Abstract
Eutrophication is considered the most important water quality problem in freshwaters and coastal waters worldwide promoting frequent occurrence of blooms of potentially toxic cyanobacteria. Removal of cyanobacteria from the water column using a combination of coagulant and ballast is a promising technique for mitigation and an alternative to the use of algaecides. In laboratory, we tested experimentally the efficiency of two coagulants, polyaluminium chloride (PAC) and chitosan (made of shrimp shells), alone and combined with two ballasts: red soil (RS) and the own lagoon sediment, to remove natural populations of cyanobacteria, from an urban brackish coastal lagoon. PAC was a very effective coagulant when applied at low doses (≤8 mg Al L−1) and settled the cyanobacteria, while at high doses (≥16 mg Al L−1) large flocks aggregated in the top of test tubes. In contrast, chitosan was not able to form flocks, even in high doses (>16 mg L−1) and did not efficiently settle down cyanobacteria when combined with ballast. The RS itself removed 33–47 % of the cyanobacteria. This removal was strongly enhanced when combined with PAC in a dose-dependent matter; 8 mg Al L−1 was considered the best dose to be applied. The lagoon sediment alone did not promote any settling of cyanobacteria but removal was high when combined with PAC. Combined coagulant and ballast seems a very efficient, cheap, fast and safe curative measure to lessen the harmful cyanobacteria bloom nuisance in periods when particularly needed, such as around the 2016 Olympics in Jacarepaguá Lagoon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities have led to major degradation of coastal waters worldwide (Kennish 2002), where eutrophication is one of the main stressors having a severe impact on water quality (Smith and Schindler 2009; Kennish et al. 2014; Paerl et al. 2014). One of the key symptoms of eutrophication is the development of harmful algal blooms (Heisler et al. 2008). There is broad scientific consensus that harmful algal blooms in coastal areas have increased worldwide over the last decades (Anderson et al. 2012; Paerl et al. 2014). Such blooms may have large consequences for ecosystem functioning and ecosystem services. Particularly, coastal lagoons with relative long water residence time are vulnerable to nutrient enrichment (Kennish et al. 2014) that may lead to such harmful algal blooms (Heisler et al. 2008). Augmented human population growth, urbanization and industrialization of the coastal area are considered the most important driver of eutrophication (Kennish 2002; Kennish et al. 2014). Cultural eutrophication is also viewed as the most common problem affecting Neotropical coastal lagoons (Esteves et al. 2008).
A severely impacted coastal lagoon is Jacarepaguá in the western part of Rio de Janeiro (Brazil), where water and sewage treatment are inadequate leading to heavy blooms of cyanobacteria, especially Microcystis aeruginosa, of which the toxins even accumulate in fish used for consumption (Magalhães et al. 2001). It is straightforward that restricting or complete stopping of the excessive external nutrient input is the first step to manage eutrophication (Cooke et al. 2005). In Jacarepaguá Lagoon, also the accumulated nutrients in the organic-rich sediments are expected to delay recovery when external load reductions have been done (Jeppesen et al. 1991; Søndergaard et al. 1999; Cooke et al. 2005). Since Jacarepaguá Lagoon is located adjacent to the 2016 Olympic Park, authorities are discussing dredging as a measure to mitigate the cyanobacterial nuisance. However, this seems shutting the stable door after the horse has bolted as external nutrient inputs have not been reduced and the water will keep rich in nutrients and infested cyanobacteria. Nowadays, cyanobacteria in Jacarepaguá Lagoon are present perennially and build more frequent and longer-lasting perennial blooms that without additional measures will maintain a high risk for ongoing cyanobacterial blooms even after dredging. Therefore, we explored the possibility for efficient, cheap, fast and safe curative measures to lessen the cyanobacteria nuisance in periods when particularly needed, such as around the 2016 Olympics.
Algaecides are among the commonly used curative measures, but their application may come with shortcomings such as toxins and nutrient release or unwanted ecotoxicological side effects (Jančula and Maršálek 2011; Merel et al. 2013). Likewise, although found effective against Planktothrix agardhii (Matthijs et al. 2012), diluted hydrogen peroxide is not considered the intervention of first choice against M. aeruginosa—the dominant cyanobacterium in Jacarepaguá Lagoon—as toxins may be liberated (Lürling et al. 2014), extracellular polymeric substances may protect Microcystis against peroxide making higher doses necessary (Gao et al. 2015), and positively buoyant cyanobacteria biomass may accumulate at the surface (Wang et al. 2012; Barrington et al. 2013) aggravating nuisance. Instead, we explored the stripping of the water column from cyanobacteria with a coagulant and ballast (e.g. Li and Pan 2013; Lürling and Van Oosterhout 2013; Noyma et al. 2016) as a promising alternative to the use of algaecides.
In this technique, cyanobacteria in the water column are flocked and the aggregates of intact cells/colonies are settled to the sediment with entrapped ballast. As ballast, natural soils and clays are commonly used (Pan et al. 2006a, b, 2011a, b; Zou et al. 2006). In stratifying inland waters with much lower external phosphorus (P) load than internal loading, cyanobacteria removal with flocculants and a lanthanum-modified clay as ballast—to chemically inactivated phosphorus released from the sediment—has also yielded very promising results (Lürling and Van Oosterhout 2013; Waajen et al. 2016). Recently, Noyma et al. (2016) showed that buoyant cyanobacteria (primarily M. aeruginosa) from the freshwater Funil Reservoir (Rio de Janeiro, Brazil) could be flocked and effectively precipitated using a combination of polyaluminium chloride (PAC) or chitosan (made of shrimp shells) with a local red soil (collected from the banks of the reservoir, consisting mainly of kaolinite clay) as ballast. Based on those results, we hypothesized that the cyanobacteria flourishing in Jacarepaguá Lagoon could also be effectively removed from the water column using a combination of PAC or chitosan with red soil as ballast. To test this hypothesis, we ran several controlled experiments in laboratory with cyanobacteria and water from Jacarepaguá Lagoon that were exposed to different concentrations of PAC and chitosan alone and in the presence of red soil. In addition, we tested the local sediment as potential ballast compound.
Material and Methods
Jacarepaguá Lagoon
The Jacarepaguá Lagoon (43° 17′–43° 30′ W, 22° 55′–23° 00′ S) is part of a lagoon complex located in the coastal plain of at the southern coast of Rio de Janeiro, Brazil (Fig. 1). This complex consists of four main elongated lakes: Jacarepaguá, Camorim, Tijuca and Marapendi (Gomes et al. 2009). The Jacarepaguá Lagoon has a surface area of 3.7 km2, an average depth of 3.3 m and little water exchange with the sea since it communicates with the sea through the Camorim and Tijuca lagoons. Jacarepaguá complex has the largest drainage area of the region (103 km2) and a water inflow from rivers of about 0.8 m3 s−1 (Gomes et al. 2009). The main tributaries of Jacarepaguá Lagoon flow through urban settlements surrounding the lagoon transporting not only sediment but also industrial and household sewage (Gomes et al. 2009).
Panel a shows the close vicinity of some of the 2016 Olympic arenas, the Olympic park of Jacarepaguá lagoon and the two sampling stations (JAC 18, JAC 20). Panel b shows some of the Olympic park buildings as seen from the lagoon. Panels c, d show the green water of the lagoon (January 19th 2015) and panel e, the main phytoplankton species (M. aeruginosa colonies and P. agardhii filaments)
Sampling
On 18th November, 16th December 2014 and 19th January 2015, samples were taken at two stations in Jacarepaguá Lagoon (JAC 18–22° 58′ 36.8″ S 43° 22′ 48.5″ W, depth 1.2 m and JAC 20–22° 59′ 14.1″ S 43° 24′ 9.6″ W, depth 1.3 m; Fig. 1a). Water transparency in each sampling station was measured using a Secchi disk. In situ, water temperature, conductivity, salinity, pH, dissolved oxygen concentrations and saturation were measured with a multiparameter sonde (YSI model 600R) at three depths: top, 0.5 m and bottom. Dissolved and total nitrogen and phosphorus, chlorophyll-a and quantitative phytoplankton samples were collected with an integration tube (4.5 cm diameter) integrating 1 m of water column. Phytoplankton samples were fixed with Lugol’s solution, and nutrient samples were kept frozen until analysis.
Sample Analysis
The soluble reactive phosphate (SRP), ammonium (N NH4 +), nitrate (N NO3 −), nitrite (N NO2 −) and total phosphorus (TP) were measured using flow injection analysis according to manufacturer instructions (FIAlab 2500, FIALab Instruments Inc., Seattle, Washington). Chlorophyll-a concentrations were measured using a PHYTO-PAM phytoplankton analyser (Heinz Walz GmbH, Effeltrich, Germany). Phytoplankton populations were enumerated according to the settling technique (Utermöhl 1958) in random fields (Uhelingher 1964) using an inverted microscope (Olympus, CKX41).
Chemicals and Materials
At station JAC 20, 10 L of surface water were collected for experiments with flocculants and ballasts. The dominant species was M. aeruginosa (Kützing) Kützing with some undergrowth of P. agardhii (Gomont) Anagnostidis & Komárek. Jacarepaguá sediment was collected with a plastic core sampler on January 19th 2015. Red soil (RS) used as ballast was collected from the banks of the Funil Reservoir (22° 30′ S, 44° 45′ W, Rio de Janeiro, Brazil). Prior to use, RS was dried and grinded using pestle and sieved over 0.5 mm. This soil has been characterized according to particle size and mineralogical composition, and consisted of 99 % fine earth (<2 mm) comprised mainly of clay (≅50 %). Kaolinite was most abundant, followed by goethite and mica (for details, see Noyma et al. 2016).
The coagulant PAC-AP (polyaluminium chloride; Aln(OH)mCl3n-m, ρ ≈ 1.37 kg L−1, 8.9 % Al, 21.0 % Cl) was obtained from Pan-Americana (Rio de Janeiro, Brazil), whereas chitosan made of shrimp shells was obtained from Polymar Ciência e Nutrição S/A (Ceará, Brazil). The chitosan was acidified with a 1 % hydrochloric acid as the protonation of amino groups makes chitosan positively charged that allows flocculation (Pan et al. 2006b). PAC was diluted 100 times in demineralised water prior to use.
Experiments
Aliquots of 60 mL cyanobacteria suspensions from Jacarepaguá Lagoon were transferred to 75-mL glass tubes (25 × 200 mm). The initial cyanobacterial chlorophyll-a (μg L−1) as well as the Photosystem II (PSII) efficiency was determined using a PHYTO-PAM phytoplankton analyser (Heinz Walz GmbH, Effeltrich, Germany). Cyanobacteria suspensions were treated with the designated compound(s) (treatment) or left untreated (controls). Suspensions were mixed at start and placed in the laboratory at 25 °C under stagnant conditions. After 1 h, 5-mL samples were taken from both the top and the bottom of the tubes in which chlorophyll-a concentrations and PSII efficiencies were measured. The pH was measured in the tubes.
In the first experiment, the effect of the flocculants PAC and chitosan on the vertical distribution of the cyanobacteria was studied. The initial cyanobacteria chlorophyll-a concentration was 205 (±8) μg L−1. The cells were in good physiological conditions as shown by the PSII efficiency of 0.40 (±0.04). PAC was dosed at 0, 1, 2, 4, 8, 16 and 32 mg Al L−1 and chitosan at 0, 1, 2, 4, 8, 16 and 32 mg L−1. We tested this range considering that these flocculants are efficient to remove algae at low doses as 2 mg L−1 (Divakaran and Sivasankara Pillai 2002; Noyma et al. 2016). Immediately after adding the flocculants from the prepared stocks, the contents in each test tube were mixed briefly using a glass rod. Tubes were left untouched for 1 h, then top and bottom samples were taken and analysed as mentioned above. The aluminium species prevailing in the PAC treatments was modelled using the program CHEAQS Next (Verweij 2015). As the input in the model were served the measured pH, the added Al concentration, a carbonate concentration of 229 mg L−1 as calculated from alkalinity and a phosphate concentration of 1 mg L−1.
In the second experiment, the effect of different concentrations of flocculants, PAC or chitosan, on the vertical distribution of the cyanobacteria was studied in the presence of a fixed dose of RS (320 mg L−1). The RS dose was applied as a slurry and the dose was based on a previous study in which 320 mg L−1 appeared sufficient to sink positively buoyant M. aeruginosa out of water from the Funil Reservoir, Brazil (Noyma et al. 2016). Similar to the first experiment, PAC was dosed from 0 to 32 mg Al L−1 and chitosan from 0 to 32 mg chitosan L−1. Both series included a control that received no RS and no coagulant. The cyanobacteria suspension used was the same as for the first experiment. Immediately after adding the RS—by making slurry with some of the water from the test tube—the designated amount of coagulant was added and the content in the test tube mixed briefly using a glass rod. Tubes were then left untouched for 1 h, after which top and bottom samples were taken and analysed as mentioned above.
In the third experiment, the effect of different concentrations of RS (0 to 320 mg L−1) in the presence of a fixed dose of PAC (8 mg Al L−1) on the vertical distribution of the cyanobacteria was studied. The PAC dose was based on the results from the previous experiments. Based on the outcomes of the previous experiments, chitosan was no longer included in this experiment. The initial cyanobacteria chlorophyll-a concentration was 222 (±2) μg L−1 with healthy cells as reflected in a PSII efficiency of 0.53 (±0.03). The experiment included a control without any compound added and was performed with three replicates per treatment. Immediately after adding the designated amount of RS, the PAC flocculent was added and the content in the test tube mixed briefly using a glass rod. Tubes were treated as mentioned before.
In the fourth experiment, wet sediment from Jacarepaguá Lagoon was tested on its ability to settle cyanobacteria out of the Jacarepaguá water. To apply the sediment, slurry was made with 10 g L−1 wet sediment of which 1.92 mL was pipetted into 60 mL cyanobacteria suspension (dose of 320 mg wet sediment L−1). In another treatment, the flocculent PAC was dosed at 8 mg Al L−1 immediately after the sediment. An additional control with only PAC addition was included. Immediately after dosing, the contents in each test tube were mixed briefly using a glass rod. The treatments were run in triplicates as outlined above.
Statistical Analyses
For the third (effect of different concentrations of RS in the presence of a fixed dose of PAC) and fourth (ability of wet sediment to settle cyanobacteria out) experiments, the chlorophyll-a concentrations in the top and those measured at the bottom of the test tubes, as well as PSII efficiencies and pH values were statistically evaluated through a one-way ANOVA. Chlorophyll-a concentrations were log-transformed to meet the requirement of homogeneity in variance (Equal Variance Test; Brown-Forsythe). An all pairwise multiple comparison was performed to distinguish means that were significantly different at the 0.05 level (Holm-Sidak method).
Results
Water Quality in Jacarepaguá Lagoon
The water of the tropical lagoon Jacarepaguá was warm, brackish, cyanobacteria-infested, with high pH and low transparency (Table 1). During the studied period, the water temperature was reached 30.5 ± 4.1 °C in the upper water layer and 28.9 ± 3.3 °C near the bottom. Despite these small differences in temperature, dissolved oxygen varied from oxic (top and 0.5 m) to hypoxic in JAC 18 near the bottom (Table 1). Low salinity (averages <5.77 ppt) and high conductivity and alkalinity were found. Considering the high concentrations of TP, chlorophyll-a and the low water transparency, the lagoon can be classified as hypereutrophic (Nürnberg 1996). During the sampling period, M. aeruginosa and P. agardhii together represented on average more than 90 % of total phytoplankton biomass (Table 1). M. aeruginosa was always present and comprised 56 to 99 % of total biomass while P. agardhii contributions reached up to 25 and 22 % in JAC 18 and JAC 20, respectively.
Effects of PAC and Chitosan on the Vertical Position of the Cyanobacteria
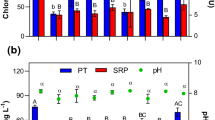
The cyanobacteria collected in Jacarepaguá had a strong positive buoyancy as in the absence of PAC (0 mg Al L−1 in Fig. 2) or chitosan (0 mg L−1 in Fig. 4); the chlorophyll-a concentrations in the top of the test tubes after 1 h were, on average, respectively 11 times and 8 times higher than in the bottom. In the PAC series, at 1 and 2 mg Al L−1, small flocks were formed that aggregated at the water surface, while at 4 and 8 mg L−1, the flocks formed were dense enough to settle the cyanobacteria to the bottom of the tubes reaching a removal efficiency of 71 and 93 %, respectively; at higher PAC dose, large fluffy aggregates accumulated at the water surface in the tubes (Fig. 2). PSII efficiency was unaffected at all PAC doses and was on average 0.52 (±0.01) in the top of the tubes and 0.51 (±0.01) in the bottom of the tubes. The pH gradually decreased with higher PAC doses; from a pH value of 9.19 (±0.09) in 0–2 mg Al L−1, pH 8.84 in 4 mg Al L−1, pH 8.20 in 8 mg Al L−1, pH 7.02, in 16 mg Al L−1 and pH 5.95 in a dose of 32 mg Al L−1 (Fig. 2).
Chlorophyll-a concentrations (μg L−1) in the top 5 mL (top light grey bars) and bottom 5 mL (lower dark grey bars) of 60 mL cyanobacteria suspension from Jacarepaguá Lagoon incubated for 1 h in the absence or presence of different concentrations of the flocculent PAC (0–32 mg Al L−1). Also included are the Photosystem II efficiencies (PSII) of the cyanobacteria collected at the water surface (filled circles) and at the bottom (open circles) as well as the pH values of the suspensions (open triangles)
The most dominant Al species in all treatments was the Al(OH)3 precipitate (Fig. 3), while in the lower PAC doses, some aluminate was present as a consequence of the higher pH. In none of the PAC treatments occurrence of Al3+ was predicted (Fig. 3).
In the chitosan series, no flocks were formed; not even at the highest dose of 32 mg L−1 which settled only 7 % of the cyanobacteria. In all treatments, most of the cyanobacteria aggregated at the water surface in the tubes (Fig. 4). PSII efficiency was unaffected at all chitosan doses and was on average 0.52 (±0.01) in the top of the tubes and 0.52 (±0.07) in the bottom of the tubes. The pH remained unaffected in the chitosan range 0 to 8 mg L−1 (pH 9.21 ± 0.03), it was slightly lower at 16 mg chitosan L−1 (pH 9.06) and pH was 8.58 at the highest chitosan dose (Fig. 4).
Chlorophyll-a concentrations (μg L−1) in the top 5 mL (top light grey bars) and bottom 5 mL (lower dark grey bars) of 60 mL cyanobacteria suspension from Jacarepaguá Lagoon incubated for 1 h in the absence or presence of different concentrations of the coagulant chitosan (0–32 mg L−1). Also included are the Photosystem II efficiencies (PSII) of the cyanobacteria collected at the water surface (filled circles) and at the bottom (open circles) as well as the pH values of the suspensions (open triangles)
Effect of Flocculants and Red Soil on the Vertical Position of the Cyanobacteria
In the experiment with different concentrations PAC in the presence of 320 mg L−1 RS, solely the RS (0 mg Al L−1 treatment) already caused a 33 % decrease in the top chlorophyll-a concentration and a 3.4 times increase at the bottom (Fig. 5). Adding PAC strongly improved the cyanobacteria removal from Jacarepaguá water; from 64 % removal from the top layer in 1 mg Al L−1 to 97 % at 8 mg Al L−1 or even 99 % removal at 32 mg Al L−1 (Fig. 5). PSII efficiency was unaffected at all PAC doses and was on average 0.52 (±0.01) in the top of the tubes and 0.52 (±0.03) in the bottom of the tubes. The pH gradually decreased with higher PAC doses; from a pH value of 9.30 in the control to pH 7.62 in the 8 mg Al L−1 dose and further down to pH 5.80 in the 32 mg Al L−1 dose (Fig. 5).
Chlorophyll-a concentrations (μg L−1) in the top 5 mL (top light grey bars) and bottom 5 mL (lower dark grey bars) of 60 mL cyanobacteria suspension from Jacarepaguá Lagoon incubated for 1 h in the absence or presence of different concentrations of the coagulant PAC (0–32 mg Al L−1) combined with a fixed dose of red soil (320 mg L−1) as ballast. Also included are the Photosystem II efficiencies (PSII) of the cyanobacteria collected at the water surface (filled circles) and at the bottom (open circles) as well as the pH values of the suspensions (open triangles)
In the experiment with different concentrations of chitosan mixed with a fixed dose of 320 mg L−1 RS, solely the RS (0 mg L−1 treatment) resulted in a 47 % decrease in the top chlorophyll-a concentration and a 3.7 times increase at the bottom of the test tube (Fig. 6). However, adding chitosan did not improve this and, actually, in the range 1 to 16 mg chitosan L−1 the reduction in the top chlorophyll-a concentration was only 26 (±3) % compared to the control without anything added. Only in 32 mg chitosan L−1 the reduction in chlorophyll-a concentration in the top of the test tube (49 %) was comparable to the solely RS treatment (Fig. 6). The PSII efficiencies were similar in the top of the tubes 0.52 (±0.01) regardless of RS and RS plus chitosan additions; the same was observed in the bottom of the test tubes with a mean PSII efficiency of 0.52 (±0.03) (Fig. 5). The pH was on average 9.07 (±0.13) in the control and the treatments up to a chitosan concentration of 16 mg L−1; only at 32 mg chitosan L−1, the pH was slightly reduced to 8.38 (Fig. 6).
Chlorophyll-a concentrations (μg L−1) in the top 5 mL (top light grey bars) and bottom 5 mL (lower dark grey bars) of 60 mL cyanobacteria suspension from Jacarepaguá Lagoon incubated for 1 h in the absence or presence of different concentrations of the coagulant chitosan (0–32 mg L−1) combined with a fixed dose of red soil (320 mg L−1) as ballast. Also included are the Photosystem II efficiencies (PSII) of the cyanobacteria collected at the water surface (filled circles) and at the bottom (open circles) as well as the pH values of the suspensions (open triangles)
Based on the former experiments, a fixed PAC dose of 8 mg Al L−1 appeared most suitable in terms of removal efficiency through flocculation, influence on the pH and no toxic Al3+ formation, but solely the flocculating colloidal-sized Al(OH)3 (s).
The effect of adding additional ballast (RS) to this fixed PAC dose showed that top chlorophyll-a concentrations were even further reduced than the 95 % reduction by solely PAC; at 40 mg RS L−1, more than 98 % was removed, and at higher dose more than 99 % of the cyanobacteria were removed from the top water layer (Fig. 7). The one-way ANOVA indicated that the differences were significant (F 6,14 = 63.7; p < 0.001), while the post hoc comparison revealed three homogenous groups that were significantly different from each other: (1) the controls, (2) the sole PAC treatment and (3) all PAC + RS treatments. Also for the chlorophyll-a concentrations measured at the bottom of the tubes, a one-way ANOVA indicated significant differences (F 6,14 = 560.6; p < 0.001), while the post hoc comparison revealed three homogenous groups that were significantly different from each other: (1) the controls with the lowest chlorophyll-a concentrations, (2) the sole PAC treatment with the highest chlorophyll-a and (3) all PAC + RS treatments (Fig. 7).
Chlorophyll-a concentrations (μg L−1) in the top 5 mL (top light grey bars) and bottom 5 mL (lower dark grey bars) of 60 mL cyanobacteria suspension from Jacarepaguá Lagoon incubated for 1 h in the absence and presence of the coagulant PAC (8 mg Al L−1) combined with different concentration of red soil (0–320 mg L−1) as ballast. Also included are the Photosystem II efficiencies (PSII) of the cyanobacteria collected at the water surface (filled circles) and at the bottom (open circles) as well as the pH values of suspensions (open triangles). Error bars indicate one standard deviation (n = 3). Similar letters indicate homogeneous groups according to the Holm-Sidak method
The PSII efficiencies showed some variability in the top of the tube, but remained fairly high (0.41–0.71), while in the bottom they were similar (0.55 ± 0.01). The pH in the controls (9.04 ± 0.01) was significantly higher than the pH values (mean 7.35 ± 0.20) in all other treatments (F 6,14 = 50.2; p < 0.001), which was confirmed by the post hoc comparison (Fig. 7).
Effect of Sediment on the Vertical Position of the Cyanobacteria
Adding only sediment did not lead to removal of cyanobacteria from the water column in the test tubes in contrast to adding PAC at a dose of 8 mg Al L−1 (Fig. 8). The one-way ANOVA indicated significant differences in both the chlorophyll-a concentration in the top of the test tubes (F 3,8 = 2290; p < 0.001) as in the bottom (F 3,8 = 853.7; p < 0.001); in both cases, the post hoc comparison revealed three homogenous groups that were significantly different from each other: (1) the control and the solely sediment treatment, (2) the sole PAC treatment and (3) the PAC + sediment treatment. The PSII efficiency in the top of the control tubes was significantly lower (F 3,8 = 26.6; p < 0.001) than in the treatments, while in the bottom PSII efficiency in the control and PAC treatment were significantly higher (F 3,8 = 28.5; p < 0.001) than in the sediment and sediment + PAC treatments (Fig. 8). Finally, the pH was significantly different (F 3,8 = 4964; p < 0.001) and clearly reduced in the two treatments with PAC added (pH 7.47 and 7.42) compared to the control (pH 9.13 ± 0.01) and the only sediment addition (pH 9.07 ± 0.01) (Fig. 8).
Chlorophyll-a concentrations (μg L−1) in the top 5 mL (top light grey bars) and bottom 5 mL (lower dark grey bars) of 60 mL cyanobacteria suspensions from Jacarepaguá Lagoon incubated for 1 h without addition (Control) or solely the coagulant PAC (8 mg Al L−1) added (PAC), solely sediment from the Lagoon (320 mg L−1) added (Sediment) or a combination of both PAC and sediment (Sediment + PAC). Also included are the Photosystem II efficiencies (PSII) of the cyanobacteria collected at the water surface (filled circles) and at the bottom (open circles) as well as the pH values of suspensions (open triangles). Error bars indicate one standard deviation (n = 3). Similar letters indicate homogeneous groups according to the Holm-Sidak method
Discussion
Our field work confirmed the strong cyanobacterial proliferation of the water in Jacarepaguá Lagoon. Jacarepaguá is an oligo-mesohaline system suffering from strong anthropogenic pressure of which eutrophication is causing massive cyanobacterial blooms (Gomes et al. 2009) that also threaten humans consuming fish from the system which have accumulated cyanobacterial toxins (Magalhães et al. 2001). Hence, there is strong pressure to reduce the harmful cyanobacteria blooms. While nutrient input control may take quite some years, we hypothesized that the cyanobacteria flourishing in Jacarepaguá Lagoon could also be effectively removed from the water column using a combination of PAC or chitosan with a RS or local sediment as ballast. Our experimental data showed an efficient removal when these ballast compounds were joined with PAC, but not for chitosan, which did not form flocks in the Jacarepaguá Lagoon water.
Chitosan is widely used in water and waste water treatment, because it is a non-toxic, non-corrosive natural product, which is efficient in cold water without causing environmental pollution (Renault et al. 2009). Deacetylation of chitin—mostly derived from shrimps and crabs—produces chitosan, which is a linear copolymer of d-glucosamine and N-acetyl-d-glucosamine that due to its rigid structure is insoluble in water. The copolymer becomes fully soluble in dilute acids when the free amino groups of chitosan are protonated that enables electrostatic interactions between the protonated amino groups of chitosan and the negatively charged cyanobacteria (Renault et al. 2009). The long chain polymers, like chitosan, can attach onto particles forming “bridging” connections. These bridging connections between the particles can together form a “net” (Tripathy and De 2006; Chen et al. 2014). However, in conditions where large amounts of negative ions gather around the protonated groups, it becomes shielded, the molecule contracts and also hampers the netting and bridging properties (Pan et al. 2006b; Qun and Ajun 2006). Consequently, chitosan is not a very efficient coagulant at high pH (Morales et al. 1985; Vandamme et al. 2013) and at high ionic strength of the water (Pan et al. 2006a). Given the high pH and considerable ionic strength of the Jacarepaguá water (see Table 1), chitosan is not an appropriate coagulant to be applied in Jacarepaguá, or any other high ionic strength and high pH water. In addition, the price of chitosan is much higher than that of aluminium salts (Granados et al. 2012).
Contrary to chitosan, polyaluminium chloride (PAC) turned out an excellent coagulant in Jacarepaguá water. In general, aluminium salts are widely used in water treatment including cyanobacteria removal (Jančula and Maršálek 2011). PAC has several advantages over other aluminium salt coagulants, such as alum: less pH reduction, lower dose needed, less residual Al, less sulphate added and better flocs at low temperature (Gebbie 2001; de Julio et al. 2010). Use of aluminium formulations is sometimes met with scepticism related to presumed toxic effects (e.g. Renault et al. 2009). However, aluminium is the most abundant metal in the Earth’s crust (>8 %) and application of aluminium formulations in waters with neutral pH can be considered safe (Jančula and Maršálek 2011). The toxicity of metals depends on speciation which is steered by pH (Stumm and Morgan 1996), where in the case of aluminium the trivalent Al3+ prevails at pH lower than 5.5 (Driscoll and Schecher 1990; Gensemer and Playle 1999). As the pH in Jacarepaguá Lagoon keep alkaline in the entire water column, low soluble aluminium will be free even near the bottom. In our experiments, no occurrence of this toxic Al species in the water was predicted. Nonetheless, it should be notified that aluminium toxicity in fish has also been ascribed to precipitation of Al(OH)3 on the gills leading to suffocation of the fish (Wauer et al. 2004). Therefore, before applying PAC to Jacarepaguá, albeit in a relative low dose of 8 mg Al L−1—field applications of Al salts are reported to be dosed at 2.6 to 45 mg L−1 (Cooke et al. 2005)—additional enclosure studies including the abundant fish Tilapia rendalli are recommended.
Red soil in itself removed 33–47 % of the cyanobacteria from the water, which may have been caused by some flocculating properties of the RS (Pan et al. 2006b). The RS is a typical laterite soil—rich in iron and aluminium—and predominantly composed of kaolinite, goethite, gibbsite and some anatase (Noyma et al. 2016). The main component, the clay kaolinite, removed 43 % of a M. aeruginosa populations when dosed at 200 mg L−1 and 88 % at 700 mg L−1 (Pan et al. 2006b), while 1000 mg L−1 removed almost 60 % of the red-tide dinoflagellate Karenia brevis (Sengco and Anderson 2004). The stickiness of M. aeruginosa may also cause attachment of clay particles resulting in sedimentation of buoyant Microcystis (Verspagen et al. 2006). However, given sediment from Jacarepaguá had no effect on settlement of M. aeruginosa, where RS did partly, stickiness seems not have been the main mechanisms operating. Hence, just RS or local sediment are not appropriate to sink the cyanobacteria out of the water column. Similarly, Li and Pan (2013) found that sand alone was ineffective to flock and sink two marine algae and also M. aeruginosa. When they added chitosan, however, removal was between 40 and 60 %, whereas Moringa oleifera (Moringaceae) extract led to more than 90 % removal (Li and Pan 2013). Also, Pan et al. (2012) recognised that soils by themselves hardly remove phytoplankton and flocculent addition was needed to facilitate removal.
Indeed, the combination of PAC and RS as ballast improved the settling efficiency at low PAC dose. At a sole PAC dose of 1 and 2 mg Al L−1, although flocks were formed, no difference in settling with the control was observed (see Fig. 2). Adding RS, however, greatly improved sedimentation of cyanobacteria flocks at these PAC doses (see Fig. 5). The choice for 8 mg Al L−1 PAC as optimum dose in combination with RS was based on the efficient removal of cyanobacteria from the water column and acceptable lowering of pH.
The dose of RS could be lowered to 80 mg L−1 without losing any efficiency (see Fig. 7). This dose of ballast is quite comparable with the 100 mg L−1 of a local sand or soil found in other studies (Pan et al. 2011a; Li and Pan 2013, 2015). The combination of flocculent and soil has already been applied successfully in an isolated bay of Lake Taihu (China), where adding 25–31 mg L−1 (40–50 g m−2) effectively cleared the water of cyanobacteria (Pan et al. 2011b). In Lake Rauwbraken (The Netherlands), PAC (0.83 mg L−1) and a lanthanum-modified bentonite (84 mg L−1) effectively removed cyanobacteria from the water column and resulted in water devoid of cyanobacteria nuisance for several years (Lürling and van Oosterhout 2013). As in this technique, entrapped cyanobacteria in flocks remain intact (Chow et al. 1999; Drikas et al. 2001)—the intactness of precipitated cells is also reflected in unaffected PSII efficiencies, no release of toxins and nutrients during treatment occurs, which is a major advantage over using algaecides in treating cyanobacterial blooms.
The experiments in laboratory scale are important to demonstrate, in rapid assays, the possible effects of the lagoon water chemistry on flocculation efficacy. Although physical factors, i.e. turbulence, can interfere in the flocculation processes, resuspending flocks, the wind-induced mixing might also be beneficial in facilitating formation of flocks. The flock and sink approach has been applied successfully to a shallow and small lake (Lake De Kuil, The Netherlands) (Waajen et al. 2016). Furthermore, this promising approach seems to be applicable for different water characteristic, although complementary studies are needed to evaluate the effectiveness of the compounds in larger scales.
Although it is widely recognised that preventing cyanobacterial and harmful algal bloom development through adequate nutrient control in the watershed is far better than eliminating existing blooms, costs and politics may delay such actions that make curative bloom control strategies inevitable (Heisler et al. 2008). Furthermore, synergistic effects of eutrophication and global change may promote an escalation in cyanobacterial and harmful algal blooms in estuarine and coastal waters (Paerl et al. 2014). Hence, efficient, cheap, fast and safe curative measures to lessen the cyanobacteria and harmful algal bloom nuisance will provide a welcome extension of the water managers’ tool-box. Combined coagulant and ballast seems a very promising tool, and it may give temporal relief from cyanobacteria or harmful algal nuisance in periods when particularly needed, such as around the 2016 Olympics in Jacarepaguá Lagoon.
Conclusions
-
Polyaluminium chloride (PAC) flocculated cyanobacteria from a brackish tropical lagoon, while chitosan appeared ineffective as flocculent.
-
Positively buoyant cyanobacteria from a brackish tropical lagoon could be flocked and effectively precipitated using a combination of PAC with red soil (RS) or local sediment as ballast.
-
Sole use of RS or sediment as ballast was inefficient in removing cyanobacteria.
-
Combined use of PAC and ballast seems a very efficient curative measure to lessen cyanobacterial bloom nuisance.
References
Anderson, D.M., A.D. Cembella, and G.M. Hallegraeff. 2012. Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science 4: 143–176.
Barrington, D.J., E.S. Reichwaldt, and A. Ghadouani. 2013. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecological Engineering 50: 86–94.
Chen, G., Zhao, L., Qi, Y., and Cui, Y. L. 2014. Chitosan and its derivatives applied in harvesting microalgae for biodiesel production. Journal of Nanomaterials.
Chow, C.W., M. Drikas, J. House, M.D. Burch, and R. Velzeboer. 1999. The impact of conventional water treatment processes on cells of the cyanobacterium Microcystis aeruginosa. Water Research 33(15): 3253–3262.
Cooke, G.D., E.B. Welch, S. Peterson, and S.A. Nichols. 2005. Restoration and management of lakes and reservoirs. Boca Raton: CRC press.
De Julio, M., D.A. Fioravante, T.S. De Julio, F.I. Oroski, and N.J.D. Graham. 2010. A methodology for optimising the removal of cyanobacteria cells from a Brazilian eutrophic water. Brazilian Journal of Chemical Engineering 27(1): 113–126.
de la Morales, J., J. Noiie, and G. Picard. 1985. Harvesting marine microalgae species by chitosan flocculation. Aquacultural Engineering 4: 257–270.
Divakaran, R., and V.N. Sivasankara Pillai. 2002. Flocculation of algae using chitosan. Journal of Applied Phycology 14: 419–422.
Drikas, M., G. Newcombe, and B. Nicholson. 2001. Water treatment options for cyanobacteria and their toxins. Proceedings Water Quality Technology Conf:2006–2033.
Driscoll, C.T., and W.D. Schecher. 1990. The chemistry of aluminum in the environment. Environmental Geochemistry and Health 12(1–2): 28–49.
Esteves, F.A., A. Caliman, J.M. Santangelo, R.D. Guariento, V.F. Farjalla, and R.L. Bozelli. 2008. Neotropical coastal lagoons: an appraisal of their biodiversity, functioning, threats and conservation management. Brazilian Journal of Biology 68(4 Suppl): 967–981.
Gao, L., X. Pan, D. Zhang, S. Mu, D.-J. Lee, and U. Halik. 2015. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Research 69: 51–58.
Gebbie, P. 2001. Using polyaluminium coagulants in water treatment. 64th Annual Water Industry Engineers and Operators Conference:39–47.
Gensemer, R.W., and R.C. Playle. 1999. The bioavailability and toxicity of aluminum in aquatic environments. Critical Reviews in Environmental Science and Technology 29(4): 315–450.
Gomes, A.M.A., P.L. Sampaio, A. Ferrão-Filho, V. Magalhães, M.M. Marinho, A.C. Pimentel de Oliveira, V. Barbosa dos Santos Domingos, P. Azevedo, and M.F.O. Sandra. 2009. Florações de cianobactérias tóxicas em uma lagoa costeira hipereutrófica do Rio de Janeiro/RJ (Brasil) e suas consequências para saúde humana. Oecologia Brasiliensis 13(2): 329–345.
Granados, M.R., F.G. Acién, C. Gómez, J.M. Fernández-Sevilla, and E. Molina Grima. 2012. Evaluation of flocculants for the recovery of freshwater microalgae. Bioresource Technology 118: 102–110.
Heisler, J., P.M. Glibert, J.M. Burkholder, D.M. Anderson, W. Cochlan, C. Dennison, Q. Dortch, C.J. Gobler, C.A. Heil, E. Humphries, A. Lewitus, R. Magnien, H.G. Marshallm, K. Sellner, D.A. Stockwell, D.K. Stoecker, and M. Suddleson. 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8: 3–13.
Jančula, D., and B. Maršálek. 2011. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85(9): 1415–1422.
Jeppesen, E., P. Kristensen, J.P. Jensen, M. Søndergaard, E. Mortensen, and T. Lauridsen. 1991. Recovery resilience following a reduction in external phosphorus loading of shallow, eutrophic Danish lakes: duration, regulating factors and methods for overcoming resilience. Memorie dell’Istituto Italiano di Idrobiologia 48: 127–148.
Kennish, M.J. 2002. Environmental threats and environmental future of estuaries. Environmental Conservation 29(1): 78–107.
Kennish, M.J., M.J. Brush, and K.A. Moore. 2014. Drivers of change in shallow coastal photic systems: an introduction to a special issue. Estuaries and Coasts 37(Suppl 1): S3–S19.
Li, L., and G. Pan. 2013. A universal method for flocculating harmful algal blooms in marine and fresh waters using modified sand. Environmental Science & Technology 47(9): 4555–4562.
Li, H., and G. Pan. 2015. Simultaneous removal of harmful algal blooms and microcystins using microorganism- and chitosan-modified local soil. Environmental Science and Technology 49(10): 6249–6256.
Lürling, M., and F. van Oosterhout. 2013. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Research 47(17): 6527–6537.
Lürling, M., D. Meng, and E.J. Faassen. 2014. Effects of hydrogen peroxide and ultrasound on biomass reduction and toxin release in the cyanobacterium, Microcystis aeruginosa. Toxins 6(12): 3260–3280.
Magalhães, V.F., R.M. Soares, and S.M.F.O. Azevedo. 2001. Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon 39: 1077–1085.
Matthijs, H.C.P., P.M. Visser, B. Reeze, J. Meeuse, P.C. Slot, G. Wijn, R. Talens, and J. Huisman. 2012. Selective suppression of harmful cyanobacteria in an entire lake withhydrogen peroxide. Water Research 46: 1460–1472.
Merel, S., D. Walker, R. Chicana, S. Snyder, E. Baurès, and O. Thomas. 2013. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International 59: 303–327.
Noyma, N., L. de Magalhães, L. Lima Furtado, M. Mucci, F. van Oosterhout, V.L.M. Huszar, M.M. Marinho, and M. Lürling. 2016. Controlling cyanobacterial blooms through effective flocculation and sedimentation with combined use of flocculents and phosphorus adsorbing natural soil and modified clay. Water Research 97: 26–38. doi:10.1016/j.watres.2015.11.057.
Nürnberg, G.K. 1996. Trophic state of clear and colored, soft-and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake and Reservoir Management 12(4): 432–447.
Paerl, H.W., N.S. Hall, B.L. Peierls, and K.L. Rossignol. 2014. Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuaries and Coasts 37: 243–258.
Pan, G., M.M. Zhang, H. Chen, H. Zou, and H. Yan. 2006a. Removal of cyanobacterial blooms in Taihu Lake using local soils. I. Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Environmental Pollution 141(2): 195–200.
Pan, G., H. Zou, H. Chen, and X. Yuan. 2006b. Removal of harmful cyanobacterial blooms in Taihu Lake using local soils III. Factors affecting the removal efficiency and an in situ field experiment using chitosan-modified local soils. Environmental Pollution 141(2): 206–212.
Pan, G., J. Chen, and D.M. Anderson. 2011a. Modified local sands for the mitigation of harmful algal blooms. Harmful Algae 10(4): 381–387.
Pan, G., B. Yang, D. Wang, H. Chen, B.H. Tian, M.L. Zhang, X.Z. Yuan, and J. Chen. 2011b. In-lake algal bloom removal and submerged vegetation restoration using modified local soils. Ecological Engineering 37(2): 302–308.
Pan, G., L. Dai, L. Li, L. He, H. Li, L. Bi, and R.D. Gulati. 2012. Reducing the recruitment of sedimented algae and nutrient release into the overlying water using modified soil/sand flocculation-capping in eutrophic lakes. Environmental Science & Technology 46(9): 5077–5084.
Qun, G., and W. Ajun. 2006. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydrate Polymers 64: 29–36.
Renault, F., B. Sancey, P.M. Badot, and G. Crini. 2009. Chitosan for coagulation/flocculation processes—an eco-friendly approach. European Polymer Journal 45(5): 1337–1348.
Sengco, M.R., and D.M. Anderson. 2004. Controlling harmful algal blooms through clay flocculation. The Journal of Eukaryotic Microbiology 51(2): 169–172.
Smith, V.H., and D.W. Schindler. 2009. Eutrophication science: where do we go from here? Trends in Ecology & Evolution 24(4): 201–207.
Søndergaard, M., J.P. Jensen, and E. Jeppesen. 1999. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 408(409): 145–152.
Stumm, W., and J. Morgan. 1996. Aquatic chemistry, chemical equilibra and rates in natural waters. Environmental Science and Technology Series.
Tripathy, T., and B.R. De. 2006. Flocculation: a new way to treat the waste water. Journal of Physical Sciences 10: 93–127.
Uhelingher, V. 1964. Étude statistique dês méthodes de dénobrement planctonique. Archive Science 77(2): 121–123.
Utermöhl, H. 1958. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitteilungen der Internationalen Vereinigung der Theoretischen und Angewandten Limnologie 9: 1–38.
Vandamme, D., I. Foubert, and K. Muylaert. 2013. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends in Biotechnology 31(4): 233–239.
Verspagen, J.M.H., P.M. Visser, and J. Huisman. 2006. Aggregation with clay causes sedimentation of the buoyant cyanobacterium Microcystis. Aquatic Microbial Ecology 44: 165–174.
Verweij, W. 2015. CHEAQS Next – Chemical Equilibria in Aquatic Systems, version P2015.3, http://www.cheaqs.eu/.
Waajen, G., F. Van Oosterhout, G. Douglas, and M. Lürling. 2016. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant—Lanthanum modified bentonite treatment. Water Research 97: 83–95. doi:10.1016/j.watres.2015.11.034.
Wang, Z., D. Li, H. Qin, and Y. Li. 2012. An integrated method for removal of harmful cyanobacterial blooms in eutrophic lakes. Environmental Pollution 160: 34–41.
Wauer, G., H.J. Heckemann, and R. Koschel. 2004. Analysis of toxic aluminium species in natural waters. Microchimica Acta 146: 149–154.
Zou, H., G. Pan, H. Chen, and X. Yuan. 2006. Removal of cyanobacterial blooms in Taihu Lake using local soils II. Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan. Environmental Pollution 141(2): 201–205.
Acknowledgments
This study was sponsored by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil, through a Science without Borders Grant, SwB (400408/2014-7) and by Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro, FAPERJ, Brasil (111.267/2014). L. De Magalhães PhD scholarship was funded by Federal Government of Brazil, Ministry of Education, through CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Ministério da Educação). V.Huszar was partially supported by CNPq (309700/2013-2). M. Mucci PhD scholarship was funded by SwB/CNPq (201328/2014-3). This study was conducted under the flag of the CAPES (Brazil)/NUFFIC (The Netherlands) project 045/12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hans W. Paerl
Rights and permissions
About this article
Cite this article
de Magalhães, L., Noyma, N.P., Furtado, L.L. et al. Efficacy of Coagulants and Ballast Compounds in Removal of Cyanobacteria (Microcystis) from Water of the Tropical Lagoon Jacarepaguá (Rio de Janeiro, Brazil). Estuaries and Coasts 40, 121–133 (2017). https://doi.org/10.1007/s12237-016-0125-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0125-x