapeake Bay (Fig. 1).
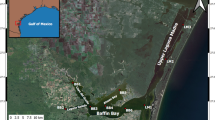
The James River segments and sampling stations (star) at the oligo-/mesohaline border (LE5.1), in the mesohaline (James River Fishing Pier), and at the meso-/polyhaline border (LE5.4). Streamflow data was collected by the United States Geological Survey (USGS) at the fall line; wind data was obtained from the Chesapeake Bay Bridge Tunnel (CBBT; cross)
Sampling Strategy
For the purpose of monitoring water quality, the James River is divided into tidal fresh (salinity 0–0.5), oligohaline (salinity 0.5–5), mesohaline (salinity 5–18), and polyhaline (salinity >18) segments (Fig. 1). Surface water quality data were obtained from station LE5.1 on the border between the oligo- and mesohaline segments of the James River and station LE5.4 in the polyhaline segment of the river. These stations are part of the long-term CBMP that have been sampled nearly monthly since 1984. Details regarding sample collection, data handling, and data downloading can be found at http://www.chesapeakebay.net/data/downloads/cbp_water_quality_database_1984_present . Surface water samples for measuring nutrient concentrations and making rate measurements (N uptake and primary productivity) were collected during DATAFLOW cruises near station LE5.1 on 1, 8, 12, and 21 September 2011 and near station LE5.4 on 31 August and 6, 13, and 20 September 2011. Surface water sampling stations and monitoring stations (LE5.1 and LE5.4) were <3 km apart and from here forward will be referred to as LE5.1 and LE5.4. There were two sampling dates after Hurricane Irene and before TS Lee and two sampling dates after TS Lee at each site. CBMP data were used also to augment this data set and assess estuarine conditions prior to and after the storms in 2011. In 2012, weekly sampling was conducted for the exact same parameters and process measurements as in 2011 but from the James River Fishing Pier in the southern portion of the mesohaline segment. For each discreet sample, surface water was collected into an acid-cleaned 20 L carboy and kept cool and in the dark while being transported to Old Dominion University (ODU) where samples were filtered, preserved, and stored and experimental incubations set up as described below.
Phytoplankton Composition
Samples to enumerate cell abundance, major taxonomic groups of phytoplankton, and Chl a concentrations were also collected weekly during HRSD’s monitoring and mapping cruises and at VA DEQ’s CBMP monitoring stations LE5.1, and LE5.4 in 2011 and 2012 (Fig. 1). Collections for the weekly samples were made at five stations in each segment and ad hoc when surface Chl a concentrations were above 10–12 μg L−1. Surface water samples were fixed with acid Lugol’s solution (0.7 %) following standard methods (Rice et al. 2012) and phytoplankton species were enumerated using light microscopy (100–600×) (Egerton et al. 2012). Algal cells were identified to the lowest taxonomic unit (species) and densities were summarized into groups and reported in cells ml−1. Biomass estimates (as μg C L−1) were calculated based on cell biovolume following Smayda (1978). Results presented were averaged for each segment and week where data was available. Chl a concentrations were averaged for each segment and week and associated with YSI Chl a data obtained at the time of each cell abundance collection. Calibrations and quality assurance/quality control (QA/QC) checks for all YSI sensors were done weekly by HRSD following standard protocols in accordance with methods described in the YSI operating manual. Details regarding sample collection, data handling, and QA/QC were made available on the VECOS website.
Nutrient Collections and Analysis
Nutrient samples were collected by pumping surface water through acid-cleaned tubing and a Supor 0.2-μm cartridge filter into sterile 50-mL centrifuge tubes on site. Samples were immediately frozen until analysis. Nutrient samples were thawed and nitrite + nitrate (NO2 − + NO3 −), NO2 −, urea, total dissolved N (TDN), and phosphate (PO4 3−) concentrations were measured using an Astoria Pacific autoanalyzer (Astoria Pacific International, Clackamas, OR) according to the manufacturer’s specifications and standard colorimetric methods (Parsons et al. 1984). The limits of detection were 0.14 μmol L−1 for NO2 − + NO3 −, 0.08 μmol N L−1 for urea, 0.57 μmol L−1 for TDN, and 0.08 μmol L−1 for PO4 3−. Ammonium (NH4 +) concentrations were measured manually using the phenol hypochlorite method and spectrophotometric detection; the limit of detection was 0.05 μmol L−1 (Solorzano 1969). Dissolved organic N (DON) concentrations were calculated as the difference between TDN and dissolved inorganic N (DIN; DIN = NH4 + + NO3 − + NO2 −) concentrations.
Nitrogen Uptake and Primary Productivity Rates
Whole water samples were dispensed into triplicate acid-cleaned 60-mL PETG bottles and N and carbon (C) uptake experiments were initiated by adding highly enriched (96–99 %) 15N-labeled NH4 +, NO2 −, and NO3 −, and 15N/13C-labeled urea (Glibert and Capone 1993; Mulholland et al. 2002). The atom % enrichment of the nutrient pool in the incubation bottles usually ranged from 3 to 20 %, a range shown to produce reliable uptake rates (Mulholland et al. 2009). Because nutrient concentrations were below the limit of detection, the atom % enrichment was >20 % in nine incubations, mainly NH4 + and NO2 − incubations, making it possible that uptake could have been stimulated during those incubation experiments. Incubation times were kept short (~30 min) and less than 10 % of the total 15N addition was recovered in the particulate pool after the short incubation period, ensuring that isotopic equilibrium was not reached (Lipschultz 2008). Isotope-amended incubation bottles were placed in walk-in incubators maintained at temperatures (20–30 °C) and light levels (50 μE m−2 s−1 on a 12 h:12 h light:dark light period supplied with cool white fluorescent bulbs) similar to those observed in the environment. While light was not measured for every experiment, this was within the range of light measured in situ during morning sampling expeditions. Incubations were terminated after 30 min by gentle filtration onto combusted (450 °C for 2 h) GF/F filters (nominal pore size of 0.7 μm). Filters were placed into sterile cryovials and frozen until analysis. Filters were dried (~2 days) at 40 °C, pelletized in tin disks, and analyzed using a Europa 20/20 isotope ratio mass spectrometer (IRMS) equipped with an automated N and C analyzer. Rates of 15N and 13C uptake were calculated using a mixing model and are detailed in Mulholland et al. (2006). Primary productivity was measured using tracer additions (~10 %) of 13C-labeled bicarbonate. Incubations were carried out as described above; however, in addition to triplicate light bottles, there were triplicate dark bottle incubations (Mulholland and Capone 2001). Primary productivity incubations were terminated after 24 h and filters were prepared, analyzed, and rates calculated as described above.
Data Analysis
In order to examine time lags associated with the transport of freshwater through an estuary, salinity is often used as a conservative tracer for mixing. However, in the case of these two storms and because nutrients are both consumed and regenerated during transport along the length of the James River, their concentrations were non-conservative and could not be estimated using a mixing model. Instead, we cross-correlated daily freshwater flow above the fall line with daily salinity measurements obtained from the Jamestown buoy (operated by NOAA’s National Data Buoy Center), located 11 km upstream from station LE5.1. We used the cross-correlation function in MATLAB (Box et al. 2015) to evaluate lag times in 2011 and 2012.
Results
Meteorological and Hydrological Observations
Hurricane Irene made landfall in southeastern Virginia as a category 1 storm on 26 August 2011, inundating the lower James River watershed as it moved landward. Rainfall associated with Hurricane Irene ranged between 2 and 25 cm along the main stem of the James River during the 3 days of the storm (Fig. 2a) and northeasterly shifting to southerly winds of up to 20 m s−1 were observed at the CBBT (Fig. 2b). In contrast, TS Lee made landfall in the Gulf of Mexico and moved north, overland reaching the upper James River, basin, 100 miles west of the coastline 6 September 2011. The bulk of the precipitation delivered (10–20 cm) to the upper James River watershed occurred over the course of 4 days (Fig. 3a). Primarily northwesterly winds measuring up to 5 m s−1 were observed at the CBBT in association with TS Lee (Fig. 3b). Based on USGS average annual streamflow data, 2011 was a drier than normal year for the upper James River watershed (Langland et al. 2013) up until the two storms. During August and September, as a result of Hurricane Irene and TS Lee, runoff in the watershed was greater than the 90th percentile for annual runoff in the state of VA in 2011. Daily maximum discharges, measured above the fall line in the James River, peaked at 91 m3 s−1 during Hurricane Irene but reached 830 m3 s−1 during TS Lee (Fig. 4). In 2012, there were peaks in discharge due to local rain events in the upper James River watershed in late August and September, but no significant watershed-wide storm event occurred (Fig. 4).
Average daily streamflow (m3 s−1) from the United States Geological Survey (USGS) streamflow gauge for the James River at the fall line from 1 August–30 September, 2011 and 2012. Gray bars indicate when Hurricane Irene (26–28 August) and Tropical Storm (TS) Lee (6–8 September) arrived over the area in 2011
The storms in 2011 differed from each other in that Hurricane Irene approached from the Atlantic Ocean making landfall just south of the Chesapeake Bay mouth in North Carolina’s Outer Banks and so delivered the largest amounts of rainfall directly to coastal areas and the tidal part of the James River watershed. In contrast, TS Lee approached Virginia overland, from the southwest, delivering precipitation to the western and northern parts of the upper James River watershed. Therefore, much of the freshwater input to the lower tidal James River delivered from this storm came through the watershed in the days and weeks after the storm had passed. As a result, average surface salinity in the meso- and polyhaline James River segments began a steady decline following Hurricane Irene that remained below those observed in late September 2012 (Fig. 5a, b). Average weekly surface water temperatures decreased in both river segments after Hurricane Irene (Fig. 5c, d). Because Hurricane Irene came from the southeast, it also created conditions favorable for offshore surface water flow and oceanic upwelling. Temperatures increased just prior to and in the weeks after TS Lee in 2011 in both river segments, typical of the seasonal heating and cooling associated with the transition from summer to fall observed in 2012. Turbidity reached a peak in the polyhaline segment of the James River the week following Hurricane Irene in 2011 (mesohaline 2011 turbidity data were lost) and this peak was significantly different from the average turbidity value in 2012 when there were no storms (p < 0.05; Fig. 5e, f). Following TS Lee, turbidity was not significantly different in either station for any sampling date when compared to 2012 turbidity (Fig. 5e, f).
Nutrient Concentrations, Biomass, and Community Composition
The lowest DIN (the sum of NH4 +, NO2 −, and NO3 −) concentrations in the meso- and polyhaline sections were observed 1 week after and during TS Lee, respectively (Fig. 6a, b), in 2011 when diatom abundances were elevated (Fig. 6c, d). The highest DIN concentrations were observed approximately 2 weeks after TS Lee in both segments. NO2 − + NO3 − was the dominant form of DIN present throughout the study in the mesohaline section while NH4 + was the dominant form of DIN prior to and in the week following Hurricane Irene in the polyhaline segment (Table 1). TDN concentrations decreased from between 18 and 20 μmol L−1 before Hurricane Irene to 8–11 μmol L−1 just after TS Lee (Table 1). Uncharacterized DON almost always comprised the bulk of the TDN pool (Fig. 6a, b) and urea concentrations remained below 1 μmol L−1 throughout the study period (Table 1). PO4 3− concentrations ranged between 0.31 and 1.18 μmol L−1 with the lowest concentrations coinciding with the low DIN concentrations (Table 1). A declining bloom of the dinoflagellate, C. polykrikoides, dominated the algal biomass prior to Hurricane Irene (Fig. 6c–f; Egerton et al. 2012), and Chl a concentrations reached an average maximum of 120 μg L−1 in the mesohaline James River. Concentrations of ≥4000 C. polykrikoides cells mL−1 were observed in the mesohaline James River for 5 weeks prior to Hurricane Irene (Egerton et al. 2012). After Hurricane Irene, the dinoflagellate bloom was effectively washed out, diatoms increased approximately 3-fold, and cyanobacteria reached an average density of 1200 cells mL−1 in the mesohaline James River. Diatoms increased over 70-fold in the polyhaline James in the 2 weeks after the storm (Fig. 6c, d). In the mesohaline segment, cyanobacteria also increased after TS Lee (Fig. 6c) but their biomass was low (Fig. 6e).
DIN and DON concentrations (μmol L−1; a, b), cell abundance (cells mL−1; c, d), and cell biomass (μg C L−1; left axis) and Chl a concentrations (μg L−1; right axis; e, f) for the mesohaline (left panel) and polyhaline (right panel) James River during 2011 storm events. Data obtained from the CBMP are marked with an asterisk. Gray bars indicate when Hurricane Irene (26–28 August) and TS Lee (6–8 September) arrived over the area in 2011
During summer and fall 2012, precipitation was much lower than in 2011 and no significant storms came through the region. Similar to 2011, there was a C. polykrikoides bloom in the James River but it was significantly larger in 2012 than in 2011 with cell abundances reaching 25,000 cells mL−1 translating to a cell biomass of 90,000 μg C L−1 (Fig. 7c–f). Overall, DIN concentrations were greater in 2012 than in 2011 (Table 1). In early August, DIN and TDN concentrations declined as the C. polykrikoides thrived in both segments (Fig. 7c, d). In mid- to late August the bloom declined corresponding to an increase in NH4 +, NO2 − + NO3 −, and urea concentrations (Table 1; Fig. 7a, b). By the end of September, NO3 − + NO2 − dominated the dissolved N pool and Chl a concentrations and cell biomass were lower than at the beginning of August (Table 1 and Fig. 7e). Diatom abundance was relatively constant, but low throughout August and September in the mesohaline segment (Fig. 7c). In contrast to 2011 when there were significant increases in diatoms and cyanobacteria abundance after the storms in the mesohaline (Fig. 6c) but not biomass (Fig. 6e), cell abundances and biomass showed similar trends to one another in 2012 when dinoflagellates were the dominant group throughout the study period (Fig. 7c, e). Because there were so few data, we were unable to assess variations in nutrient concentrations at weekly time scales in the polyhaline segment. However, DON concentrations were greater than DIN concentrations, and at the end of September, NO3 − + NO2 − concentrations were high and dominated the DIN pool (Table 1). Diatom and dinoflagellate cell abundance and biomass increased again towards the middle of September (Fig. 7d, f), and were higher than during the same time period in 2011 (Fig. 6d, f). As in 2011, PO4 3− concentrations were generally lower than 1 μmol L−1 in both segments throughout the study period (Table 1).
DIN and DON concentrations (μmol L−1; a, b), cell abundance (cells mL−1; c, d), and cell biomass (μg C L−1; left axis) and Chl a concentrations (μg L−1; right axis; e, f) for the mesohaline (left panel) and polyhaline (right panel) James River during fall 2012. Data obtained from the CBMP are marked with an asterisk
Nutrient Uptake and Primary Productivity Rates
In 2011, rates of N uptake and primary productivity in the mesohaline section of the James River were greatest in the week after Hurricane Irene and decreased by almost half after 2 weeks (Fig. 8a). In contrast, rates of N uptake and primary productivity increased and were similar one and 2 weeks after TS Lee. While NO3 − was the most abundant form of DIN present at the mesohaline station (Table 1), NH4 + was the dominant form of N taken up throughout the study period, accounting for greater than 40 % of the total measured N uptake (Fig. 8a). After TS Lee, urea uptake accounted for up to 38 % of the total measured N uptake, whereas NO3 − uptake rates accounted for 20–37 % of the N uptake. NO2 − accounted for less than 8 % of the total N uptake rate after Hurricane Irene and was near the limit of analytical detection after TS Lee.
N uptake rates for NH4 +, NO2 −, NO3 −, urea (μmol N L−1 h−1; left axis) and primary productivity rates (μmol C L−1 h1; right axis) near mesohaline station LE5.1 (a) and polyhaline station LE5.4 (b) in 2011 and at the James River fishing pier in the polyhaline section in 2012 (c) for individual sampling dates
As for the mesohaline section, total measured N uptake and primary productivity rates in the polyhaline segment were coupled in 2011 (Fig. 8b). Overall, total measured N uptake rates were much lower in the polyhaline section (Fig. 8b), consistent with the lower cell abundance (Fig. 6b) at that station. In contrast to the mesohaline segment, primary productivity and N uptake rates were the highest 2 weeks after Hurricane Irene in the polyhaline segment and decreased in the 2 weeks following TS Lee. NH4 + was taken up at the highest rates (>40 % of the total N uptake rate) except during the sampling point 2 weeks following Hurricane Irene, when it accounted for just 10 % of the total N uptake and NO3 − and urea accounted for 29 and 49 %, respectively, of the total measured N uptake. Similar to the mesohaline segment, NO2 − accounted for less than 10 % of the total N uptake at the polyhaline segment and was usually near the limit of analytical detection.
In 2012, N uptake rates and primary productivity in the lower mesohaline segment were greater than rates in 2011 (Fig. 8c). High N, in particular NH4 +, uptake rates were observed during the C. polykrikoides bloom in 2012 and declined after its demise. During late August and September 2012, rates of N uptake and primary productivity were higher than in 2011. Unlike 2011, during mid-September of 2012, the primary form of N taken up was urea. No N uptake or primary productivity rates were available in the polyhaline segment in 2012.
Discussion
Storm Influence on Water Quality
The effects of large storms (e.g., tropical and extra-tropical storms and depressions) on water quality and productivity in aquatic ecosystems are still poorly characterized, largely because these events are under-sampled during routine monitoring programs. Short- and long-term environmental impacts from storms are generally not discernable because (1) sampling is not frequent enough to capture their effects, (2) monitoring is generally done during quiescent periods to ensure the safety of personnel, and (3) monitoring programs lack statistical representation of these events because they are sporadic. Because of this gap in our knowledge, water quality management does not incorporate these massive disturbances into projected water quality plans and models (Mallin and Corbett 2006). Although we know that stochastic events contribute disproportionately to nutrient loading in aquatic systems (Mallin and Corbett 2006; Paerl et al. 2006), this may not always translate into productivity and accumulation of biomass if the load is rapidly transported through the system or if the system is not poised to respond to the load.
Storms exert different effects depending on when and where they make landfall, the direction and speed of the wind, the duration and geographical extent of the storm with respect to the watershed, and the condition of the ecosystem when the storm arrives (Mallin and Corbett 2006; Mullaugh et al. 2013). Even though nutrient loading from stochastic events can dominate the nutrient inputs (Paerl et al. 2001; Paerl et al. 2006; Mullaugh et al. 2013), the results from this study suggest that nutrient loads and ecosystem responses are dependent on the timing of storms with respect to seasonally variable estuarine conditions and on the residence time of the estuarine system. Hurricane Irene introduced large amounts of rain over the entire James River watershed which had a basin-scale impact on salinity but was accompanied by only a slight increase in flow at the fall line. In contrast, during TS Lee, most of the rainfall was delivered to the upper James River watershed above the fall line and there was little rainfall and runoff flowing directly into the meso- and polyhaline James River. This generated high freshwater flow through the fall line and a time lag in the response of the lower James River estuary to TS Lee. However, because salinity in the lower James River estuary was already depressed from Hurricane Irene, it continued to decrease after TS Lee as freshwater moved down the watershed. Because the two storms were only 14 days apart, it was difficult to attribute longer-term impacts to the individual storms; however, the immediate and combined effects of the storms were evident when compared to variability in water quality parameters over the same time frame in 2012, when there were no major storms. We observed changes in salinity, shifts in algal species composition, fluctuations in Chl a and biomass concentrations, and changes in nitrogen concentrations, uptake rates, and primary productivity rates in the mesohaline segment after the 2011 storms. Because of the short residence time of the James River system during high flow, we observed a washout of dinoflagellate populations from the system and an emergence of diatom and cyanobacteria-dominated phytoplankton assemblages in 2011. In contrast, in 2012, the bloom of C. polykrikoides persisted into mid-August and was followed by another surge of dinoflagellate growth in September. Because many dinoflagellates in the lower Chesapeake Bay are potentially harmful (Marshall and Egerton 2009), the 2011 storms may have actually improved local water quality in the lower James River.
Community Composition Shifts
In the mesohaline segment, and to a lesser extent in the polyhaline segment of the lower James River, the shift in species composition from dinoflagellates and diatoms to a mix of diatoms and cyanobacteria following Hurricane Irene and TS Lee was atypical for the late summer/early fall when dinoflagellates typically dominate the phytoplankton assemblage (Marshall and Lacouture 1986; Marshall et al. 2009). While C. polykrikoides and other dinoflagellates can tolerate certain levels of turbulence associated with upwelling and coastal fronts (Margalef 1978; Smayda 2002), high winds and turbulence related to typhoons and hurricanes can disrupt these populations (Lee 2008; Morse et al. 2014; Lim et al. 2015). However, meteorological disturbances may promote proliferation of diatoms and certain cyanobacteria, depending on the timing of the storm with respect to the phytoplankton community and the type of disturbance (Paerl et al. 2010). Diatoms are fast-growing, have a high affinity for NH4 + and NO3 − (Eppley et al. 1969; McCarthy et al. 1975; Lomas and Glibert 2000), are ubiquitous in the southern Chesapeake Bay and its tributaries, and dominate phytoplankton communities in late winter/early spring (Marshall and Lacouture 1986; Marshall et al. 2009). Diatoms generally prefer more turbulent water and cooler water temperatures and thrive following storm events when there is high discharge and short residence times in the Chesapeake Bay (Paerl et al. 2006). While diatom abundances increased considerably, this did not translate into higher biomass because the community was likely made up of small diatoms.
While the sampling frequency was not high enough to determine what exactly fueled the increase in diatoms and cyanobacteria, their growth coincided with substantial decreases in DIN concentrations in the mesohaline segment (12 September 2011). In the freshwater, oligohaline, and upper mesohaline portions of Chesapeake Bay and James River, cyanobacteria abundance increases in summer due to high temperatures and stratification and their affinity for reduced N compounds (Kemp et al. 2005; Marshall et al. 2009). Cyanobacteria generally thrive when water residence times are long and temperature is high (e.g., in summer or during periods of drought) (Kemp et al. 2005; Marshall et al. 2006) so their emergence after TS Lee in the mesohaline was surprising since residence times were shortened due to increased flow. We suspect that the increase in cyanobacteria abundance in the mesohaline James River after TS Lee resulted from transport of cells from the oligohaline and tidal fresh portion of the river since most of the freshwater during TS Lee was delivered to the upper part of the watershed. While we were unable to determine whether the cyanobacteria were actually growing, the lower salinity in the mesohaline segment after the storm could have enabled cyanobacterial growth (Robson and Hamilton 2003).
During the more quiescent fall in 2012, nutrient concentrations were much higher than in 2011, particularly following the decline of the dinoflagellate bloom when nutrients were likely regenerated from decaying algal biomass. In fall, a mixed dinoflagellate/diatom assemblage emerged. While the community composition did shift after the 2011 storms, the overall algal biomass and rates of N uptake and primary productivity were considerably lower than those measured during a similar time frame in 2012. This could have been because residence times in the James River decreased enough during the storms to washout algal populations. Streamflow after TS Lee in the headwaters of the James River was almost nine times greater than the 4-year average (2007–2010) streamflow for August through September (133 m3 s−1) and more than twice the average annual peak streamflow, which occurs in March (340 ± 322 m3 s−1; 2007–2010; USGS streamflow data not shown). In the upper portion of the river, using cross-correlation, we estimated that the time lag for the freshwater flow at the fall line near Richmond, VA, to depress salinity at the Jamestown buoy (near station LE5.1) was on the order of days (1–6 days) in 2011 (Fig. 9a). This is consistent with estimates for James River residence times of 35 days or less under high flow conditions (>700 m3 s−1) (Shen and Lin 2006). In 2012, when there were no storms, there was no salinity disturbance at the buoy (Fig. 9b). Storms can also result in high turbidity that can inhibit photosynthesis and the autotrophic uptake of DIN. Indeed, average turbidity in the James River mesohaline segment 1 week following Hurricane Irene was 20.7 ± 46.8 NTU (range was 2 to 1261 NTU). While we do not have direct evidence from the James River, a large sediment plume was observed over the upper portion of the Chesapeake Bay in the weeks following TS Lee (Hirsch 2012; Cheng et al. 2013; Palinkas et al. 2014).
The autocorrelation coefficient and the time lag (days) between daily streamflow at the fall line and averaged daily salinity at the Jamestown buoy (11 km upstream of station LE5.1) between 1 August and 30 September for 2011 (a) and 2012 (b). Solid horizontal lines indicate upper and lower 95 % confidence boundaries
In the short term, high streamflow coupled with the depressed salinity and short residence times suggest that there may have been “wash out” of algal biomass in the estuary, thereby resulting in lower overall Chl a, cell biomass, and N uptake and primary productivity rates after the 2011 storms compared to 2012. Community composition changed after the storms, but this did not translate into high rates of productivity. In contrast, in 2012 when there was little rain and storm activity, dinoflagellates continued to dominate the algal biomass as summer turned to fall, nutrients remained plentiful, and algal diversity remained low (Egerton et al. 2014). While in the longer residence time Neuse River/Pamlico Sound estuarine system, storms exacerbated eutrophication through the delivery and retention of nutrients and biomass, in the James River estuary, short residence times and associated factors such as turbidity and light attenuation may combine to suppress algal growth and improve water quality. The timing of the storms with respect to seasonal successions of plankton communities and nuisance or harmful algal blooms may also contribute to the overall ecosystem impacts of individual storm systems and whether they are positive or negative. In addition, we only addressed short-term effects of storms in this study; long-term effects of storms have yet to be determined. If nutrients and biomass are retained in the James River system over time, they may contribute to eutrophication over time. There is speculation that a large Heterocapsa triquetra bloom in the mesohaline James River in Spring 2012 (7 weeks earlier and 3 times greater cell abundance compared to 2011) and the C. polykrikoides bloom in summer 2012 (reached higher in the mesohaline James River compared to 2011) (Egerton et al. 2012) may have been lag-responses due to disturbances from Hurricane Irene and TS Lee.
Conclusion
Our results show that short-term meteorological disturbances can alter phytoplankton community assemblages through the delivery of freshwater and associated biomass in the James River estuary. Because routine monitoring lacks the resolution to track impacts from extreme meteorological events such as tropical storms and hurricanes, high-intensity storm-response sampling, coupled with long-term monitoring is crucial to understanding of how these events impact aquatic systems. Climate variability results in interannual differences in freshwater and nutrient inputs and estuarine productivity but a large fraction of the nutrient loads to estuaries are probably delivered during short, high-intensity events (Paerl et al. 2010). Storms can lead to shifts or interruptions in the typical succession of phytoplankton assemblages over annual cycles s (Paerl et al. 2014), but impacts may be more severe in systems with longer residence times than the James River. Overlain on climate variability is long-term climate change, which could profoundly change meteorology in the mid-Atlantic region. Storms are projected to increase and become more extreme in the mid-Atlantic region as a result of climate change (Kunkel et al. 2013), storm-induced and tidal flooding are also likely to increase as sea levels continue to rise. It is projected that increased storminess will be punctuated by longer periods of drought in the region (Goldenberg et al. 2001; Emanuel 2005; Webster et al. 2005; Najjar et al. 2010), resulting in storms arriving to drought-hardened landscapes that enhance overland flow into the estuary. In the Chesapeake Bay watershed, it is predicted that there will be up to a 4 % increase in precipitation during the winter and spring months by 2099 (Najjar et al. 2010). Better and more data are crucial for understanding and modeling the short and long-term responses of estuarine systems to storms.
References
Blake, E.S., Landsea, C.W., Gibney, E.J. 2011. The deadliest, costliest, and most intense United States tropical cyclones from 1851 to 2010 (and other frequently requested hurricane facts). National Weather Service, National Hurricane Center.
Box, G.E., G.M. Jenkins, G.C. Reinsel, and G.M. Ljung. 2015. Time series analysis: forecasting and control: John Wiley & Sons.
Bukaveckas, P., and W. Isenberg. 2013. Loading, transformation, and retention of nitrogen and phosphorus in the tidal freshwater James River (Virginia). Estuaries and Coasts 36: 1219–1236.
Cheng, P., M. Li, and Y. Li. 2013. Generation of an estuarine sediment plume by a tropical storm. Journal of Geophysical Research: Oceans 118: 856–868.
Dolan, R., H. Lins, and B. Hayden. 1988. Mid-Atlantic coastal storms. Journal of Coastal Research: 417–433.
Dwight, R.H., J.S. Caplan, M.V. Brinks, S.N. Catlin, G. Buescher, and J.C. Semenza. 2011. Influence of variable precipitation on coastal water quality in southern California. Water Environment Research 83: 2121–2130.
Egerton, T.A., H.G. Marshall, and W.S. Hunley. 2012. Integration of microscopy and underway chlorophyll mapping for monitoring algal bloom development. Paper presented at IEEE Proc. Oceans, 2012.
Egerton, T.A., R.E. Morse, H.G. Marshall, and M.R. Mulholland. 2014. Emergence of algal blooms: the effects of short-term variability in water quality on phytoplankton abundance, diversity, and community composition in a tidal estuary. Microorganisms 2: 33–57.
Emanuel, K. 2005. Increasing destructiveness of tropical cyclones over the past 30 years. Nature 436: 686–688.
Eppley, R., J. Coatsworth, and L. Solorzano. 1969. Studies of nitrate reductase in marine phytoplankton. Limnology and oceanography: 194–205.
Glibert, P.M. and D.G. Capone. 1993. Mineralization and assimilation in aquatic, sediment, and wetland systems. Nitrogen Isotope Techniques: 243–272.
Goldenberg, S.B., C.W. Landsea, A.M. Mestas-Nuñez, and W.M. Gray. 2001. The recent increase in Atlantic hurricane activity: causes and implications. Science 293: 474–479.
Greening, H., P. Doering, and C. Corbett. 2006. Hurricane impacts on coastal ecosystems. Estuaries and Coasts 29: 877–879.
Heisler, J., P.M. Glibert, J.M. Burkholder, D.M. Anderson, W. Cochlan, W.C. Dennison, Q. Dortch, C.J. Gobler, C.A. Heil, and E. Humphries. 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8: 3–13.
Hirsch, R.M. 2012. Flux of nitrogen, phosphorus, and suspended sediment from the Susquehanna River basin to the Chesapeake Bay during Tropical Storm Lee, September 2011, as an indicator of the effects of reservoir sedimentation on water quality. U.S. Geological Survey Scientific Investiagations Report. 2012–5185
Howarth, R., D. Swaney, E. Boyer, R. Marino, N. Jaworski, and C. Goodale. 2006. The influence of climate on average nitrogen export from large watersheds in the northeastern United States. Biogeochemistry 79: 163–186.
Kemp, W.M., W.R. Boynton, J.E. Adolf, D.F. Boesch, W.C. Boicourt, G. Brush, J.C. Cornwell, T.R. Fisher, P.M. Glibert, and J.D. Hagy. 2005. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series 303: 1–29.
Kunkel, K.E., T.R. Karl, D.R. Easterling, K. Redmond, J. Young, X. Yin, and P. Hennon. 2013. Probable maximum precipitation (PMP) and climate change. Geophysical Research Letters. doi:10.1002/grl.50334.
Langland, M.J., Blomquist, J.D., Moyer, D.D., Hyer, K.E., Chanat, J.G. 2013. Total nutrient and sediment loads, trends, yields, and nontidal water-quality indicators for selected nontidal stations, Chesapeake Bay Watershed, 1985–2011: U.S. Geological Survey Open-File Report 2013–1052. USGS.
Lee, D.-K. 2008. Cochlodinium polykrikoides blooms and eco-physical conditions in the South Sea of Korea. Harmful Algae 7: 318–323.
Lim, A.S., H.J. Jeong, T.Y. Jang, N.S. Kang, S.H. Jang, and M.J. Lee. 2015. Differential effects of typhoons on ichthyotoxic Cochlodinium polykrikoides red tides in the South Sea of Korea during 2012–2014. Harmful Algae 45: 26–32.
Lin, N., J.A. Smith, G. Villarini, T.P. Marchok, and M.L. Baeck. 2010. Modeling extreme rainfall, winds, and surge from Hurricane Isabel (2003). Weather and Forecasting 25: 1342–1361.
Lipschultz, F. 2008. Isotope tracer methods for studies of the marine nitrogen cycle. In Nitrogen in the marine environment, edited by D. Capone, et al.: Academic Press.
Lomas, M.W., and P.M. Glibert. 2000. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. Journal of Phycology 36: 903–913.
Lucas, L.V., J.R. Koseff, S.G. Monismith, J.E. Cloern, and J.K. Thompson. 1999. Processes governing phytoplankton blooms in estuaries. II: the role of horizontal transport. Marine Ecology Progress Series 187: 17–30.
Mallin, M.A., and C.A. Corbett. 2006. How hurricane attributes determine the extent of environmental effects: multiple hurricanes and different coastal systems. Estuaries and Coasts 29: 1046–1061.
Mallin, M.A., M.H. Posey, M.R. McIVER, D.C. Parsons, S.H. Ensign, and T.D. Alphin. 2002. Impacts and recovery from multiple hurricanes in a Piedmont-Coastal Plain river system. Bioscience 52: 999–1010.
Margalef, R. 1978. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanologica acta 1.
Marshall, H.G., and T.A. Egerton. 2009. Phytoplankton blooms: their occurrence and composition within Virginia’s tidal tributaries. Virginia Journal of Science 60: 149–164.
Marshall, H.G., and R. Lacouture. 1986. Seasonal patterns of growth and composition of phytoplankton in the lower Chesapeake Bay and vicinity. Estuarine, Coastal and Shelf Science 23: 115–130.
Marshall, H.G., R.V. Lacouture, C. Buchanan, and J.M. Johnson. 2006. Phytoplankton assemblages associated with water quality and salinity regions in Chesapeake Bay, USA. Estuarine, Coastal and Shelf Science 69: 10–18.
Marshall, H.G., M.F. Lane, K.K. Nesius, and L. Burchardt. 2009. Assessment and significance of phytoplankton species composition within Chesapeake Bay and Virginia tributaries through a long-term monitoring program. Environmental Monitoring and Assessment 150: 143–155.
McCarthy, J.J., W.R. Taylor, and J.L. Taft. 1975. The dynamics of nitrogen and phosphorus cycling in the open waters of the Chesapeake Bay. Marine Chemistry of the Coastal Environment 664–681.
Middleton, A.P. 1984. Tobacco coast: a maritime history of Chesapeake Bay in the colonial era: Johns Hopkins University Press.
Morse, R., J. Shen, J. Blanco-Garcia, W. Hunley, S. Fentress, M. Wiggins, and M. Mulholland. 2011. Environmental and physical controls on the formation and transport of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in the lower Chesapeake Bay and its tributaries. Estuaries and Coasts 34: 1006–1025. doi:10.1007/s12237-011-9398-2.
Morse, R.E., M.R. Mulholland, W.S. Hunley, S. Fentress, M. Wiggins, and J.L. Blanco-Garcia. 2013. Controls on the initiation and development of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in lower Chesapeake Bay and its tributaries. Harmful Algae 28: 71–82.
Morse, R.E., M.R. Mulholland, T.A. Egerton, and H.G. Marshall. 2014. Phytoplankton and nutrient dynamics in a tidally dominated eutrophic estuary: daily variability and controls on bloom formation. Marine Ecology Progress Series 503: 59–74.
Mulholland, M.R., R.E. Morse, G.E. Boneillo, P.W. Bernhardt, K.C. Filippino, L.A. Procise, J.L. Blanco-Garcia, H.G. Marshall, T.A. Egerton, W.A. Hunley, K.A. Moore, D.L. Berry, and C.J. Gobler. 2009. Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries and Coasts 32: 734–747. doi:10.1007/s12237-009-9169-5.
Mulholland, M.R., and D.G. Capone. 2001. Stoichiometry of nitrogen and carbon utilization in cultured populations of Trichodesmium IMS101: implications for growth. Limnology and Oceanography 46: 436–443.
Mulholland, M.R., C.J. Gobler, and C. Lee. 2002. Peptide hydrolysis, amino acid oxidation, and nitrogen uptake in communities seasonally dominated by Aureococcus anophagefferens. Limnology and Oceanography 47: 1094–1108.
Mulholland, M.R., P.W. Bernhardt, C.A. Heil, D.A. Bronk, and J.M. O’Neil. 2006. Nitrogen fixation and release of fixed nitrogen by Trichodesmium spp. in the Gulf of Mexico. Limnology and Oceanography 51: 1762–1776.
Mullaugh, K., J. Willey, R. Kieber, R. Mead, and G. Avery Jr. 2013. Dynamics of the chemical composition of rainwater throughout Hurricane Irene. Atmospheric Chemistry and Physics 13: 2321–2330.
Najjar, R.G., C.R. Pyke, M.B. Adams, D. Breitburg, C. Hershner, M. Kemp, R. Howarth, M.R. Mulholland, M. Paolisso, and D. Secor. 2010. Potential climate-change impacts on the Chesapeake Bay. Estuarine, Coastal and Shelf Science 86: 1–20.
Paerl, H.W., J.D. Bales, L.W. Ausley, C.P. Buzzelli, L.B. Crowder, L.A. Eby, J.M. Fear, M. Go, B.L. Peierls, and T.L. Richardson. 2001. Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ largest lagoonal estuary, Pamlico Sound, NC. Proceedings of the National Academy of Sciences USA 98: 5655–5660.
Paerl, H.W., L.M. Valdes, B.L. Peierls, J.E. Adolf, and L.W. Harding Jr. 2006. Anthropogenic and climatic influences on the eutrophication of large estuarine ecosystems. Limnology and Oceanography 51: 448–462.
Paerl, H.W., K.L. Rossignol, S.N. Hall, B.L. Peierls, and M.S. Wetz. 2010. Phytoplankton community indicators of short-and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuaries and Coasts 33: 485–497.
Paerl, H.W., N.S. Hall, B.L. Peierls, and K.L. Rossignol. 2014. Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuaries and Coasts 37: 243–258.
Palinkas, C.M., J.P. Halka, M. Li, L.P. Sanford, and P. Cheng. 2014. Sediment deposition from tropical storms in the upper Chesapeake Bay: field observations and model simulations. Continental Shelf Research 86: 6–16.
Parsons, T.R., Y. Maita, and C.M. Lalli. 1984. A manual of chemical and biological methods for seawater analysis, Oxford. Oxford: Pergamon.
Peierls, B.L., R.R. Christian, and H.W. Paerl. 2003. Water quality and phytoplankton as indicators of hurricane impacts on a large estuarine ecosystem. Estuaries and Coasts 26: 1329–1343.
Rice, E.W., R.B. Baird, A.D. Eaton, and L.S. Clesceri. 2012. Standard methods for the examination of water and wastewater, 22nd edn. Washington: American Public Health Association, American Water Works Association, Water Environment Federation.
Robson, B.J., and D.P. Hamilton. 2003. Summer flow event induces a cyanobacterial bloom in a seasonal Western Australian estuary. Marine and Freshwater Research 54: 139–151.
Sellner, K.G. 2004. Hurricane Isabel in perspective, edited by K. G. Sellner and N. Fisher. MD: Chesapeake Research Consortium.
Shen, J., and J. Lin. 2006. Modeling study of the influences of tide and stratification on age of water in the tidal James River. Estuarine, Coastal and Shelf Science 68: 101–112. doi:10.1016/j.ecss.2006.01.014.
Smayda, T.J. 1978. From phytoplankters to biomass, 273–279. Paris: Phytoplankton Manual. UNESCO.
Smayda, T.J. 2002. Turbulence, watermass stratification and harmful algal blooms: an alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 1: 95–112.
Solorzano, L. 1969. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceanography 14: 799–801.
U.S. Environmental Protection Agency. 2010. Chesapeake Bay total maximum daily load for nitrogen, phosphorus, and sediment. https://www.epa.gov/chesapeake-bay-tmdl/chesapeake-bay-tmdl-document. Accessed January 2012.
Webster, P.J., G.J. Holland, J.A. Curry, and H.-R. Chang. 2005. Changes in tropical cyclone number, duration, and intensity in a warming environment. Science 309: 1844–1846.
Wetz, M.S., and H.W. Paerl. 2008. Estuarine phytoplankton responses to hurricanes and tropical storms with different characteristics (trajectory, rainfall, winds). Estuaries and Coasts 31: 419–429.
Acknowledgments
The authors would like to thank HRSD for their financial and in-kind support for this project. In particular, we would like to thank HRSD’s boat crew, Scott Fentress, Mike Wiggins, John Hicks, and Bruce Weckworth; VA DEQ; ODU’s Phytoplankton laboratory; and members of the Mulholland Laboratory, in particular Ryan Morse, for the sample collection and for the help during sampling periods.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Deana Erdner
Rights and permissions
About this article
Cite this article
Filippino, K.C., Egerton, T.A., Hunley, W.S. et al. The Influence of Storms on Water Quality and Phytoplankton Dynamics in the Tidal James River. Estuaries and Coasts 40, 80–94 (2017). https://doi.org/10.1007/s12237-016-0145-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0145-6