Abstract
The invasive cordgrass, Spartina densiflora, has demonstrated a high tolerance to salinity stress. However, no attempt has yet been made to assess the intra-specific variation in salinity tolerance among S. densiflora populations. The aim of this study was to analyze the effects of provenance and salinity (0, 80, 170, 510, and 1023 mM NaCl) on germination, seedling survival, growth, and physiological performance of S. densiflora adult plants from three populations of the Gulf of Cadiz (Odiel, Tinto, and Piedras, SW Spain). Our results indicate that a salinity increment decreased germination and seedling survival of all S. densiflora populations, but these impairments were also dependent on the original population of the seeds. Accordingly, the Odiel population showed greater germination (70 %) and seedling survival (86 %) percentages at 510 mM NaCl than Tinto and Piedras populations, with mean percentage values of 57 % germination and 55 % seedling survival. These differences were also clearly reflected in long-term measurements, such as those regarding total dry mass, relative growth rate, and tiller number, which were mainly determined by the extent of photosynthetic area rather than the variations in net photosynthetic rate. Also, the higher tolerance of the Odiel population could be partly explained by a reduced level of Na in its tissues. Consequently, all populations might have the ability to colonize brackish marshes and river banks, but their invasion would be severely limited under hypersaline conditions, except in the case of the Odiel population, which would have a greater capacity for invasion of sub-optimal habitats, such a salt pans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spartina densiflora is a halophyte cordgrass with a very wide latitudinal distribution. It is native to South America, where it appears from Sao Paulo state (Brazil; ca. 23° 20′ S to) to Rio Gallegos city (Argentina; ca. 51° 33′S), and it is even invading salt marshes in Chile (ca. 33° 30′ S–42° 46′ S). In the northern hemisphere, it is colonizing marshes in California (ca. 37° 56′ N) and Washington states (ca. 49° 20′ N) in the USA, British Columbia (Vancouver Island; ca. 49° 28′ N) in Canada, in North Africa (Morocco; ca. 34° 50′ N), and in the southwest (SW) Iberian Peninsula (ca. 36° 02′ N–37° 21′ N) (Bortolus 2006; Saarela 2012; Castillo et al. 2014). In its native area, it is found from the lowest to the highest topographic levels of tidal marshes, including ecotones with adjacent terrestrial ecosystems (Costa et al. 2003). Similarly, in Mediterranean marshes, it can grow in soils ranging from well to very poorly oxygenated, as well as with a wide range of conductivity levels, varying from brackish to saline, or temporarily hypersaline, as occurs in salt pans (Nieva et al. 2001).
Many aspects of S. densiflora have hitherto been analyzed, a major conclusion being that its invasive potential derives from its prolific seed production and from its clonal growth (Kittelson and Boyd 1997; Bortolus 2006; Nieva et al. 2001). In addition, its high ecological (Bortolus 2006), phenotypic (Bortolus et al. 2004; Bortolus 2006; Castillo et al. 2008, 2014), and physiological (Mateos-Naranjo et al. 2007, 2011; Idazkin and Bortolus 2011) plasticity apparently allows S. densiflora to tolerate wide ranges of salinity, tidal submergence, drainage, and nutrient availability (Alberti et al. 2010; Costa et al. 2003; Castillo et al. 2005; Mateos-Naranjo et al. 2007). Nevertheless, despite the extensive information on these aspects, further investigations on the responses of different populations to the main environmental factors that are known to influence plant distribution and growth in coastal mashes, particularly salinity (Nestler 1977; DeLaune et al. 1983), will provide us with a greater insight into the invasive potential of this species. From an ecological perspective, this is particularly important because the invasiveness of an exotic species can depend on the specific population (Fortune et al. 2008) and the different impact of environmental factors such as salinity on each population. In this regard, the contrasting invasiveness reported for S. densiflora in different regions worldwide suggests a similar situation (Bortolus 2006) in the Gulf of Cadiz (SW of Spain).
The main goal of this study was, thus, to identify differences in salinity tolerance between S. densiflora populations. In addition, the majority of the experimental studies related to intra-specific variability within Spartina species have focused on adult plants, and no attention has been paid to other stages of the life cycle of these halophytic species. This aspect is of remarkable interest because the disconnection between optimum salinity ranges for germination, seedling, and adult stages has been identified for many halophytes (Ungar 1978; Woodell 1985). The specific objectives were to (1) investigate how an increase in salinity affected seed germination and early survival of seedlings from each population of S. densiflora; (2) analyze the growth responses of the populations of S. densiflora and ascertain the extent to which the effects on the photosynthetic apparatus (PSII) and gas exchange parameters determine plant performance; and, finally, (3) analyze the concentrations of Na, Ca, K, and Mg in all the tissues of S. densiflora.
Materials and Methods
Seed Collection and Salinity Characterization According to Origin
In December 2008, ripe spikes of S. densiflora were collected from three different populations (from 20 different clumps each) located in Tinto, Odiel, and Piedras marshes, respectively (Huelva, SW Spain; Fig. 1). Caryopses were stripped from the spikes, and those containing seeds were selected and stored under dark conditions at 4 °C. Furthermore, for the seed selections, we considered the study of Nieva (1996), which found a great variability in mature seed production at individual plant level. To avoid this effect, all seeds employed in our germination experiment were checked that it had mature embryos, and also, we did not observe differences in weight between them (data not presented).
Map showing a the location of Odiel, Tinto, and Piedras salt marshes (Huelva, SW Spain) and b the sampling sites. Adapted from Mateos-Naranjo et al. (2011)
Prior to seed collection, samples of the first 10 cm of sediment were taken on the sites of each of the three selected S. densiflora populations for their characterization in relation to salinity conditions in soil. All sediment samples were placed into individual plastic bags and subsequently carried to the laboratory. Conductivity of sediment samples (n = 5) was measured with a conductivity meter (Crison-522, Spain) after mixing each sample with distiller water (1:1; Mateos-Naranjo et al. 2008). Sediment conductivity was reported using the Practical Salinity Scale (Lewis 1980).
Seed Germination Experiment
Three months after seed storage, four 25-seed replicates of each Spartina population were placed on filter paper in 5 cm petri dishes. Five solutions of 0, 80, 170, 510, and 1023 mM NaCl were added to each dish, made up with distilled water (four petri dishes per population × five NaCl concentration combinations; n = 60). Dishes were wrapped in paraffin to avoid water loss through evaporation, placed in a germinator (ASL Aparatos Científicos M-92004, Madrid, Spain) and subjected to a regime of 16 h of light (25 °C, 400–700 nm, 35 μmol photon m−2 s−1) and 8 h of dark (12 °C). This temperature regime was chosen to replicate temperatures of SW Spain marshes in spring, when this species germinates. Dishes were daily inspected for 30 days and seed germination acknowledged after cotyledon appearance. Likewise, water level was also adjusted daily by adding distilled water to avoid changes in NaCl concentration. The three lower NaCl concentrations were chosen to reproduce the electrical conductivity values recorded in each sampling population, and the two higher were added to reproduce temporarily hypersaline conditions in the Gulf of Cadiz marshes, as occurs in salt pans (Nieva 2001).
Two germination characteristics were determined: final germination percentage and mean time to germination (MTG). MTG was calculated using the equation
where n is the number of seeds germinated by day i, d is the observation period in days, and N is the total number of germinating seeds (Redondo-Gómez et al. 2007).
Seedling Survival and Plant Tolerance Response Experiment
Germinated seedlings (n = 20) from the germination experiment on each population were immediately transferred to individual plastic pots (9 cm height and 11 cm diameter) filled with pearlite and placed in the greenhouse with a temperature range of 21–25 °C, 40–60 % relative humidity, and natural daylight (maximum light flux: 1000 μmol m−2 s−1). Seedlings were allocated to the same NaCl treatments under which they had germinated. Salinity treatments of 0, 80, 170, and 510 mM NaCl were established by using 20 % modified Hoagland’s solution (Hoagland and Arnon 1938) and NaCl of the appropriate concentration. Salinity concentration of 1023 mM NaCl was not used at this stage due to the previous lack of seed germination. Saline solutions were placed down to a depth of 1 cm in plastic trays, and plastic pots containing the transplanted seedlings were placed in these trays and distributed randomly in the glasshouse (20 plants per population × four NaCl concentration combinations; n = 80). During the experiment, tray levels were monitored and topped up to the marked level with 20 % Hoagland’s solution (without additional NaCl) to limit the change of NaCl concentration caused by water evaporation from nutrient solution. In addition, the entire solution (including NaCl) was changed on a weekly basis. To avoid border effect and the influence of their placement in the glasshouse, the position of trays was randomly changed every 2 days.
Fifty days after seedling sowing, the number of surviving seedlings was recorded. Moreover, in order to assess the response of plants from each population with a more advanced state of development to salinity, nine seedlings from each treatment were kept under the same salinity conditions for over 3 months (100 days) (nineplants per population × four NaCl concentration combinations; n = 36). At the end of the experiment, i.e., 150 days after seedling sowing, whole plants (roots and leaves included) from each treatment were dried at 80 °C for 48 h and then weighed. In addition, 5 days before harvest, when plants appeared to show a stable growth rate, measurements of gas exchange and chlorophyll fluorescence were performed on the youngest fully expanded leaves. Finally, nutrient concentrations in tissues were also analyzed.
Gas Exchange
Gas exchange measurements were performed at midday on randomly selected, fully expanded penultimate leaves (n = 9, one measurement per plant) using an infrared gas analyzer in an open system (LI-6400, LI-COR Inc., Neb., USA). Net photosynthetic rate (A N), intercellular CO2 concentration (C i), and stomatal conductance to CO2 (g s) were determined at ambient CO2 concentration of 380 ppm CO2, temperature of 20 °C, 50 ± 5 % relative humidity, and a photon flux density of 1000 μmol m−2 s−1. A N, C i, and g s were calculated using standard formulae of Von Caemmerer and Farquhar (1981). Photosynthetic area was approximately estimated as the area of a trapezium. Intrinsic water-use efficiency (iWUE) was calculated as the ratio between A N and g s.
Chlorophyll Fluorescence
Chlorophyll fluorescence was randomly measured in fully developed penultimate leaves (n = 9, one measurement per plant) using a portable modulated fluorometer (FMS-2, Hansatch Instruments Ltd., England). Light- and dark-adapted fluorescence parameters were measured at dawn (stable, 50 μmol m−2 s−1 ambient light) and at midday (1600 μmol m−2 s−1) to investigate whether salt concentration affected the sensitivity of plants to photoinhibition (Qiu et al. 2003).
Plants were dark-adapted for 30 min by using leaf clips exclusively designed for this purpose. The minimal fluorescence level in the dark-adapted state (F 0) was measured using a modulated pulse (<0.05 μmol m−2 s−1 for 1.8 μs) which was too small to induce significant physiological changes in the plants. Thus, the stored data were average values taken over 1.6 s periods. Maximal fluorescence in this state (F m) was measured after applying a saturating actinic light pulse of 15,000 μmol m−2 s−1 for 0.7 s. The value of F m was recorded as the highest average of two consecutive measurements. Values of the variable fluorescence (F v = F m − F 0) and maximum quantum efficiency of PSII photochemistry (F v/F m) were calculated from F 0 and F m values. This ratio of variable to maximal fluorescence correlates with the number of functional PSII reaction centers, allowing dark-adapted values of F v/F m to be used to quantify photoinhibition (Maxwell and Johnson 2000).
The same leaf section of each plant was used to measure light-adapted parameters. Steady-state fluorescence yield (F s) was recorded after adapting plants to ambient light conditions for 30 min. A saturating actinic light pulse of 15,000 μmol m−2 s−1 for 0.7 s was then used to produce the maximum fluorescence yield (F m′) by temporarily inhibiting PSII photochemistry.
Quantum efficiency of PSII (ΦPSII = (F m′ − F s) / F m′) was calculated using the fluorescence parameters determined for light- and dark-adapted states.
Elemental Analysis
At the end of the plant tolerance response experiment, leaf and root samples of S. densiflora plants were separately dried at 80 °C for 48 h and ground. Leaves and roots were carefully washed with distilled water before any further analysis. Then, 0.5 g samples were taken from a leaf or root mixture from the nine plants of each treatment. Tissue samples were tripicately digested with 6 ml HNO3 (3:1 v/v), 0.5 ml HF, and 1 ml H2O2 at 130 °C for 5 h. Finally, Na, Ca, K, and Mg concentrations were measured by inductively coupled plasma (ICP) spectroscopy (ARL-Fison 3410, USA).
Statistical Analysis
Statistical analyses were carried out using Statistica v. 6.0 (Statsoft Inc.). Data were analyzed using one- and two-way analysis of variance (ANOVA test). Data were first tested for normality with the Kolmogorov-Smirnov test and for homogeneity of variance with the Brown-Forsythe test. Significant test results were followed by Tukey tests for identification of important contrasts. One-way analysis of variance was performed at salinity of 510 mM NaCl to compare differences between S. densiflora population in order to avoid the statistical influence of the lowest salinity data and the lack of information at 1020 mM NaCl, where the weight of the population variable was statistically insignificant. Finally, the comparison between measurements of fluorescence at dawn and midday was performed by Student’s test (t test).
Results
Soil Salinity of the Three Spartina densiflora Populations
Electrical conductivity values indicated that salinity was consistently higher in the soil from the Odiel population, with values of 15.4 ± 0.8 mS cm−1 (a. 170 mM NaCl), than in Tinto and Piedras soils, with values of 9.7 ± 0.6 (a. 110 mM NaCl) and 6.9 ± 0.6 mS cm−1 (a. 80 mM NaCl), respectively.
Seed Germination Experiment and Seedling Survival
Final germination decreased significantly with increasing external salinity concentration in all S. densiflora populations (two-way ANOVA, salinity: F 3,36 = 12.7, p < 0.001; Table 1), with overall lack of germination at the highest concentration. While the site of origin of the seeds and the interaction between salinity and provenance showed no significant effects in our two-way ANOVA model (population: F 2,36 = 2.6, p = 0.08; salinity × population: F 6,36 = 1.5, p > 0.05; Table 1), at 510 mM NaCl, however, the percentage of germinated seeds from the Odiel population (71 ± 1 %) was significantly higher than those from Tinto (62 ± 4 %) and Piedras (53 ± 3 %) populations, the latter showing the lowest final germination percentage (one-way ANOVA, population: F 2,9 = 6.8, p < 0.05).
Fifty days after seed sowing, seedling survival was adversely affected at 510 mM NaCl in all S. densiflora populations; however, this negative effect was considerably greater in seedlings from the Piedras population (Table 1), with a survival reduction of 55 %.
Plant Tolerance Response Experiment
Growth Analysis
There was a significant interaction between salinity and origin of seeds on S. densiflora growth. Thus, total dry mass was substantially reduced at the highest salinity concentration (510 mM NaCl) across all populations (two-way ANOVA, salinity: F 3,96 = 60.5, p < 0.05; Fig. 2a), but this reduction was more pronounced in plants from Tinto and Piedras populations. Also, at 170 mM NaCl, plants from the Odiel and Tinto populations showed greater total dry mass than plants from the Piedras population. The same trends were evident in relative growth rate (RGR) (Fig. 2b), the effects of salinity and origin of seeds being highly significant on RGR after 150 days of treatment (two-way ANOVA, salinity × population: F 6,96 = 4.0, p < 0.001). Finally, the number of tillers also decreased at 510 mM NaCl (two-way ANOVA, salinity: F 3,96 = 42.3, p < 0.001; Fig. 2c) but was affected neither by the origin of seeds nor by an interaction between salinity and seed origin. However, at 510 mM NaCl, the number of tillers was greater in plants from the Odiel than from the Piedras population (one-way, population: ANOVA, F 2,24 = 6.5, p < 0.05).
Gas Exchange
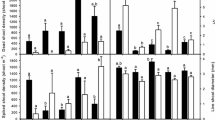
Overall, net photosynthetic rate (A N) data were similar across all salinity treatments at each of the three S. densiflora populations (two-way ANOVA, salinity × population: F 6,96 = 0.79, p > 0.05; Fig. 3a), showing values c. 14 μmol m−2 s−1 CO2 in all cases.
Net photosynthetic rate, A N (a), stomatal conductance, g s (b), intercellular CO2 concentration, C i (c) and intrinsic water-use efficiency, iWUE (d) in randomly selected, fully expanded penultimate leaves of adult plants from three S. densiflora populations in response to a range of NaCl concentrations for 150 days. Shown values are mean ± S.E, n = 9
Stomatal conductance (g s) and intercellular CO2 concentration (C i) were greater in plants grown in the presence of NaCl, while iWUE decreased under salinity conditions (two-way ANOVA, salinity: F 3,96 = 15.2, p < 0.001 for g s; F 3,96 = 6.5, p < 0.001 for C i and F 3,96 = 34.9, p < 0.001 for iWUE, respectively; Fig. 3b, c, d). Furthermore, those variables showed no significant differences between populations (two-way ANOVA, population: F 2,96 = 0.9 for g s; F 2,96 = 0.1 for C i and F 2,96 = 0.4 for iWUE, p > 0.05).
Chlorophyll Fluorescence
F v/F m and Φ PSII values at dawn were uniformly high at all external NaCl concentrations, with values around 0.87 and 0.84 for all S. densiflora populations, respectively (Table 2). F v/F m and Φ PSII values at midday were always lower than at dawn (t test, p < 0.05), probably due to the lower F m and qP values at midday than at dawn (data not presented). Also, both parameters did not show differences among S. densiflora populations and salinity treatments at midday (two-way ANOVA, salinity × population: F 6,96 = 0.4 for F v/F m and F 6,96 = 0.2 for Φ PSII, p > 0.05; Table 2).
Chemical Analysis of Plant Samples
Mineral analysis data showed that tissue Na concentration increased markedly with external NaCl concentration across all S. densiflora populations, this increment being more obvious in plants from the Tinto and Piedras populations (two-way ANOVA, salinity × population: F 6,24 = 92.5 for roots and F 6,24 = 94.2 for leaves, p < 0.001; Fig. 4a, b). Contrastingly, overall tissue Ca, K, and Mg concentrations decreased with increasing salinity in all S. densiflora populations. Finally, no significant differences were found among populations (two-way ANOVA population, Ca: F 2,24 = 3.6 for roots and F 2,24 = 2.9 for leaves; K: F 2,24 = 3.9 for roots and F 2,24 = 4.1 for leaves; Mg: F 2,24 = 3.3 for roots and F 2,24 = 2.31 for leaves, p > 0.05; Figs. 4c, d, e, f, g, h).
Discussion
The capacity of S. densiflora seeds to germinate is a primary determinant of the invasive potential of this species. Our results indicate that seed germination was completely inhibited at 1020 mM NaCl for all S. densiflora populations, and final germination was lower at 510 mM NaCl. However, in the latter case, we found a certain variability among S. densiflora populations, with seeds collected from Odiel population showing a greater percentage of germination than those from the Tinto and Piedras populations. The decrease in S. densiflora final germination percentage at higher NaCl concentrations agrees with previous studies on S. densiflora (Nieva et al. 2001) and with the general trend for other halophytes (Ungar 1978; Woodell 1985; Baskin and Baskin 1998). Also, in line with our results, intra-specific variability in germination rates between plant populations of halophytic species such as Phragmites autralis, Atriplex halimus, Cakile maritime, and Polypogon monspeliensis has been already reported (Mauchamp and Mésleard 2001; Debez et al. 2001; Ghars et al. 2009; Atia et al. 2011).
Additionally, the establishment period is also a crucial stage of the life cycle of plants under saline conditions (Khan and Gulzar 2003; Perez et al. 1998; Waisel 1972). In accordance with the results regarding germination, seedling survival was adversely affected at 510 mM NaCl across all S. densiflora populations after 50 days of seed sowing, the seedlings from the Odiel population showing nonetheless a greater percentage of survival than those from the Tinto and especially from the Piedras population. These differences in survival were also clearly reflected in long-term plant tolerance responses.
Concerning growth, our results reveal that although high salinity concentrations impaired the growth of all S. densiflora populations, there were significant differences. Thus, the biomass of plants from Odiel, though reduced, was still a 58 % superior to the biomass of the other two populations at 510 mM NaCl and a 22 % superior to that of the Piedras population at 170 mM NaCl. This trend was also apparent in the RGR and number of tillers. These findings are consistent with those of Pezeshki (1991), who observed differences in leaf area between Spartina patens populations. Furthermore, population differentiation in terms of salinity tolerance has also been identified in Spartina alterniflora (Pezeshki and DeLaune 1995) and in other emergent macrophytes (Howard 2010).
Regarding gas exchange measurements and photochemical efficiency of PSII, A N and F v/F m and Φ PSII responses did not correspond to those of RGR, as might have been expected. Therefore, differences in growth between S. densiflora populations could be due to developmental differences of photosynthetic area rather than to variations in A N. Hence, similar A N could be more than compensated for by a greater photosynthetic area in plants from Odiel population at 170 and 510 mM NaCl.
On the other hand, differences in salt tolerance often originate from reduced levels of Na absorption or accumulation. Thus, halophytes can control tissue Na concentration through several processes, such as ion exclusion at the root level and secretion of ions from leaves through salt glands (Anderson 1974; Flowers 1985; Bradley and Morris 1991). Our chemical analysis showed a progressive accumulation of Na with increasing salinity in all S. densiflora populations, indicating the effective compartmentation of salt in the vacuoles, which is indeed a hallmark of halophytes (Munns 2002). However, the most remarkable finding arising from these results was that higher Na concentrations in tissues were measured in salt-treated plants from Piedras and Tinto populations, this revealing that the higher tolerance to salinity of Odiel plants could be partly accounted for by a reduced level of Na absorption or accumulation.
Despite the fact that seeds in our experiment were not kept within individuals separate, being able to be considered as a pseudoreplicate, the use of multiple pooled to produce a population sample of seeds to look for germination and survival could be fair representation of the population, since prior evidences did not show any statistical differences in germination capacity between mature seeds in individual plants of S. densiflora in the same area (Mateos-Naranjo et al. 2008, 2011). Thus altogether, the results of this study manifest a differential response of the three populations of S. densiflora to salinity-induced stress. The Odiel population has developed better responses to environmental salinity than the other two populations and is able to preserve the capacity of its seeds to germinate and grow to a greater extent, despite the very high salt concentration in its growth substrate. Therefore, this population might have an advantage over the others, which lack strategies to successfully deal with high salt concentrations. This greater salt tolerance appears to be partly accomplished by Na absorption or accumulation regulation in its tissues.
Perspectives on Invasion and Management of S. densiflora in Relation with Population Responses to Salinity
On a regional scale, in the Gulf of Cádiz (SW of Spain), S. densiflora invasion shows a lack of correspondence between the degree of invasion and the location of the marshes, so that marshes very close to each other (like the highly invaded Odiel Marshes and the scarcely invaded Piedras marshes) show very different degrees of invasion (Mateos-Naranjo et al. 2009). From a perspective of management of S. densiflora invasion, this discontinuous distribution pattern of the invasion makes it necessary to identify areas of new invasions and characterize populations in relation with their invasiveness potential in order to prioritize the interventions. In this respect, the results of this experiment are particularly relevant, since they prove that although the invasiveness in terms of tolerance to salinity stress was greater in the Odiel population, plants from all populations of S. densiflora were able to germinate, survive, and grow at higher salinity levels than those present in their soils of origin. This capacity for active colonization of brackish marshes and river banks is limited in areas which are hypersaline or close to the sea. The most remarkable finding regarding the less pronounced limitation of the Odiel population was the carryover of this differential salinity tolerance from S. densiflora seeds to the subsequent stages of seedling establishment and adult plant development. Thus, this population would have also a greater ability for invasion of sub-optimal habitats, such a salt pans, from which it could begin to colonize new non-invaded estuaries on account of its acknowledged powers of clonal growth, such as non-invaded marshes in Guadiana and Guadalquivir estuaries (Gulf of Cadiz, SW Spain) and marshes of Ria Formosa (S Portugal). This aspect should be taken into account to pay priority attention to this population in the management strategies of the invasion in the Gulf of Cadiz and to prevent new invasions in European and North African salt marshes. Furthermore, future research on the interaction of salinity with other important environmental factors in the marshes, such as flooding and tidal cycles, should be considered to improve our capacity to predict future patterns of invasion of S. densiflora.
References
Alberti, J., A. Méndez Casariego, P. Daleo, E. Fanjul, B. Sillimam, M. Bertness, and O. Iribarne. 2010. Abiotic stress mediates top-down and bottom-up control in a Southwestern Atlantic salt marsh. Oecologia 163: 181–191.
Anderson, C.E. 1974. A review of structure in several North Carolina salt marsh plants. In Ecology of halophytes, ed. R.J. Reimold and W.H. Queens, 307–344. New York: Academic Press.
Atia, A., A. Smaoui, Z. Barhoumi, C. Abdelly, and A. Debez. 2011. Differential response to salinity and water deficit stress in Polypogon monspeliensis (L.) Desf. provenances during germination. Plant Biology 13: 541–545.
Baskin, C., and J.M. Baskin. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press.
Bortolus, B. 2006. The austral cordgrass Spartina densiflora Brong.: Its taxonomy, biogeography and natural history. Journal of Biogeography 33: 158–168.
Bortolus, A., P. Laterra, and O. Iribarne. 2004. Crab-mediated phenotypic changes in Spartina densiflora Brong. Estuarine, Coastal and Shelf Science 59: 97–107.
Bradley, P.M., and J.T. Morris. 1991. The influence of salinity on NH4? uptake in Spartina alterniflora. Oecologia 85: 375–380.
Castillo, J.M., E. Rubio-Casal, S. Redondo, A.A. Álvarez-López, T. Luque, C. Luque, F.J. Nieva, E.M. Castellanos, and M.E. Figueroa. 2005. Short-term responses to salinity of an invasive cordgrass. Biological Invasions 7: 29–35.
Castillo, J.M., E. Mateos-Naranjo, F.J. Nieva, and M.E. Figueroa. 2008. Plant zonation at salt marshes of the endangered cordgrass Spartina maritima invaded by Spartina densiflora. Hydrobiologia 614: 363–371.
Castillo, J.M., B.J. Grewell, A. Pickart, A. Bortolus, C. Peña, E. Figueroa, and M. Sytsma. 2014. Phenotypic plasticity of invasive Spartina densiflora (Poaceae) along a broad latitudinal gradient on the pacific coast of North America. American Journal of Botany 101: 448–458.
Costa, C.S.B., J.C. Marangoni, and A.M.G. Azevedo. 2003. Plant zonation in irregularly flooded salt marshes: Relative importance of stress tolerance and biological interactions. Journal of Ecology 91: 951–965.
Debez, A., W. Chaibi, and S. Bouzid. 2001. Effet de NaCl et de régulateurs de croissance sur la germination d’Atriplex halimus L. Cahiers Agricultures 10: 135–138.
DeLaune, R.D., C.J. Smith, and W.H. Patrick Jr. 1983. Relationship of marsh elevation, redox potential and sulfide to Spartina alterniflora productivity. Soil Science Society of America Journal 47: 390–395.
Flowers, T.J. 1985. Physiology of halophytes. Plant and Soil 89: 41–56.
Fortune, P.M., K. Schierenbeck, D. Ayres, A. Bortolus, O. Catrice, S. Brown, and M.L. Ainouche. 2008. The enigmatic invasive Spartina densiflora: A history of hybridizations in a polyploidy context. Molecular Ecology 14: 4304–4316.
Ghars, M.A., A. Debez, and C. Abdelly. 2009. Interaction between salinity and original habitat during germination of the annual seashore halophyte Cakile maritima. Communications in Soil Science and Plant Analysis 40: 3170–3180.
Hoagland, D., and D.I. Arnon. 1938. The water culture method for growing plants without soil. California Agricultural Experiment Station Bulletin 347: 1–39.
Howard, R.J. 2010. Intraspecific variation in growth of marsh macrophytes in response to salinity and soil type: Implications for wetland restoración. Estuaries and Coasts 33: 127–128.
Idazkin, Y.L., and A. Bortolus. 2011. Does low temperature prevent Spartina alterniflora from expanding toward the austral-most salt marshes? Plant Ecology 212: 553–561.
Khan, M.A., and S. Gulzar. 2003. Germination responses of Sporobolus ioclados: A saline desert grass. Journal of Arid Environments 55: 453–464.
Kittelson, P.M., and M.J. Boyd. 1997. Mechanism of expansion for an introduced species of cordgrass, Spartina densiflora, in Humbolt Bay, California. Estuaries 20: 770–778.
Lewis, E.L. 1980. The Practical Salinity Scale 1978 and its antecedents. IEEE Journal of Oceanic Engineering 5: 3–8.
Mateos-Naranjo, E., S. Redondo-Gómez, J. Silva, R. Santos, and M.E. Figueroa. 2007. Effect of prolonged flooding on the invader Spartina densiflora Brong. Journal of Aquiatic Plant Management 45: 121–123.
Mateos-Naranjo, E., S. Redondo-Gómez, C.J. Luque, E.M. Castellanos, A.J. Davy, and M.E. Figueroa. 2008. Environmental limitations on recruitment from seed in invasive Spartina densiflora on a southern European salt marsh. Estuarine Coastal Shelf Science 79: 727–732.
Mateos-Naranjo, E., S. Redondo-Gómez, C.J. Luque, E.M. Castellanos, and M.E. Figueroa. 2009. Biological Invasions. Implications for the biodiversity in wetland ecosystems. In Advances in Environmental Research, ed. Albert T. Riley, 1–11. Hauppauge: Nova Science Publishers.
Mateos-Naranjo, E., L. Andrades-Moreno, and S. Redondo-Gómez. 2011. Comparison of germination, growth, photosynthetic responses and metal uptake between three populations of Spartina densiflora under different soil pollution conditions. Ecotoxicology and Environmental Safety 74: 2040–2049.
Mauchamp, A., and F. Mésleard. 2001. Salt tolerance in Phragmites australis populations from coastal Mediterranean marshes. Aquatic Botany 70: 39–52.
Maxwell, K., and G.N. Johnson. 2000. Chorophyll fluorescence—a practical guide. Journal of Experimental Botany 51: 659–668.
Munns, R. 2002. Comparative physiology of salt and water stress. Plant, Cell and Environment 25: 239–250.
Nestler, J. 1977. Interstitial salinity as a cause of ecophenic variation in Spartina alterniflora. Estuarine, Coastal and Marine Science 5: 707–714.
Nieva, F.J.J. 1996. Aspectos ecológicos de Spartina densiflora Brong. PhD Thesis, Universidad de Sevilla, Spain.
Nieva, F.J., A. Diaz-Espejo, E.M. Castellanos, and M.E. Figueroa. 2001. Field variability of invading populations of Spartina densiflora Brong grown in different habitats of the Odiel marshes (SW Spain). Estuarine, Coastal and Shelf Science 52: 515–527.
Perez, T., C. Moreno, G.L. Seffino, A. Grunber, J. Bravo, and A. Zenoff. 1998. Salinity effects on the early development stages of Panicum coloratum: Cultivar differences. Grass and Forage Science 53: 270–278.
Pezeshki, S.R. 1991. Population differentiation in Spartina patens: gas-exchange responses to salinity. Marine Ecology Progress series 72: 125–130.
Pezeshki, S.R., and R.D. Delaune. 1995. Variations in response of 2 us gulf-coast populations of Spartina alterniflora to hypersalinity. Journal of Coastal Research 11: 89–95.
Qiu, N., Q. Lu, and C. Lu. 2003. Photosynthesis, photosystem II efiiciency and teh Xanthophyll cycle in the salt-adapted halophyte Atriples centralasiatica. New Phytologist 159: 479–486.
Redondo-Gómez, S., E. Mateos-Naranjo, A.J. Davy, F. Fernández-Muñoz, E. Castellanos, T. Luque, and M.E. Figueroa. 2007. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Annals of Botany 100: 555–563.
Saarela, J.M. 2012. Taxonomic synopsis of invasive and native Spartina (Poaceae, Chloridoideae) in the Pacific Northwest (British Columbia, Washington and Oregon), including the first report of Spartina x townsendii for British Columbia, Canada). Phytokeys 10: 25–82.
Ungar, I.A. 1978. Halophyte seed germination. Botanical Review 44: 233–264.
Von Caemmerer, S., and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 377–387.
Waisel, Y. 1972. Biology of halophytes. New York: Academic Press.
Woodell, S.R.J. 1985. Salinity and seed germination patterns in coastal halophytes. Vegetatio 61: 223–229.
Acknowledgments
We are grateful to Dr. Raquel F. Lo Faso and Antonio Ruiz Rico for revising the English version of this manuscript. We also thank the Spanish Science and Technology Ministry and Junta de Andalucía for their support (projects CTM2008-04453 and RNM07274) and Seville University Glasshouse General Services for their collaboration. We also thank the useful comments of two anonymous reviewers who greatly helped to improve the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Edwin DeHaven Grosholz
Rights and permissions
About this article
Cite this article
Mateos-Naranjo, E., Redondo-Gómez, S. Interpopulation Differences in Salinity Tolerance of the Invasive Cordgrass Spartina densiflora: Implications for Invasion Process. Estuaries and Coasts 39, 98–107 (2016). https://doi.org/10.1007/s12237-015-9956-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-9956-0