Abstract
West Falmouth Harbor, a shallow lagoon on Cape Cod, has experienced a threefold increase in nitrogen load since the mid- to late 1990s due to input from a groundwater plume contaminated by a municipal wastewater treatment plant. We measured the exchange of nitrogen and phosphorus between the harbor and the coastal waters of Buzzards Bay over several years when the harbor was experiencing this elevated nitrogen load. During summer months, the harbor not only retained the entire watershed nitrogen load but also had a net import of nitrogen from Buzzards Bay. During the spring and fall, the harbor had a net export of nitrogen to Buzzards Bay. We did not measure the export in winter, but assuming the winter net export was less than 112 % of the load, the harbor exported less than half of the watershed nitrogen load on an annual basis. For phosphorus, the harbor had a net import from coastal waters in the spring and summer months and a net export in the fall. Despite the large increase in nitrogen load to the harbor, the summertime import of phosphorus from Buzzards Bay was sufficient to maintain nitrogen limitation of primary productivity during the summer. Our findings illustrate that shallow systems dominated by benthic producers have the potential to retain large terrestrial nitrogen loads when there is sufficient supply of phosphorus from exchange with coastal waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activity has greatly increased nitrogen (N) pollution in many coastal waters over the past several decades (NRC 2000; Rabalais 2002; Howarth 2008; Billen et al. 2011; Howarth et al. 2011) causing the degradation of two thirds of the estuaries in the USA (NRC 2000; Bricker et al. 2007). Shallow estuaries are particularly sensitive (Valiela et al. 1997; NRC 2000; Nixon et al. 2001) and have a more complicated response to nutrient enrichment than deeper systems (Nixon et al. 2001; McGlathery et al. 2007; Howarth et al. 2011). In shallow systems, light penetrates to the sediment which results in primary production dominated by benthic plants, algae, and cyanobacteria rather than phytoplankton. Since these benthic primary producers (particularly seagrasses and macroalgae) can accumulate a large standing stock of biomass during the growing season, seasonal retention of nutrients can be high compared to phytoplankton-dominated systems, while nutrient concentrations in the water column tend to be lower (Duarte and Cebrián 1996; Nixon et al. 2001; McGlathery et al. 2007).
Nitrogen (N), more than phosphorus (P), is the primary driver of eutrophication in many coastal ecosystems, particularly in the temperate zone (Vitousek and Howarth 1991; Nixon 1995; Howarth and Marino 2006; Conley et al. 2009). One mechanism for maintaining N limitation despite large terrestrial N inputs associated with anthropogenic activity is exchange with coastal waters that often have relatively high phosphorus (P) concentrations and low N/P ratios as a result of denitrification on the continental shelf (Howarth et al. 2011). For example, both Chesapeake Bay (Boynton et al. 1995) and the Yangtze River (Li et al. 2011) receive large inputs of P compared to N from exchange with coastal waters.
The percentage of the N load to an estuary that is exported downstream to coastal seas varies greatly across estuaries. The N that is not exported is commonly referred to as being retained within the estuary (Nixon et al. 1996; Billen et al. 2011), where “retention” includes the loss through denitrification and burial as well as seasonal storage in primary producer biomass. In this paper, we refer to the percentage net export of the watershed N load, which is the inverse of “retention” as used by Nixon et al. (1996) and Billen et al. (2011). The percentage of the watershed N load that is exported across a range of estuaries is well predicted as a function of the ratio of depth to water residence time, with less export in more shallow ecosystems and in systems with longer water residence times (Nixon et al. 1996; Billen et al. 2011). The estuaries in these studies were predominately deeper, plankton-dominated systems, and N retention was largely ascribed to denitrification. However, there are fewer studies of N export in shallow estuaries where benthic primary producers such as seagrasses and macroalgae are dominant. Assimilation of N by these benthic producers would be expected to reduce N export, at least on a seasonal basis (McGlathery et al. 2007).
West Falmouth Harbor (WFH) on Cape Cod, MA (USA) provides an ideal location to study N retention and the exchange of N and P with coastal waters in a shallow system. The N load to this small, well-bounded lagoon began increasing in the late 1990s, increasing dramatically by 2004 or 2005, and has been stable since then. This dramatic increase in N load resulted from the input of nitrate from groundwater contaminated by a municipal wastewater treatment facility (Howes et al. 2006) and was accompanied by little or no increase in P load. As part of a larger study of coupled element cycling and the biogeochemical feedbacks in WFH in response to the high inorganic N input from this contamination, we here report on our estimates of the total N load to WFH, the exchange of N and P between WFH and Buzzards Bay, and the net export of N from WFH.
Methods
Study Site
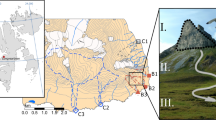
WFH is a shallow lagoon with an area of ~76 ha at mean high tide in Falmouth, MA on Cape Cod (41.36° N, 70.38° W; Table 1). The system is tidally dominated and exchanges water with the adjacent coastal waters of Buzzards Bay through a single 3-m deep narrow inlet. Precipitation and groundwater are the only major inputs of freshwater. Approximately half of the groundwater enters WFH through Mashapaquit Creek (an intertidal salt marsh) and directly into Snug Harbor, the northeastern lobe of WFH (Fig. 1; Howes et al. 2006; Kroeger et al. 2006). The mean water residence time is approximately 1 day (Howes et al. 2006; Howarth et al. 2013). Seagrass beds (Zostera marina L.) cover approximately 60 % of the outer reaches of the harbor (the region all water flows through on its way to and from Buzzards Bay) and approximately 25 % of the harbor as a whole (Hayn 2012).
Map of West Falmouth Harbor illustrating the single outlet to Buzzards Bay, the location where the contaminated wastewater plume enters the harbor and the location of the wastewater treatment facility in relation to the harbor. The dotted lines represent the watershed divides for harbor sub-portions. The arrows represent the approximate relative magnitudes of N entering Snug Harbor and Mashapaquit Creek from the wastewater plume. The star illustrates the ISCO sampler deployment location
WFH receives a large amount of nitrate from a contaminated groundwater plume that originates at a sewage treatment facility approximately 1.5 km away. Construction of this facility, which treats wastewaters that originate outside of the WFH watershed, was completed in 1986. The facility discharged secondarily treated wastewater into the groundwater from 1986 to 2005 (Howes et al. 2006; Town of Falmouth 2011). Total discharge was initially low, and increased for several years, reaching a steady rate by the mid-1990s (Howes et al. 2006). This groundwater takes an estimated at 7–10 years to travel to WFH (Kroeger et al. 2006), and nearly all of the P is removed from the effluent by sorption on aquifer minerals (Weiskel and Howes 1992). Although tertiary treatment of the wastewater for N removal began in late 2005, the high-nitrate groundwater plume has continued to flow into WFH through at least February 2013 (unpublished data). Thus, throughout the period of our study (2005 through 2009), WFH received a highly elevated N load. Virtually all of this contaminated plume enters through Mashapaquit Creek and Snug Harbor (Fig. 1; Howes et al. 2006; Hayn 2012).
Nitrogen Load Estimation
We estimated the average N load to WFH (N Loadtotal) between 2005 and 2009 by separately considering three components; the elevated load from the contaminated groundwater plume (N Loadplume), the background load coming from all other sources in the watershed (both anthropogenic and natural, including septic tanks, lawn fertilizer, street runoff, and atmospheric deposition onto the watershed; N Loadback), and a term for the direct atmospheric deposition of N onto the water surface of the harbor (N Loaddep):
We used literature values for N Loadback and N Loaddep. For N Loadback, we used the estimates of Kroeger et al. (2006) and Howes et al. (2006), which are estimates of total landscape nitrogen load obtained from empirically validated land use models, not including inputs from the wastewater facility. Kroeger et al. (2006) gave a value of 0.8 kmol N day−1, and Howes et al. (2006) estimated 1.0 kmol N day−1. We used the average of these two independent estimates for the background load from the watershed, or 0.9 kmol N day−1. Approximately half of N Loadback is composed of nitrate, considering data from all parts of WFH except Snug Harbor where we cannot separate background from wastewater load (Kroeger et al. 2006). There has been very little land use change or development in the watershed since these estimates were made. We estimated N Loaddep as 0.2 kmol N day−1 by applying the average deposition rate of 15 kg N ha−1 year−1 on Cape Cod from Bowen and Valiela (2001) to the mean surface area for WFH. This may be a slight overestimate, since N deposition has decreased over the past two decades on average in the northeastern USA, but the deposition term is in any case small.
We expect N Loadplume to be composed primarily of nitrate and calculate here only the plume load in this form, thus providing a conservative estimate for N Loadplume. We separately evaluated the two portions of WFH that receive water from the plume, Snug Harbor and Mashapaquit Creek:
where N Loadplume:MC and N Loadplume:SH are the nitrate loads in the groundwater plume from the treatment plant that enter Mashapaquit Creek and Snug Harbor, respectively. Q MC and Q SH are the mean groundwater flow rates into Mashapaquit Creek and Snug Harbor, C MC and C SH are the average concentrations of nitrate in groundwater flowing into Mashapaquit Creek and Snug Harbor, and B NO3:MC and B NO3:SH are the portion of the N background load to Mashapaquit Creek and Snug Harbor that is composed of nitrate and that is not attributable to the contamination from the sewage treatment plant.
Using the background total nitrogen load to each part of the harbor and the fraction of groundwater N as nitrate from Kroeger et al. (2006), we estimate 0.07 kmol N day−1 for B NO3:MC and 0.12 kmol N day−1 for B NO3:SH. We used the estimate of Ganju (2011) of 1,700 m3 day−1 for Q MC. For Q SH, we used an estimate for total freshwater input to WFH from Ganju et al. (2012), 18,100 m3 day−1, apportioned by the result of Kroeger et al. (2006) that 35 % of all freshwater inputs to WFH enter through Snug Harbor. We therefore calculated Q SH as 6,350 m3 day−1.
We estimated C MC and C SH from a conservative mixing model based on simultaneous measurements of salinity and nitrate in 2011 and nitrate + nitrite in 2012 in Mashapaquit Creek and Snug Harbor; the zero-salinity intercept gave an estimate of the nitrate or nitrate + nitrite in the freshwater entering the saline receiving waters. We collected detailed data along spatially representative transects during the winter just before and following low tide, when biological activity was low and there would have been the smallest dilution of groundwater by saline water. We used a Satlantic ISUS in situ optical nitrate sensor in 2011 to measure nitrate, which utilizes UV absorption technology (Johnson and Coletti 2002) to provide real-time data, and verified the readings using standard colorimetric methods for nitrate + nitrite described in the following section. We measured salinity using a calibrated YSI datasonde. In 2012, we collected discrete samples and analyzed nitrate + nitrite using standard colorimetric methods and salinity using an Orion 140 benchtop conductivity meter.
Nutrient Sampling and Analysis
We collected water samples for nutrient analysis at the inlet between WFH and Buzzards Bay using a Teledyne ISCO 6712 portable sampler. We sampled just to the south of the main boat channel, approximately 45 m from shore (Fig. 1) in water that was approximately 2.9 m deep at mean high tide. This ISCO sampler was moored in an inflatable raft and sampled water from a height of 0.7 m above the sediment surface. This single location was representative of water flowing between WFH and Buzzards Bay (Hayn 2012). The waters at the inlet are not stratified, and estuarine return flow there is minimal. Samples were taken hourly over 23-h periods on 24 days between July 2005 and August 2009. We sampled throughout the year except in winter, with the highest sampling intensity during the summer season when the response to nutrient enrichment is of greatest interest. Samples were stored on ice in the ISCO sampler during the sampling period, and then brought back to the lab. For each sample, we transferred one subsample to an acid-washed HDPE bottle and froze it for later analysis of total N (TN) and total P (TP). Two additional sets of subsamples were filtered through 0.45-μm Supor membrane filters and either stored frozen for later analysis of nitrate + nitrite or refrigerated and run within 24 h for ammonium or 48 h for soluble reactive P (SRP).
We analyzed ammonium and SRP using standard colorimetric methods on a Cary Model 50 spectrophotometer based on reactions with phenolhypochlorite and phosphomolybdate reagents, respectively (Solorzano 1969; Koroleff 1983). Our limits of detection, defined as the concentration of analyte that is significantly different from the blank to 97.5 % confidence, were 0.1 μM for both ammonium and SRP (Hayn 2012). We analyzed nitrate + nitrite on an Astoria Analyzer using a modification of the Astoria Pacific standard methodology for cadmium reduction in saline samples (A177), with an artificial seawater carrier matched to the salinity of the samples and an increased sample time to increase accuracy at concentrations below 1 μM, an imidazole buffer to prolong cadmium column life, and a decreased buffer/sample ratio to improve the detection limit. Our limit of detection for nitrate + nitrite was 0.1 μM (Hayn 2012). Dissolved inorganic N (DIN) was calculated as the sum of the ammonium and nitrate + nitrite concentrations.
We analyzed TN and TP using a dual digestion with persulfate reagent, based on the methodology of Koroleff (1983), adjusted to provide optimum recovery in estuarine systems following Marino (2001). Phosphorus in the digested samples was analyzed as SRP, again using the phosphomolybdate colorimetric assay (Koroleff 1983). For N, nitrate + nitrite in digested samples was analyzed using a modification of the method described above for saline samples, with a distilled water carrier to reduce salt load through the column. Since concentrations of TN were never below 1 μM, matrix matching the carrier to the salinity of the samples was not necessary. Our limits of detection for TN and TP were 1.5 μM and 0.3 μM, respectively (Hayn 2012).
Water and Nutrient Exchange with Buzzards Bay
We estimated water exchange rates between WFH and Buzzards Bay from changes in water volume (V) within WFH at any given time after consideration of changes due to input and loss of freshwater:
where Q V is the rate of water inflow to WFH from Buzzards Bay (with negative values reflecting outflow from WFH), V 1 and V 2 are the volumes of water in WFH at times t 1 and t 2, R is the direct input to WFH as rainfall, G is groundwater input, and E is evaporation from the surface waters of WFH. We used the estimates of Ganju et al. (2012) for G and E, 0.21 and 0.05 m3 s−1, respectively. Our estimates of water exchange were made on days with no precipitation, so R was zero.
We can calculate V at any given time from the water depth at that time and from detailed bathymetric data for WFH (Hayn 2012). We measured water depth at 5-min intervals at the Town of Falmouth dock on the eastern side of WFH using a Global Water WL16 vented, pressure- and temperature-compensated water level logger (accurate to 0.009 m). This one location well represents all of WFH, as lags in depth with the changing tide across WFH are very small (Howes et al. 2006; Ganju et al. 2012). We produced a new, detailed bathymetric map of WFH based on depth soundings with the assistance of CR Environmental, Inc. using a Trimble AgGPS Model 132 DGPS with submeter accuracy to collect horizontal coordinates, and an ODEC Bathy500MF precision survey fathometer to provide depth soundings. Data were collected along parallel transects with extra resolution in the intertidal zone, and perpendicular transects were run to assess survey accuracy. Soundings were processed in Hypack-4.3A Gold hydrographic survey software, which compensates for water level changes during the survey, adjusts for sound velocity, and computes accuracy statistics. We interpolated the bathymetric map using ArcGIS 9.2 and the Geostatistical Analyst extension to generate a grid with 5-m horizontal resolution. Further details on field methods, statistical methods used to generate the bathymetric map, and quality control procedures can be found in Hayn (2012).
We estimated the instantaneous nutrient exchange between WFH and Buzzards Bay at a given time by multiplying the concentration of a nutrient at that time (C) by the rate of water flux corresponding to that time (Q V ). The total nutrient exchange over a given time period (Ex) is then the integral of the short-term exchanges over the period of interest:
Since the water depths used to calculate changes in Q v were collected at 5-min intervals and the nutrient concentrations (C) hourly, we used linear interpolation between sample points on both data sets. We then used Eq. 5 with a dt of 2 min to calculate Ex for individual tidal cycles, obtaining multiple estimates for tidal cycles within each day by using a 30-min moving window for the start time. The number of estimates evaluated on a given day ranged from 6 to 25, determined by the daily tidal amplitude and differences between the elevations of consecutive high and low tides within the sample day. To determine whether there were biases in the rate calculation from the number of daylight hours within a tidal cycle, we examined the individual tidal cycle calculations within each day prior to averaging. We found no significant correlation between number of daylight hours in a tidal cycle and the calculated nutrient flux, and so have used a straight average of nutrient exchange for all tidal cycle calculations for each sampling day with error bars representing the 95 % confidence interval of the estimates to obtain daily nutrient exchange estimates. Further details on these calculations can be found in Hayn (2012).
Results and Discussion
Nitrogen Load
Winter nitrate and nitrate + nitrite concentrations at low tide were well correlated with salinity in both Snug Harbor and Mashapaquit Creek (Fig. 2; P < 0.00001). The zero-salinity intercepts yielded estimates for concentrations in the freshwater entering the harbor of 262 ± 2 μM for Snug Harbor and 111 ± 2 μM for Mashapaquit Creek (95 % confidence intervals indicated). There was no statistical difference in the nitrate concentrations calculated from 2011 data and the nitrate + nitrite concentrations from 2012 data, so the data were aggregated for this analysis. For simplicity, we refer to this input as nitrate, although a small portion may come from nitrite.
For Mashapaquit Creek, we used 111 μM as the average concentration of groundwater nitrate (C MC) and Eq. 2 to calculate the load from the contaminated groundwater plume (N Loadplume:MC) as 0.12 kmol N day−1. For Snug Harbor, freshwater comes from two sources; groundwater flow directly into Snug Harbor and water flow from Mashapaquit Creek. We therefore used a weighted average to calculate C SH, the concentration of nitrate directly entering Snug Harbor from groundwater, as follows:
where C SH-observed is the intercept from the mixing curve for Snug Harbor in Fig. 2 (262 μM). From Eq. 6, we estimated C SH as 300 μM. This is most likely a conservative estimate, since some of the freshwater present when we made our measurements likely originated in areas of the harbor with lower nitrate inputs and entered Snug Harbor on flooding tides. Using 300 μM for C SH and Eq. 3, we calculated the load to Snug Harbor from the contaminated groundwater plume (N Loadplume:SH) as 1.8 kmol day−1. Summing the values of the plume loading to Mashapaquit Creek and to Snug Harbor, we estimated the total mean N load to WFH attributable to the sewage treatment facility plume (N Loadplume) as 1.9 kmol N day−1. Details of temporal trends in this pollutant N load are presented elsewhere; the inter-annual variation is less than 10 % of the mean (Howarth et al. 2013). We also sampled shoreline wells and found that the concentrations of nitrate + nitrite in groundwater near Snug Harbor were high and relatively constant over our study period (Foreman et al., manuscript in preparation).
Adding our values for N Loadplume, N Loadback, and N Loaddep as in Eq. 1 resulted in an estimate for the entire load to WFH of 3.0 kmol N day−1, with almost two thirds of this attributable to the groundwater contamination from the wastewater facility. Since we believe that the other terms in the total N load have not changed markedly since the mid-1990s, WFH has experienced an approximately threefold increase in N loading between then and the period we studied, 2005 through 2009. Because the N pollution entering the groundwater at the sewage treatment facility has been largely reduced since the implementation of tertiary treatment in late 2005, we anticipate that the N load to WFH will be significantly lower by 2016, since the groundwater transit time between the facility and WFH is believed to be, at most, 10 years. Since N Loadplume is made up entirely of nitrate, approximately 50 % of N Loadback is composed of DIN, and N Loaddep is a very small term, we estimated that DIN makes up approximately 80 % of the total N load to WFH.
The N load to WFH is moderately high, high enough for the estuary to be classified as eutrophic (NRC 1993, 2000). West Falmouth Harbor has experienced serious degradation from this N load, including a die-off of seagrasses in Snug Harbor in 2010 (Howarth et al. 2013).
Water Exchange
Over our study period, the observed tidal range varied between 0.7 and 1.9 m at neap and spring tide, respectively (Table 1; Hayn 2012). The bottom topography of WFH is characterized by a relatively flat, shallow plain ~1.3 m deep at mean water level, with a deep channel running through the center as a result of past dredging (Hayn 2012). By combining the bathymetric data with the water level data, we calculated a mean exchange of tidal waters with Buzzards Bay of 8.0 × 105 m3 on each tidal cycle, just over 50 % of the total volume of water in the harbor at mean high water.
The majority of our estimates for the instantaneous water exchange rate between WFH and Buzzards Bay (Q v ) ranged from 95 to −95 m3 s−1 (positive rates indicate water entering WFH), with a median of 0. Water flux rates were normally distributed during the sampling period. We independently validated these instantaneous water exchange rates by comparing them over a 1-month period in 2010 with data obtained using standard USGS acoustic methods at the inlet and an index velocity method to estimate tidal water fluxes (Ruhl and Simpson 2005; Ganju et al. 2012). These two independent approaches were highly correlated (P < 0.0001) and in excellent agreement, showing only a 2 % deviation from the 1:1 line (Hayn 2012; Ganju et al. 2012).
Nutrient Concentrations
Figure 3 illustrates patterns of DIN, SRP, TN, and TP over a 24-h period of sampling, in this case for a typical date in late September. Note that on this day, SRP made up a substantial percentage of TP, while TN was much greater than DIN, reflecting a dominance by organic N forms as was often the case during the times we sampled. On this date, both DIN and TN tended to be higher during periods when water level was falling within WFH, reflecting the falling tide and indicating an export of N from WFH. Both SRP and TP were less dynamic over the tidal cycle, probably reflecting less net exchange of P between WFH and Buzzards Bay on this particular day. Data for other representative days are presented in Hayn (2012).
Overall, DIN concentrations at the inlet to WFH tended to be low during all seasons, with a mean of 0.7 μM and a median of 0.5 μM (Fig. 4). TN concentrations were always much higher, averaging 13.3 μM. On the majority of sampling dates, at least 85 % of TN was composed of organic forms, and during the summer months TN was composed almost entirely of organic forms. There was no statistical difference in DIN concentrations among seasons sampled; however, mean TN was significantly (if slightly) higher in the summer than either spring or fall. SRP concentrations varied the least of all measured nutrient parameters (Fig. 4), with a mean and median of 0.6 μM. Unlike the relationship between DIN and TN, SRP made up 50 % on average of the TP. TP showed a mean concentration of 1.2 μM and ranged from 0.1 to 2.3 μM. Seasonally, both SRP and TP were lower in the spring than either the summer or fall (Fig. 4).
Boxplots showing the median (white bar), middle 50 % (shaded box), and full range of observations of N and P concentrations at the inlet to the harbor over the study period by season alongside the mean (diamond) and 95 % confidence interval on the mean. The charts for SRP and TP have the same vertical scale, illustrating that much of the TP is inorganic. However, the large difference in the scales of the N plots illustrates that most of the total N is organic
It is noteworthy that the minimum observed SRP concentration was 0.1 μM and that no samples were below the detection limit (0.1 μM). Conversely, 39 samples were below the limit of detection for both ammonium and nitrate + nitrite (0.1 μM). These very low DIN values are consistent with observations from other shallow systems, where rapid uptake by benthic primary producers under N-limiting conditions can result in very low levels of water column DIN (Duarte and Cebrián 1996; Nixon et al. 2001; McGlathery et al. 2004).
The very low molar ratio of inorganic N/P in WFH (averaging ~1:1) is strongly indicative of N limitation of net primary productivity (NRC 2000; Howarth and Marino 2006; Souchu et al. 2010). That WFH remained N limited despite the large increase in the N load from the wastewater contaminated plume, with no concomitant increase in the P load, is perhaps surprising. Importation of water with a low availability of N relative to P (as indicated by an N/P ratio well below that needed by the primary producers) into estuaries is one mechanism that can lead to N limitation even when the N/P ratio of watershed loads is high (Howarth and Marino 2006; Howarth et al. 2011). As presented below, this mechanism is consistent with our data, as WFH in fact imports a substantial quantity of P from Buzzards Bay during summer months.
Nutrient Exchanges with Buzzards Bay
Variation in the net exchanges of TN and TP among sampling days during the same season was high (Fig. 5). This should not be surprising, given the inherent error in teasing out the small signal of a net flux in the face of massive back-and-forth flows of nutrients with tidal waters exchanging half the volume of WFH on average with each tide, in addition to factors such as day-to-day variation in net nutrient uptake by primary producers resulting from variations in weather (Howarth et al. 2013). The variability was greatest in summer, again perhaps not surprisingly given variation in uptake by primary producers during that time.
Daily estimates of net TN exchange (a) and TP exchange (b). Each bar represents the average daily estimate from one sampling date. Every sampling date during the 5-year period of the study is shown, with error bars indicated 95 % confidence limits for the averages on each day. Negative values indicate export from WFH to coastal waters, and positive values indicate an import from coastal waters. The data indicate a general trend for net import of N to the WFH from coastal waters in the summer and net export in the spring and fall. The dotted line represents the N export if all the watershed N load were to be exported with no retention. A trend towards net TP export in the spring and net import in the summer and fall is also apparent
In Fig. 6, we illustrate the seasonally averaged exchange of DIN, TN, SRP, and TP between WFH and Buzzards Bay. WFH was on average a net importer of TN from Buzzards Bay in the summer and a net exporter of TN during the spring and fall. The fluxes of DIN tended to parallel those of TN. DIN made up a majority of the TN export in the fall, but the N fluxes in the other seasons were dominated by organic N rather than DIN. Since approximately 80 % of the total N load to WFH was DIN, the dominance of organic N in the exchange terms with Buzzards Bay indicates substantial biological processing of the N coming into the harbor. WFH imported TP from Buzzards Bay both during the summer and fall, with a much larger import during the summer, and exported TP in the spring. As with N, the P fluxes were dominated by inorganic forms (SRP) in the fall and by organic forms in the spring and summer.
Seasonally averaged fluxes of DIN, TN, SRP, and TP. Positive fluxes indicate a net import from coastal waters. Error bars are 95 % confidence intervals. Generally, we see a net import of DIN, TN, SRP, and TP during the summer, a net export in the spring, and a net export of N but net import of P in the fall
During summer months, the net imports of TN and TP into WFH from Buzzards Bay were 0.5 and 0.08 kmol day−1, respectively (Fig. 6). The N/P ratio of this import was approximately 6:1, well below the Redfield ratio of 16:1 for phytoplankton, and even lower than the N/P ratio commonly observed in seagrasses and macro-algae (which is highly variable but averages 30:1; Atkinson and Smith 1983; Duarte 1995). As discussed previously, an import of nutrients with a low N/P ratio from coastal ocean waters into estuaries is one mechanism that can help maintain N limitation (Howarth and Marino 2006; Howarth et al. 2011). This rate of P import during the summer, 0.08 kmol P day−1, was sufficient to support a rate of N assimilation in net primary productivity by seagrasses and macro-algae of 2.4 kmol N day−1 (assuming an N/P ratio of 30:1 in seagrasses and macro-algae; Duarte 1995). Thus, the potential rate of N uptake in macrophyte primary production was 1.9 kmol N day−1 more than the N imported as TN into WFH from Buzzards Bay, or the equivalent of the increased N load to WFH from the wastewater-contaminated groundwater plume (Loadplume). This plume contained virtually no P due to removal during the long transit from the sewage facility to WFH. Unfortunately, we lack reliable information on the watershed load of P to WFH that would correspond to the background N load (N Loadback), and so we cannot provide a complete analysis of the balance of the N and P budgets for WFH from all sources. Nonetheless, the import of P from Buzzards Bay was clearly an important driving factor in maintaining N limitation. Faster recycling of P compared to N in the water column as well as preferential release of P compared to N from sediments are other mechanisms demonstrated to help maintain N limitation in some estuaries (Nixon 1995; Boynton et al. 1995; Howarth and Marino 2006).
Net Nitrogen Export
During the summer months, WFH not only retained the entire watershed N load and inputs of N from direct atmospheric deposition onto the harbor (N Loadtotal, or 3.0 kmol day−1), the ecosystem also had a net import of 0.5 kmol N day−1 from Buzzards Bay (Fig. 6), and retained an additional amount of N added to the ecosystem through N fixation by epiphytic cyanobacteria (estimated as negligible in some years but as much as 0.4 kmol day−1 in 2008 during summer months, and highly variable across years; Marino et al., ms. in prep.). That is, WFH had a net N retention rate during the summer of at least 3.5 kmol day−1. Assimilation by seagrasses and algae probably was responsible for much of this retention, as well as denitrification (McGlathery et al., ms. in prep).
During the spring and fall, WFH was a net exporter of N to Buzzards Bay (Fig. 6), although the net export was substantially less than the total load (N Loadtotal). An estimated 40 % of the load was exported in the fall and 60 % in the spring. We do not have reliable data on the N exchange with Buzzards Bay during the winter due to logistical difficulties with deploying the ISCO sampler. We would expect winter export to be high due to low biological activity, certainly no less than the 60 % observed in the spring and perhaps approaching 100 %. Using a range of export from 60 % to 100 % for the winter, we calculated an annual rate of N export from WFH of 37 % to 47 %, or a mean of 42 % (Fig. 7). The annual estimate for net export of the N load remains below 50 % so long as the assumed wintertime export is less than 112 % of the load.
The percentage of watershed N load exported across seasons. Measurements in the spring, summer, and fall are shown with 95 % confidence intervals. During the winter, we assume that between 60 % and 100 % of the load to the harbor is exported to Buzzards Bay, represented by the hashed bar with error bars representing the range of this estimate. Annually, this results in an estimated total export of 42 % of the watershed N load to coastal waters, with the variation in this estimate from the assumption of winter export shown in the hashed region
We can compare annual N export from WFH with other estuaries using the relationship developed by Nixon et al. (1996) and updated by Billen et al. (2011), where export is a function of the ratio of estuary depth to water residence time (Fig. 8). Here we show the N that was not exported, which is equivalent to “retention” in the language of these previous studies, on an annual basis. Most of the N that was not exported on this annual time scale was probably denitrified with a lesser amount “permanently” buried in the sediment. On an annual scale, net accumulation in living biomass or in recently produced detritus must be small, although it could be quite large and provide an important N sink during the summer. The percentage of the N load not exported on an annual basis increases with longer water residence times and increases with shallower water depths, reflecting greater interaction between N in the water and sediments, which favors greater rates of denitrification (Nixon et al. 1996; Billen et al. 2011). Most of the estuaries in Fig. 8 are relatively deep, phytoplankton-based ecosystems. The percentage of the N load exported from WFH on an annual basis was far less than for these deeper ecosystems.
Conclusions
West Falmouth Harbor has provided a unique opportunity to study the effects of a dramatically increased N load without an accompanying increase in P load on a shallow lagoonal ecosystem. Through the time of our study (2005 to 2009), WFH was able to retain the entire terrestrial N load during the summer, as well as additional N imported from the coastal waters of Buzzards Bay.
In the spring and fall, WFH exported N to Buzzards Bay, but the net export was less than the N load from the watershed. If we assume that the net export during winter is less than 112 % of the load, then on an annual timescale WFH exported less than half of the total N load. This is a small export compared to other estuaries, given the short water residence time of WFH, and suggests that even more so than deeper planktonic-based systems, shallow, benthic producer-dominated systems have the potential to retain significant amounts of terrestrially derived nitrogen, thus reducing its impact on nearshore waters.
Although nutrient limitation in estuaries can switch from N to P under conditions of high N loading (EPA/SAB 2008; Conley et al. 2009; Howarth et al. 2011), such a switch did not occur in WFH. Primary productivity in WFH remained N limited throughout our study, as indicated by low concentrations of DIN and low DIN/SRP ratios. Despite the high N/P ratio in watershed inputs to the harbor, N limitation was maintained in part by the net import of coastal water with a low N/P ratio (6:1) during the summer when primary productivity was highest. This suggests that P import from nearshore waters could be an important factor toward maintaining N limitation in other shallow lagoons with short residence times, contributing to higher productivity and degradation of water quality within these systems as a result of increased terrestrial N loading from human activities.
References
Atkinson, M.J., and S.V. Smith. 1983. C:N:P ratios of benthic marine plants. Limnology and Oceanography 28: 568–574.
Billen, G., M. Silvestre, B. Grizzetti, A. Leip, J. Garnier, M. Voss, R. Howarth, F. Bouraoui, H. Behrendt, A. Lepisto, P. Kortelainen, P. Johnes, C. Curtis, C. Humborg, E. Smedberg, O. Kaste, R. Ganeshram, A. Beusen, and C. Lancelot. 2011. Nitrogen flows from European regional watersheds to coastal marine waters. Chapter 13, European Nitrogen Assessment. Cambridge: Cambridge University Press.
Bowen, J.L., and I. Valiela. 2001. Historical changes in atmospheric nitrogen deposition to Cape Cod, Massachusetts, USA. Atmospheric Environment 35: 1039–1051.
Boynton, W.R., J.H. Garber, R. Summers, and W.M. Kemp. 1995. Inputs, transformations and transport of nitrogen and phosphorus in Chesapeake Bay and selected tributaries. Estuaries 18: 285–314.
Bricker, S., B. Longstaff, W. Dennison, A. Jones, K. Boicourt, C. Wicks, and J. Woerner. 2007. Effects of nutrient enrichment in the nation’s estuaries: a decade of change. NOAA Coastal Ocean Program Decision Analysis Series No. 26. Silver Spring: National Centers for Coastal Ocean Science.
Conley, D.J., H.W. Paerl, R.W. Howarth, D.F. Boesch, S.P. Seitzinger, K.E. Havens, C. Lancelot, and G.E. Likens. 2009. Controlling eutrophication: Nitrogen and phosphorus. Science 323: 1014–1015.
Duarte, C.M. 1995. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41: 87–112.
Duarte, C.M., and J. Cebrián. 1996. The fate of marine autotrophic production. Limnology and Oceanography 41: 1758–1766.
EPA Science Advisory Board. 2008. Hypoxia in the northern gulf of Mexico: An update by the EPA Science Advisory Board. EPA-SAB-08-003. Washington, D.C: US Environmental Protection Agency, Science Advisory Board.
Ganju, N.K. 2011. A novel approach for direct estimation of fresh groundwater discharge to an estuary. Geophysical Research Letters 38, L11402.
Ganju, N.K., M. Hayn, S. Chen, R.W. Howarth, P.J. Dickhudt, A.L. Aretxabaleta, and R. Marino. 2012. Tidal and groundwater fluxes to a shallow, microtidal estuary: constraining inputs through field observations and hydrodynamic modeling. Estuaries and Coasts 35(5): 1285–1298.
Hayn, M. 2012. Exchange of nitrogen and phosphorus between a shallow estuary and coastal waters. M.S. dissertation. Cornell University.
Howarth, R.W. 2008. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 8: 14–20.
Howarth, R.W., and R. Marino. 2006. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnology and Oceanography 51: 364–376.
Howarth, R.W., F. Chan, D.J. Conley, J. Garnier, S.C. Doney, R. Marino, and G. Billen. 2011. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Frontiers in Ecology and the Environment 9: 18–26.
Howarth, R.W., M. Hayn, R.M. Marino, K. Foreman, P. Berg, A.E. Giblin, K. McGlathery, and D. Walker. 2013. Metabolism of a nitrogen-enriched coastal marine lagoon during the summertime. Biogeochemistry (in press).
Howes, B., S.W. Kelley, J.S. Ramsey, R. Samimy, D. Schlezinger, and E. Eichner. 2006. Linked watershed-embayment model to determine critical nitrogen loading thresholds for West Falmouth Harbor, Falmouth, Massachusetts. Boston: Massachusetts Estuaries Project, Massachusetts Department of Environmental Protection.
Johnson, K.S., and L.J. Coletti. 2002. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide, and bisulfide in the ocean. Deep-Sea Research I 49: 1291–1305.
Koroleff, F. 1983. Simultaneous oxidation of nitrogen and phosphorus compounds by persulfate. In Methods of seawater analysis, 2nd ed, ed. K. Grasshoff, M. Eberhardt, and K. Kremling, 168–169. Weinheimer: Verlag Chemie.
Kroeger, K.D., M.L. Cole, J.K. York, and I. Valiela. 2006. N transport to estuaries in wastewater plumes: Modeling and isotopic approaches. Ground Water 44: 188–200.
Li, X., Z. Yu, X. Song, X. Cao, and Y. Yuan. 2011. Nitrogen and phosphorus budgets of the Changjiang River estuary. Chinese Journal of Oceanology and Limnology 29(4): 762–774.
Marino, R. 2001. An experimental study of the role of phosphorus, molybdenum, and grazing as interacting controls on planktonic nitrogen fixation in estuaries. Ph.D. dissertation. Cornell University.
McGlathery, K.J., K. Sundback, and I.C. Anderson. 2004. The importance of primary producers for benthic N and P cycling. In The influence of primary producers on estuarine nutrient cycling, ed. S.L. Nielsen, G.M. Banta, and M.F. Pedersen. Dordrecht: Kluwer Academic Publishers.
McGlathery, K.J., K. Sundback, and I.C. Anderson. 2007. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Marine Ecological Progress Series 348: 1–18.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199–219.
Nixon, S.W., J.W. Ammerman, L.P. Atkinson, V.M. Berounsky, G. Billen, W.C. Boicourt, W.R. Boynton, T.M. Church, D.M. DiToro, R. Elmgren, J.H. Garber, A.E. Giblin, R.A. Jahnke, N.J.P. Owens, M.E.Q. Pilson, and S.P. Seitzinger. 1996. The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35: 141–180.
Nixon, S.W., B. Buckley, S. Granger, and J. Bintz. 2001. Responses of very shallow marine ecosystems to nutrient enrichment. Human and Ecological Risk Assessment 7: 1457–1481.
NRC (National Research Council). 1993. Managing wastewater in coastal urban areas. Washington, D.C.: National Academy Press.
NRC (National Research Council). 2000. Clean coastal waters: understanding and reducing the effects of nutrient pollution. Committee on the Causes and Management of Coastal Eutrophication. Washington, D.C.: National Academy Press.
Rabalais, N.N. 2002. Nitrogen in aquatic ecosystems. Ambio 31: 102–112.
Ruhl, C.A., and M.R. Simpson. 2005. Computation of discharge using the index velocity method in tidally affected areas: U.S. Geological Survey Scientific Investigations Report 2005–5004, 31p.
Solorzano, L. 1969. Determination of ammonia in natural waters by the phenol hypochlorite method. Limnology and Oceanography 14: 799–801.
Souchu, P., B. Bec, V.H. Smith, T. Laugier, A. Fiandrino, L. Benau, V. Orsoni, Y. Collos, and A. Vaquer. 2010. Patterns in nutrient limitation and chlorophyll a along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Canadian Journal of Fisheries and Aquatic Science 67: 743–753.
Town of Falmouth. 2011. Wastewater department website: 2. Current wastewater management in Falmouth. http://www.falmouthmass.us/depart.php?depkey=wastewater. Accessed 1 Dec 2011.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgae blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Vitousek, P.M., and R.W. Howarth. 1991. Nitrogen limitation on land and in the sea. How can it occur? Biogeochemistry 13: 87–115.
Weiskel, P.K., and B.L. Howes. 1992. Differential transport of sewage derived nitrogen and phosphorous through a coastal watershed. Environmental Science and Technology 26: 352–360.
Acknowledgments
Primary funding was provided by the Biocomplexity Program of the National Science Foundation. Additional support came from the Woods Hole SeaGrant Program and from an endowment given by David R. Atkinson to Cornell University. We thank Pat Sullivan and Chris Sherwood for helpful discussions and advice. Thanks to Eli Perrone for assistance with bathymetric mapping and CR Environmental, Inc. for their expertise and use of their surveying equipment and software. We appreciate the assistance provided in the lab and field by Jane Tucker, Clara Funk, Laura Keeling, Sam Kelsey, Jeff Walker, Marina Molodovskaya, and Neil Bettez.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Wayne S. Gardner
Rights and permissions
About this article
Cite this article
Hayn, M., Howarth, R., Marino, R. et al. Exchange of Nitrogen and Phosphorus Between a Shallow Lagoon and Coastal Waters. Estuaries and Coasts 37 (Suppl 1), 63–73 (2014). https://doi.org/10.1007/s12237-013-9699-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9699-8