Abstract
Significant progress has been made recently towards a better understanding of the nature, causes, and consequences of anthropogenic eutrophication of shallow coastal systems. It is well established that, in pristine systems dominated by seagrasses, incipient to moderate eutrophication often leads to the replacement of seagrasses by phytoplankton and loose macroalgal mats as the dominant producers. However, less is known about the interactions between phytoplankton and loose macroalgae at intense eutrophication. Using a combination of original research and literature data, we provide support for the hypothesis that substantial macroalgal decline may occur at intense eutrophication due to severe water column shading. Our results suggest that such declines may be widespread. However, we also show that intense eutrophication is not always necessarily conducive to severe water column shading and large macroalgal declines, possibly due to short water residence time and/or elevated grazing on phytoplankton. Furthermore, we provide support to the hypothesis that the occurrence of hypoxic/anoxic conditions in eutrophication-driven shifts in dominant primary producer assemblages influences the nature and extent of functional change in the system. Focusing on the macroalgal blooms and seagrass decline that often occur at incipient/moderate eutrophication, we show the blooms have a positive effect on epifaunal abundance under well-oxygenated conditions, but a negative effect if pervasive anoxic/hypoxic conditions develop with the bloom. These findings provide support to prior suggestions that secondary productivity in shallow coastal systems may increase as seagrasses get replaced by loose macroalgal stands if the stands remain well oxygenated. In concert, our results contribute to an improvement of our current model of eutrophication of shallow coastal systems and suggest that further effort should be put on ascertaining the mechanisms that may prevent severe water column shading and large macroalgal decline at intense eutrophication, as well as thorough documentation of the impacts of anoxic/hypoxic conditions on system functionality at different stages of eutrophication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication of coastal waters is recognized as one of the most widespread, pervasive impacts of the anthropogenic development and exploitation of coastal watersheds (Cloern 2001; Cole et al. 2006; Teichberg et al. 2010). About 23 % of the global human population lived within 100 km from the coastline by 1990 (Nicholls and Small 2002). As humans occupy coastal watersheds, they profoundly transform the landscape by replacing native forests and marshlands with developed land including roads, parking lots, residences, farms, and amenity resorts such as golf courses. Inevitably, such conversion entails higher nutrient loads into receiving coastal waters through altered flow regimes and nutrient concentrations in runoff, groundwater, and point sources (Bricker et al. 2008). This problem has generated an impressive wealth of research over the past decades (Valiela 2006).
It is well documented that, in pristine shallow systems populated with seagrass beds, increased nutrient loads often lead to the overgrowth of algal producers to the detriment of the seagrass beds (Valiela et al. 1997; Hauxwell and Valiela 2004; Burkholder et al. 2007). These eutrophication-fueled algal producers described to inflict detrimental impacts on seagrasses include phytoplankton, epiphytes, and loose macroalgae (i.e., fast-growing filamentous or laminate genera such as Ulva, Enteromorpha, Cladophora, Gracilaria, Ectocarpus/Pilayella). The understanding as to why these algal producers overgrow and decimate seagrass beds is well developed. Phytoplankton, epiphytes, and loose macroalgae normally feature higher nutrient uptake rates than do seagrasses (Iizumi and Hattori 1982; Pedersen and Borum 1996, 1997; Pedersen et al. 2010), and thus, the algal producers readily take additional nutrients and build up biomass as nutrient availability increases. High biomass accumulations of these algal producers may exert a number of detrimental effects on seagrasses including intense light limitation, competition for dissolved inorganic carbon, anoxic/hypoxic conditions in the water column and within algal canopies, exacerbated anoxia in the sediment, and harmful biogeochemical conditions (Hauxwell et al. 2001, 2003; Liu et al. 2005; Burkholder et al. 2007; Holmer and Nielsen 2007; Rasmussen et al. 2012; Mvungi et al. 2012). As these deleterious impacts take hold, seagrasses decline and may eventually disappear.
At any rate, the response of algal producers to nutrient over-enrichment and resulting impacts on seagrasses is far from simple. Algal producers do not always increase in a clear quantitatively predictable way with increased nutrient loading since factors other than nutrient availability also control algal abundance. For instance, grazers may exert substantial pressure on epiphytes, thereby largely palliating increases of epiphyte biomass and negative cascading impacts on seagrasses under increased nutrient loading (Heck et al. 2000, 2006; Hughes et al. 2004; Cebrian et al. 2013). The response of loose macroalgal stands to increased nutrient loading can also be quite variable. Environmental factors such as temperature, salinity, sediment resuspension and water clarity, protection to wave exposure, and grazing can profoundly modify the response of loose macroalgae to increased nutrient availability (e.g., Collado-Vides et al. 2007; Engelsen et al. 2010; Hessing-Lewis et al. 2011; Paalme et al. 2011; Piñon-Gimate et al. 2012). Similarly, several factors may curtail phytoplankton biomass accumulation in shallow coastal systems following increased nutrient delivery. Recent experiments have added renewed evidence to the notion that intense grazing by filter feeders may, by removing large quantities of phytoplankton biomass, help maintain adequate levels of water clarity and healthy seagrass populations in nutrient-enriched conditions (Bruschetti et al. 2008; Xu et al. 2010; Wall et al. 2011). Another important factor that can regulate the extent of phytoplankton biomass accumulation under eutrophic conditions is water residence time, since phytoplankton will not have time to develop large standing stocks in shallow coastal waters if the rate at which those waters are advected out to the open ocean (i.e., flushing rate) is much faster than the generation rate of the phytoplankton population (Valiela et al. 2000a; Oberg 2005; Xu et al. 2010).
These complex responses of epiphytes, loose macroalgae, and phytoplankton to anthropogenic nutrient enrichment and resulting impacts (or lack of) on seagrass abundance in shallow coastal systems are well represented in our current understanding and conceptual model of coastal eutrophication, as it has been recently reviewed by Burkholder et al. (2007) and McGlathery et al. (2007). With incipient to moderate eutrophication of seagrass-dominated pristine shallow coastal systems, epiphytes, loose macroalgae, and phytoplankton may accumulate to different degrees depending on a variety of environmental and biotic factors and cause corresponding impacts on the seagrasses. However, we know much less about the interactions that occur between phytoplankton and loose macroalgae at intense levels of eutrophication in shallow coastal systems. When both loose macroalgae and phytoplankton accumulate and cause seagrass decline following nutrient enrichment of pristine shallow sites, several authors have postulated that, as nutrient enrichment increases further and reaches intense levels, phytoplankton would accumulate to higher levels, thereby building large standing stocks that would impose severe shading and lead to the decline of the loose macroalgae underneath (Valiela et al. 1997, 2000a; McGlathery et al. 2007). There exists some evidence that supports this hypothesis. In a comparison of 27 coastal sites in Denmark, Nielsen et al. (2002) found strong correlations between water nitrogen concentration, water turbidity, and the depth limit of macroalgal and seagrass distribution, with higher nutrient concentrations associated with more turbid waters and shallower depth limits. Similarly, in a series of papers, Krause-Jensen et al. found reduced macroalgal abundance at a fixed depth range with higher eutrophication intensity and more turbid waters across Danish coastal sites (Krause-Jensen et al. 2007a, b), a pattern that also occurred in other locations (Krause-Jensen et al. 2008). Other works also provide support that intense increases in water turbidity due to environmental degradation may cause large macroalgal declines (Trimmer et al. 2000; Shepherd et al. 2009; Rinne et al. 2011). Generating new evidence that intense eutrophication in shallow coastal systems can lead to the demise of loose macroalgae due to severe shading by phytoplankton will help improve our current conceptual model of the nature, patterns, and consequences of anthropogenic eutrophication in these systems. This is the first goal of this paper.
As the second goal of this paper, we intend to further contribute to our current conceptual model of eutrophication by providing new evidence that the occurrence of hypoxic/anoxic conditions in eutrophication-driven shifts in producer assemblages may condition functional change in the system. We focus on the loose macroalgal canopies that may form in seagrass beds under eutrophied conditions. Drastically reduced water renewal and high organic matter accumulation occurs within thick and stagnant canopies, which causes severe oxygen depletion and high concentrations of toxic reduced compounds such as sulfides and ammonium within the canopy and sediment underneath (Hauxwell et al. 2001, 2003; Burkholder et al. 2007). These adverse biogeochemical conditions may also extend into the water column above the canopy during calm, hot summer nights in shallow estuaries (Sfriso et al. 1992; D’Avanzo and Kremer 1994). Under these conditions, high mortality and large reductions of the infaunal and epifaunal invertebrate communities in relation to pre-macroalgal blooms levels have been reported (Hauxwell et al. 1998; Franz and Friedman 2002; Gray et al. 2002; Arroyo et al. 2012).
However, some macroalgal canopies do not stay stagnant, but frequently roll over due to the action of waves and currents (i.e., rolling macroalgal mats; Norkko et al. 2000; Österling and Pihl 2001; Rasmussen et al. 2013). Some other times, despite restricted movement, the canopies are quite lax, depending on the morphology, architecture, and degree of entanglement of the forming macroalgae (Hessing-Lewis et al. 2011; Rasmussen et al. 2012). Under these conditions, one could expect well-oxygenated macroalgal canopies and, possibly, little detrimental impact (or perhaps a total lack of) on infaunal and epifaunal invertebrate communities. Published reports on macroalgal–seagrass interactions suggest this may well be the case. For instance, ulvoid additions twice the level of ambient canopies did not have a detrimental impact on the abundance of eelgrass (Zostera marina) in the marine reaches of Coos Bay, Oregon (Hessing-Lewis et al. 2011), and the authors attributed this to the observation that the increased ulvoid canopies were still sufficiently lax and seemingly did not create deleterious oxygen and light conditions. Similarly, Höffle et al. (2011) observed little effect of canopies of the coarsely branched red macroalga Gracilaria vermiculophylla on eelgrass performance, and they attributed their findings to the lax morphology of these canopies (“openly structured mat,” as coined by the authors) compared to the tighter packing found in dense mats of fine filamentous or sheet-like macroalgae, although the short duration of their experiments (i.e., 3 weeks) could also help explain the lack of detrimental impacts. Here we test the hypotheses that stagnant canopies tend to have negative impacts on epifaunal communities, whereas drifting canopies do not.
We pursue the two goals by using a combination of original field work and novel analyses done with information culled from the literature. The results help implement our current understanding and conceptual model of anthropogenic eutrophication of shallow coastal systems by (1) providing evidence that intense eutrophication may cause macroalgal decline via severe water column shading and (2) the impacts of nutrient-fuelled macroalgal canopies on epifaunal communities depend on the degree of motility of the canopy (stagnant versus drifting).
Materials and Methods
Interactions Between Loose Macroalgae and Phytoplankton Under Intense Levels of Eutrophication
We used a dual approach combining original data from a comparative field study among estuaries in Cape Cod (Massachusetts) and analyses of data obtained from literature surveys. The field study examines whether trends exist between the intensity of eutrophication, the degree of light attenuation, and loose macroalgal abundance across the estuaries compared that are consistent with the hypothesis that intense eutrophication may lead to macroalgal decline due to severe water column shading. We complement this field study with two analyses of literature data. First, we synthesize information from mesocosm studies to investigate whether experimental large increases in nutrient loading lead to macroalgal declines due to intense shading by the water column. Second, we compile data from a number of shallow coastal systems worldwide to examine whether a positive association exists between nutrient loading rates and macroalgal abundance at incipient and moderate eutrophication when light availability remains adequate, but the association disappears at high eutrophication intensity due to intense water column shading.
Field Comparison Among Estuaries in Southwest Cape Cod (Massachusetts)
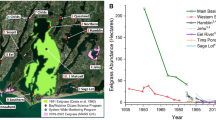
We sampled seven estuaries in southwestern Cape Cod (Massachusetts, USA; Fig. 1). The estuaries differ largely in the extent of human development in the estuarine watershed (Valiela et al. 2000b). Estuaries that differ largely in watershed human occupation are likely to differ in the loading rate of nutrients from the watershed into the estuarine waters as well as in the concentrations of nutrients in the estuarine water column, with more developed estuaries having higher loading rates and concentrations (Boynton et al. 1996). For estuaries in Southwest Cape Cod, the relationship between larger watershed occupation and higher water column nutrient concentrations mostly applies to dissolved inorganic nitrogen and not to dissolved organic nitrogen since undeveloped estuaries may have higher loads and concentrations of dissolved organic nitrogen than developed estuaries (Valiela et al. 2000a). Valiela et al. (2004) found a strong positive association between watershed development, nitrogen loading rates, and water column concentrations of dissolved inorganic nitrogen across seven estuaries in Southwest Cape Cod, with two of those estuaries being included in this study (Childs River and Quashnet River). Based on our knowledge of the estuaries in this region, we chose to measure water column nitrate concentration to characterize the differences in nitrogen loading due to watershed development among the estuaries studied here. More largely developed estuaries showed higher nitrate concentrations (Table 1), indicating that water column nitrate concentration is in this case an adequate proxy for the intensity of anthropogenic eutrophication.
Sampling was carried out in August 1999, when phytoplankton and macroalgae typically display high levels of abundance in Cape Cod estuaries (Hauxwell et al. 1998; Tomasky et al. 1999; Fox et al. 2008). In each estuary, six sampling stations were randomly distributed throughout the saline reaches in an effort to minimize the impacts of varying salinity on our results. At each station, we measured the depth of the water column using a labeled lead line and oxygen concentrations, salinity, and temperature at the surface and bottom using a hand-held YSI (Model 85). We sampled macroalgae with a 15 × 15-cm2 Ekman grab attached to a 2-m-long pole at four points around each station, for a total of 24 macroalgal samples per estuary. The samples were put on ice and brought back to the laboratory, where the algae were rinsed through a 2-mm mesh sieve, sorted out, and dried at 80 °C. Macroalgal biomass was expressed as gram dry weight per square meter.
At each station, we took duplicate samples for the determination of water column nitrate concentrations. These samples were collected in acid-washed bottles and kept on ice during transportation to the laboratory. Upon arrival, the samples were filtered through 47-mm-diameter GF/F (0.7 μm) filters and the filtrate frozen until analysis. Nitrate concentration was measured with a Quick Chem AE autoanalyzer (LACHAT Instruments) using standard colorimetric methods (Strickland and Parsons 1972). We also measured the light extinction coefficient throughout the water column at each station. In Timms Pond, Childs River, Quashnet River, and Pinquickset Bay, we used a LI-COR 1000 data logger with a submersible quantum sensor. Light intensity was measured just underneath the water surface and every 10 cm throughout the water column. Duplicate light profiles were taken at each station for a total of 12 per estuary. For each profile, the light extinction coefficient (z) was derived as the slope of the Beer–Lambert's equation fitted to the data:
where c is a constant, I z is the light intensity at depth z, I 0 is the light intensity just underneath the water surface, and z is water depth. Technical problems, however, prevented us from using the LI-COR in the other estuaries. Hence, at Shoestring Bay, Perch Pond, and Mashpee River, we took duplicate Secchi depth (SD) measurements at each sampling station, for a total of 12 measurements per estuary. To remove personal bias, the same individual did all the Secchi depth measurements. Secchi depth measurements were converted into estimates of light extinction coefficient (k) using the equation (Malone 1977; Grillini and Lazzara 1978):
Bottom irradiance (micromole photons per square meter per second) in Shoestring Bay, Perch Pond, and Mashpee River was estimated with Eq. 1 using the mean value of I 0 for the other four estuaries. The conversion factor between k and SD varies depending on the relative amount of light scattering to absorption in the water column. In waters with large quantities of inorganic suspended matter and high levels of scattering, the conversion factor is <1.7, and in waters with large amounts of light-absorbing particulate and dissolved organic matter, the factor is >1.7 (Koenings and Edmundson 1991; Pierson et al. 2008; Gallegos et al. 2011). Here we used a conversion factor of 1.7 for the three estuaries where we indirectly derived the highest levels of k. If these three estuaries had much higher amounts of inorganic suspended matter in their water columns, the calculated k values for those estuaries would have been inflated, and thus, the differences in k values between these three and the other four estuaries would be questionable. However, this is highly unlikely since in general there are no large differences in the factors that control inorganic suspended matter, such as sediment type, fetch area, predominant wind direction, and riverine inputs, among the estuaries compared here (Valiela et al. 2000b; Hauxwell et al. 2003). Rather, if anything we would expect higher concentrations of light-absorbing matter in the three estuaries with the indirectly derived highest levels of k (Tomasky et al. 1999; Hauxwell et al. 2001), which would render the differences in light attenuation with other estuaries even more prominent. Thus, our conclusions regarding higher light attenuation in the three estuaries with indirectly calculated k values should be robust. Differences in light extinction coefficient and bottom irradiance among estuaries were analyzed with one-way ANOVA followed by post hoc Tukey tests comparing pairs of estuaries. Water column nitrate concentration and macroalgal biomass did not comply with the requirements of ANOVA, even after data transformation. Thus, differences in these variables among estuaries were analyzed with the nonparametric Kruskal–Wallis test followed by Q tests for comparisons between pairs of estuaries (Zar 1984).
Interactions Between Nutrient Enrichment, Water Column Shading, and Macroalgal Abundance in Mesocosm Studies
We resort to published mesocosm studies to offer more evidence that intense eutrophication can lead to macroalgal decline due to severe water column shading in shallow coastal systems. The studies examined here are Burkholder et al. (1992, 1994), Taylor et al. (1995a, b), and Taylor et al. (1999) for a total of 14 trials. These studies reflect realistic field conditions and integrate the communities of producers and consumers found in the mimicked shallow bays, lagoons, and estuaries. They all are nutrient enrichment studies and have the common theme of investigating how higher nutrient inputs than those observed under ambient conditions affect a number of structural and dynamic system properties, including community composition and abundance, intra- and interspecific competition, trophic relationships between producers and consumers, system metabolism, and the turnover/recycling of energy and materials in the system.
We delved into the data provided by these studies to provide further supporting evidence that macroalgal abundance increases at moderate eutrophication levels, but may decrease at high eutrophication levels due to intense water column shading. Namely, using information reported in these studies, we test the hypothesis that, under moderate nutrient enrichment, light penetration through the water column will be adequate for macroalgal growth and higher macroalgal biomass values will be found in enriched than in non-enriched conditions; however, under intense nutrient enrichment, severe light attenuation through the water column will occur, thereby greatly limiting macroalgal growth and resulting in lower macroalgal biomass values in enriched than in non-enriched conditions. We culled the following information out of the 14 nutrient enrichment trials: nutrient loading rates for the enriched treatments; macroalgal biomass in the non-enriched and enriched treatments at the beginning and end of the experiment; light intensity at the top of the macroalgal canopy at the beginning of the experiment; and mean light intensity at the top of the macroalgal canopy throughout the experiment in non-enriched and enriched treatments. With this information, we plotted for each of the trials the change in macroalgal biomass in enriched in relation to non-enriched conditions versus the shift in light intensity received by the macroalgae in the enriched treatment during the experiment. We overlaid on this plot as insets the loading rates of the enrichment treatments and the mean light intensities reaching the macroalgal canopy in the non-enriched treatments during the experiment. The plot allows for a clear depiction of whether macroalgal biomass increases under moderate nutrient enrichment in relation to non-enriched conditions owing to adequate levels of light availability, and whether macroalgal biomass decreases under intense nutrient enrichment in parallel with drastic reductions in light availability in relation to non-enriched conditions. In addition, using t tests, we did two comparisons between enrichment trials receiving low to moderate loading rates and those receiving high loading rates. We first compared between the two groups of trials the mean light intensity received by macroalgae in enriched conditions during the experiment, and second, we compared the change in macroalgal biomass in enriched conditions in relation to non-enriched conditions that occurred over the experiment.
Relationship Between Nutrient Loading Rate, Water Column Light Attenuation, and Macroalgal Biomass in Shallow Coastal Systems
As a third effort to provide evidence that intense eutrophication may lead to macroalgal declines due to severe water column shading in shallow coastal systems, we compiled data on nitrogen loading rates, light extinction coefficient through the water column, and macroalgal biomass for 23 systems (Table 2). The systems range in depth from 0.2 to 10 m and encompass a wide range of eutrophication intensity as revealed by their differences in nutrient loading. We only accepted reports that (1) provided yearly nitrogen loading rates for the entire system and (2) had adequate spatial sampling coverage and provided well-balanced, spatially weighted values of macroalgal biomass and water column light extinction coefficients representative of the system as a whole. Most of the systems compiled are temperate, and thus, the values of macroalgal biomass and light extinction coefficient in those systems correspond to mean values for the growing season.
Our hypothesis was that, at low to moderate nitrogen loading levels, light availability would remain adequate for macroalgal growth, and thus, macroalgal biomass would increase with increasing loading rates. Under intense loading levels, however, severe light limitation would occur and macroalgal biomass would show reduced values. To test this hypothesis, we regressed macroalgal biomass versus nitrogen loading rates across the systems. We expected that, at low to moderate loading rates, macroalgal biomass would be positively associated with loading rates, and the systems following that association would also feature low to moderate coefficients of water column light extinction. However, we expected this association to be disrupted at intense loading rates, where severe light limitation (as shown by the water column light extinction coefficient in the system) would result in depressed macroalgal biomass.
Stand Mobility Dependence of the Impacts of Macroalgal Stands on Epifaunal Communities: Stagnant Versus Drifting Stands
To further contribute to improving our current conceptual model of anthropogenic eutrophication of shallow coastal systems, we again resort to the literature to generate evidence that the impacts of nutrient-fueled macroalgal blooms on epifaunal populations are dependent upon the degree of mobility of the bloom through occurrence of anoxic/hypoxic conditions. Namely, we expect that if the blooms form dense, stagnant mats, then adverse biogeochemical conditions would develop (hypoxia/anoxia, high sulfide and ammonium concentrations), thereby causing substantial mortality of epifaunal populations. Oppositely, if the algal mats are mobile (drifting mats), we could expect persistent sufficient oxygenation within the algal canopy and, therefore, little to no mortality of epifaunal populations. In other words, we would expect detrimental impacts of dense, stagnant mats on the abundance of epifaunal populations, but this would not be the case for drifting mats.
To test this hypothesis, we compiled a number of studies of macroagal blooms in shallow coastal systems (Tagliapietra et al. 1998; Norkko et al. 2000; Boström and Bonsdorff 1997; Franz and Friedman 2002; Österling and Pihl 2001; Hauxwell et al. 1998). The studies focus on diverse ecological impacts of the blooms, but they all provide data on bloom macroalgal biomass, epifaunal abundance for the bloom canopy and control substrate (i.e., seagrass or bare sediment around the macroalgal blooms), water oxygen concentration within the bloom canopy and on surrounding control area, and whether the bloom algal mat is stagnant or drifting. The studies compiled refer to temperate locations and encompass most of the growing season for macroalgae and epifauna. They also include most of the epifaunal community, if not all of it. With these data, we compared the percent change in epifaunal abundance that occurred with the bloom in relation to control substrate (expressed as the ratio of mean epifaunal abundance for the macroalgal canopy to mean epifaunal abundance for the control substrate multiplied by 100) between canopies with persistent hypoxic/anoxic conditions and canopies with persistent well-oxygenated conditions. We expected to find negative changes (i.e., lower epifaunal abundance for the macroalgal canopy than for the control substrate) under persistent hypoxic/anoxic conditions and such changes to be associated with stagnant canopies. In contrast, we did not expect this to be the case under persistent well-oxygenated conditions featured by drifting canopies. The percent change in epifaunal abundance that occurred with the bloom in relation to control substrate was compared between both types of conditions (hypoxic/anoxic versus normoxic) using a t test. We also noted the biomass (as grams of dry weight per square meter) of the macroalgal mat to see if it was related to its mobility and occurrence of hypoxic/anoxic conditions.
Results
Interactions Between Loose Macroalgae and Phytoplankton Under Intense Levels of Eutrophication
Field Comparison Among Estuaries in Southwest Cape Cod (Massachusetts)
The estuaries examined are shallow, with mean depth ranging from 0.2 (Pinquickset Bay) to 1.5 m (Perch Pond) among them (Table 1). Mean oxygen concentrations in the water column differed somewhat among the estuaries, and they ranged from 5.7 (Pinquickset Bay) to 11.9 mg L−1 (Perch Pond). Mean water temperature displayed typical summer values, ranging little among estuaries (from 22.5 °C in Pinquickset Bay to 26.2 °C in Timms Pond). Mean salinity varied to some extent among estuaries, from 17.0 ppt in Mashpee River to 28.4 ppt in Timms Pond, but in general, these data indicate that our sampling took place within the saline reaches of the estuaries.
In contrast mean water column nitrate concentration varied largely (approximately by 50-fold) among the estuaries (Table 1). We found a gradient towards increasing concentrations from Timms Pond/Pinquickset Bay to Childs River/Quashnet River to Shoestring Bay to Mashpee River/Perch Pond, although the pairwise comparisons among these groups were not always statistically significant (Fig. 2). The mean water column light extinction coefficient varied approximately by twofold among estuaries, and extinction coefficient values were higher in Shoestring Bay, Mashpee River, and Perch Pond than in the rest of the estuaries (Fig. 2). Mean bottom irradiance varied approximately by 20-fold among estuaries, and in accordance to the differences found in light extinction through the water column, values of bottom irradiance were generally lower in Shoestring Bay, Mashpee River, and Perch Pond than in the rest of estuaries (Fig. 2). Mean macroalgal biomass varied approximately by 150-fold among estuaries, and values of macroalgal biomass were higher in Childs River and Quashnet River than in the rest of estuaries (Fig. 2).
Box plots of nitrate concentration (micromolar), light extinction coefficient (per meter), bottom irradiance (micromole per square meter per second), and macroalgal biomass (gram dry weight per square meter) for the Cape Cod sub-estuaries. Letters on boxes denote significant differences with post hoc pairwise tests (see “Results”). Letters on the X-axis denote the sub-estuaries (see Fig. 1)
We plotted the mean light extinction coefficient, bottom irradiance, and macroalgal biomass versus nitrate concentration across the estuaries studied to explore if trends existed among these variables that are supportive of our hypothesis (Fig. 3). Mean light extinction in the water column remained relatively low (<1.5 m−1) in estuaries with a mean water column nitrate concentration <2 μM (i.e., Timms Pond, Pinquickset Bay, Childs River, and Quashnet River), but it abruptly increased to values >2.5 m−1 in estuaries with a mean concentration >2 μM (i.e., Shoestring Bay, Mashpee River, and Perch Pond). In accordance with this trend, mean bottom irradiance remained >300 μmol photons m−2 s−1, albeit quite variable, for estuaries with a mean water column nitrate concentration <2 μM, but it abruptly decreased to ≤100 μmol photons m−2 s−1 for estuaries with a mean water column nitrate concentration >2 μM. Mean values of bottom irradiance for all estuaries with mean water column nitrate concentration >2 μM were much closer to the 75 % percentile of a range of compensation irradiance values (i.e., irradiance at which algal photosynthesis equals respiration) reported in the literature for macroalgal species found in our estuaries than were mean values of bottom irradiance for estuaries with a mean nitrate concentration <2 μM (Fig. 3). Mean macroalgal biomass increased from Timms Pond/Pinquickset Bay (mean nitrate concentrations 0.2 and 0.4 μM, respectively) to Childs River/Quashnet River (mean nitrate concentrations 0.7 and 1.1 μM, respectively), but it decreased abruptly to very low values in the estuaries with mean nitrate concentration >2 μM (Fig. 3).
Relationship between mean light extinction coefficient (per meter); bottom irradiance (micromole per square meter per second); and macroalgal biomass (gram dry weight per square meter) and nitrate concentration (micromolar) across the Cape Cod sub-estuaries studied. Dashed lines on the bottom irradiance panel correspond to the 25 % (10 μmol m−2 s−1) and 75 % (35 μmol m−2 s−1) percentiles of a compilation of values of compensation irradiance for some of the species of macroalgae found in the sub-estuaries. Sources are Gordon et al. (1980), Arnold and Murray (1980), Gacia et al. (1996a, b), Dawes and Koch (1990), Dawes et al. (1999), Vergara et al. (1997, 1998), Israel et al. (1995), Orduña-Rojas et al. (2002), Beach et al. (1995), Chisholm and Jaubert (1997), Chisholm et al. (2000), Sand-Jensen (1988), Johansson and Snoeijs (2002), Gayol et al. (1995), Rosenberg and Ramus (1982), Peckol and Rivers (1995), Levavasseur et al. (1991), Henley (1992) and Henley et al. (1991)
Interactions Between Nutrient Enrichment, Water Column Shading, and Macroalgal Abundance in Mesocosm Studies
Figure 4 depicts for each trial the change in macroalgal biomass in the enriched in relation to the non-enriched treatment versus the change in mean light availability for the macroalgae in the enriched treatment during the experiment. To do that, we represent two points for each of the 14 trials, which are connected by an arrow. The first point corresponds on the Y-axis to the percent of macroalgal biomass in the enriched treatment in relation to the non-enriched treatment at the start of the experiment, and on the X-axis to the light intensity that reaches the algal canopy at the start of the experiment. The second point corresponds on the Y-axis to the percent of macroalgal biomass in the enriched treatment in relation to the non-enriched treatment at the end of the experiment, and on the X-axis to the average light intensity at the top of the algal canopy in the enriched treatment during the experiment. Overlaid as insets are the loading rates for the enriched treatments and the average light intensities at the top of the algal canopy in the non-enriched treatments during the experiment.
Change in macroalgal biomass in enriched treatments in relation to non-enriched treatments for a number of mesocosm experiments. Values for each enrichment trial are connected with arrows. The first point corresponds on the Y-axis to the percent of macroalgal biomass in the enriched treatment in relation to the non-enriched treatment at the start of the experiment and on the X-axis to the light intensity that reaches the algal canopy at the start of the experiment. The second point corresponds on the Y-axis to the percent of macroalgal biomass in the enriched treatment in relation to the non-enriched treatment at the end of the experiment and on the X-axis to the average light intensity at the top of the algal canopy in the enriched treatment during the experiment. Overlaid as insets are the loading rates for the enriched treatments and the average light intensity at the top of the algal canopy in the non-enriched treatment during the experiment (i.e., mean control light). Dashed lines as in Fig. 3
Burkholder et al. (1994) had only one enriched treatment with a nitrogen load of 2.6 mmol N m−2 day−1. The enriched macroalgae received abundant light during the experiment, en par with non-enriched algae, and reached higher biomass than non-enriched algae. Burkholder et al. (1992) had three enrichment levels with loads of 1.8, 3.6, and 18 mmol N m−2 day−1. The enriched macroalgae in all enrichment treatments received plentiful light during the experiment, similar to the light levels received by the algae in the non-enriched treatment. Enriched macroalgae reached higher biomass than non-enriched algae in two (1.8 and 3.6 mmol N m−2 day−1) out of the three enrichment treatments. Taylor et al. (1999) applied four enrichment levels (1.3, 2.3, 4.3, and 8.3 mmol N m−2 day−1). The macroalgae in all enrichment treatments received light values en par or above the values received by the non-enriched algae during the experiment, which, although not as high as in other experiments, was still sufficient to permit macroalgal growth. The macroalgae in all enrichment treatments reached higher biomass than in the non-enriched treatment. Taylor et al. (1995a, b) applied one single level of enrichment (8.3 mmol N m−2 day−1) to six different trials. The enriched macroalgae in all trials received drastically reduced light in relation to the light received by the algae under non-enriched conditions. Light levels received by enriched macroalgae were in line with or below the 75 % percentile of the range of compensation irradiance values compiled, which suggests that the enriched algae suffered severe growth limitation. Accordingly, enriched algae reached a much smaller biomass than non-enriched algae.
All together, this analysis offers support to the hypothesis that light availability remains adequate and macroalgal growth is stimulated under incipient to moderate eutrophication in relation to non-eutrophied conditions, but intense eutrophication may lead to severe light limitation and reductions in macroalgal biomass. The trials with lower nitrogen loading rates included in this comparison (1.3, 1.8, 2.3, 2.6, 3.6, and 4.3 mmol N m−2 day−1) all maintain adequate light availability and are conducive to increases in macroalgal biomass in relation to non-enriched conditions. Out of the eight trials receiving relatively high nitrogen loading rates (8.3 and 18 mmol N m−2 day−1), six of them experienced large reductions in light availability and macroalgal biomass in relation to non-enriched conditions, one of them had good light availability and increased macroalgal biomass in relation to non-enriched conditions, and another one had good light availability and almost no change in macroalgal biomass in relation to non-enriched conditions (Fig. 4). Overall, the trials with low loading rates (1.3, 1.8, 2.3, 2.6, 3.6, and 4.3 mmol N m−2 day−1) showed much higher light availability for enriched macroalgae than trials with high loading rates (8.3 and 18 mmol N m−2 day−1) (t test comparing mean light intensity received by enriched algae between the two groups of trials, P < 0.05). Trials with low loading rates also showed much larger enriched/non-enriched macroalgal biomass ratios at the end of the experiment than trials with high loading rates (t test, P < 0.05). In concert, these mesocosm experiments suggest that moderate eutrophication in shallow coastal systems maintains adequate light availability and increases macroalgal biomass, but intense eutrophication induces severe light limitation and decreased macroalgal biomass. However, intense eutrophication may not always lead to reduced light availability and algal biomass, as shown by two of the trials receiving high loading rates.
Relationship Between Nutrient Loading Rate, Water Column Light Attenuation, and Macroalgal Biomass in Shallow Coastal Systems
Mean macroalgal biomass varied by three orders of magnitude (i.e., from ca. 1 to 1,500 g DW m−2) across the systems compared (Table 2). We adjusted a linear regression equation to all systems except the two with the highest nitrogen loading rates (Fig. 5), which corresponded to:
Relationship between macroalgal biomass (gram dry weight per square meter) and nitrogen loading rate (millimole nitrogen per square meter per day) across shallow coastal systems. Open symbols correspond to systems fitted to the regression equation (solid line), and closed symbols correspond to systems not fitted to the equation. Included as an inset is the histogram of light extinction coefficient values for the systems fitted to the regression equation (dashed line on histogram corresponds to median value). Full references for the systems compared are provided in Table 2
(n = 21, R 2 = 0.51, P < 0.05)
Water column light extinction coefficients for the systems included in the regression equation ranged from 0.2 to 1.9 m−1. The two systems not included in the regression equation clearly lied off the linear trend of increasing macroalgal biomass with higher nutrient loading rates identified for all other systems. Along with having the highest loading rates, those two systems had very low macroalgal biomass (i.e., about 100 times lower than predicted from the regression equation) and high extinction coefficients (2.65 and 2.70 m−1; Table 2). These results offer further support to the hypothesis that moderate eutrophication maintains adequate light availability and leads to increased macroalgal biomass in shallow coastal systems, but severe water column shading and large reductions in macroalgal biomass may occur at intense eutrophication.
Stand Mobility Dependence of the Impacts of Macroalgal Stands on Epifaunal Communities: Stagnant Versus Drifting Stands
A total of ten macroalgal bloom events were extracted from the reports examined (Fig. 6). Persistent or recurrent anoxia/hypoxia was found within the canopies of five of the blooms, and correspondingly, epifaunal abundance was lower for the macroalgal bloom canopy than for the surrounding substrate (bare sediment or seagrass). In contrast, five other blooms featured well-oxygenated conditions within the canopy, and epifaunal abundance for those canopies was higher than for the surrounding substrate. Indeed, the ratio of epifaunal abundance for the macroagal canopy to epifaunal abundance for the control substrate was higher for well-oxygenated (higher than 1 in all cases) than for anoxic/hypoxic canopies (lower than 1 in all cases) (t test, P < 0.05). These differences did not seem to be influenced by the type of control substrate (bare sediment or seagrass; Fig. 6). All blooms that featured persistent or recurrent anoxia/hypoxia and depressed epifaunal abundance were stagnant or, at best, had highly restricted mobility (Fig. 6). Their biomass ranged from 100 to 225 g DW m−2. Interestingly, the blooms that featured well-oxygenated conditions and promoted epifaunal abundance could be stagnant or drifting, but drifting blooms displayed higher biomass (ranging from 220 to 480 g DW m−2) than stagnant blooms (ranging from 60 to 71 g DW m−2). This suggests that stagnant blooms, if not too thick (i.e., having relatively reduced biomass levels), can be well oxygenated and harbor large epifaunal abundance.
Change in epifaunal abundance with macroalgal blooms in relation to surrounding substrate (i.e., seagrasses or bare sediment). The change is expressed as the ratio of mean epifaunal abundance for the algal canopy to mean epifaunal abundance for the surrounding substrate (control epifaunal abundance) multiplied by 100. The studies compiled are divided in two types: those featuring persistent hypoxic/anoxic conditions and those featuring well-oxygenated conditions in the bloom. Numbers on the X-axis correspond to the study number. The studies are further differentiated into whether the blooms are stagnant or drifting. Numbers within the bins denote the mean macroalgal biomass (in gram dry weight per square meter) in the study, and letters denote the surrounding substrate (S seagrasses, B bare sediment)
Discussion
With this work, we intended to contribute to our current conceptual model of eutrophication of shallow coastal systems. The current model represents well the complex responses by epiphytes, loose macroalgae, and phytoplankton to incipient/moderate eutrophication (Burkholder et al. 2007; McGlathery et al. 2007). When pristine shallow coastal systems are eutrophied, these algal producers may increase their biomass to varying degrees depending on a number of factors such as grazing, temperature, salinity, exposure to wave energy, and, in the case of phytoplankton, water residence time (e.g., Collado-Vides et al. 2007; Engelsen et al. 2010; Piñon-Gimate et al. 2012; Xu et al. 2010). Accordingly, cascading impacts on seagrasses can also be quite variable. Nevertheless, the interactions that occur between phytoplankton and loose macroalgae at intense eutrophication when they both accumulate at incipient/moderate eutrophication in shallow coastal systems have been little studied. Here we provide evidence that loose macroalgae may decline at intense eutrophication due to severe water column shading. We also help improve the current conceptual model by providing evidence that the impacts of eutrophication-induced shifts of producer assemblages on system function are contingent upon the co-occurrence of anoxic/hypoxic conditions. Focusing on loose macroalgal blooms, we show that well-oxygenated blooms promote epifaunal abundance, but poorly oxygenated blooms depress epifaunal abundance in relation to surrounding habitat. Below we discuss these two contributions to our current conceptual model of shallow coastal systems.
Three efforts are presented here (i.e., comparison of Cape Cod estuaries, analysis of a suite of published mesocosm studies, and compilation of published data for shallow coastal systems) to provide support to the hypothesis that moderate eutrophication in shallow coastal systems may, by maintaining adequate light availability, increase macroalgal abundance, but intense eutrophication may lead to macroalgal decline due to severe light limitation. Each of these efforts bears its own caveats. For instance, even though we took our samples in the Cape Cod estuaries in August, a period with maximum biological activity and where macroalgal and phytoplankton biomass should be highest in the year (Hauxwell et al. 1998; Tomasky et al. 1999; Fox et al. 2008), we only sampled the estuaries one time. Despite the gradients in water column nitrate concentrations and light extinction coefficients found among the estuaries with our one-time sampling, these parameters may vary widely through time for one given estuary (Cloern 2001; Hauxwell et al. 2001). Thus, in-depth characterization of differences in eutrophication intensity and resulting interactions between water column shading and macroalgal biomass among the estuaries examined would require more sampling. At any rate, the three efforts presented here are convergent in presenting evidence that intense eutrophication via severe water column shading may result in macroalgal declines in shallow coastal systems. The diversity of approaches, systems, measurements, and designs encompassed by the three efforts may offer further reassurance for this hypothesis.
Several authors have previously suggested the trend that, as moderately eutrophic shallow coastal systems are further eutrophied, phytoplankton biomass could accumulate to large levels and exert intense shading on the underlying macroalgae, thereby leading to their decline. Sand-Jensen and Borum (1991) and Duarte (1995) were among the first ones to suggest this trend based on a number of empirical observations. They also provided reasoning for it in that phytoplankton, due to its higher internal nutrient demands, tends to have higher nutrient uptake rates in comparison with macroalgae. In turn, macroalgae, due to their bulkier architecture and self-shading on their photosynthetic cells, tend to have higher light requirements (i.e., higher photosynthesis–respiration compensation irradiances) in comparison with phytoplankton. Thus, as nutrient inputs increase to intense levels, phytoplankton biomass will accumulate to large values owing to its higher nutrient uptake rates. This will intensify shading on underlying macroalgae, which may suffer severe growth light limitation and biomass decline due to their inherently higher light requirements. Valiela et al. (1997, 2000a) provided renewed evidence by showing that, when a number of coastal systems covering a broad range of nitrogen loading rates are compared, the contribution of phytoplankton to total system's primary productivity increases, but the contribution of macroalgae decreases, as we move from moderate to intense loading rates (Figure 6 in Valiela et al. 2000a). McGlathery et al. (2007) established this apparent trend as one of the building premises of our current eutrophication model for shallow coastal systems, although they also recognized that more research supporting the apparent trend was needed. Here we provide further support for this trend.
However, our results also suggest that intense eutrophication in shallow coastal systems is not always conducive to severe water column shading and macroalgal decline. Out of the eight trials receiving high nitrogen loading rates in the mesocosm studies, six of them showed drastically reduced light availability and large decreases in macroalgal biomass in relation to non-enriched conditions, but two of them showed good light availability and one of them increased macroalgal biomass in relation to non-enriched conditions. Short water residence time (i.e., high flushing rates) is a potential reason as to why intense nutrient loading may not be always conducive to severe water column light limitation and ensuing macroalgal decline in shallow coastal ecosystems. Fast advection rates (i.e., short water residence time) can substantially limit the accumulation of phytoplankton biomass and shading effects on benthic producers in coastal systems, even under high nutrient inputs (Valiela et al. 2000a; Oberg 2005; Xu et al. 2010). Other factors such as grazing on phytoplankton by filter feeders (Bruschetti et al. 2008; Xu et al. 2010; Wall et al. 2011) and sediment resuspension and light limitation of phytoplankton growth (Monbet 1992) can also curtail phytoplankton build-up under intense eutrophication. Here it seems unlikely that water residence time is the dominant mechanism accounting for the disparity observed in water column shading and macroalgal abundance among intense loading rates in the mesocosm studies, since all the studies compiled had similar flushing rates in the experimental tanks (i.e., 5–10 % of the tank volume per day). Unfortunately, the information provided in the studies is not sufficient for a deep analysis of this disparity.
The replacement of seagrasses by loose macroalgae and phytoplankton as dominant producers when pristine shallow coastal systems are eutrophied and large accumulations of phytoplankton and decline of loose macroalgae as the system is further eutrophied suggest important functional implications. In a recent review, McGlathery et al. (2007) synthesized some implications for the retention and fate of organic matter. Pools of refractory organic matter should be reduced because phytoplankton decomposes more quickly and stores smaller pools of recalcitrant detritus (on an areal basis) than do loose macroalgae, which in turn decompose more quickly and produce less recalcitrant detritus than do seagrasses and bulky macroalgae (Enriquez et al. 1993; Banta et al. 2004; Cebrian and Lartigue 2004). However, hypoxic/anoxic conditions that may occur with large accumulations of loose macroalgae and/or phytoplankton could influence refractory matter storage, since decomposition proceeds more slowly under those conditions (Kristensen et al. 1995; Kristensen 2000; Middelburg et al. 2004). Export of organic matter from shallow coastal systems to neighboring waters should also be altered as dominant producers in the system shift from seagrasses to loose macroalgae/phytoplankton to phytoplankton. Loose macroalgae are unattached and can be transported by waves and currents more easily than seagrasses, which are anchored to the sediment via roots and rhizomes and typically only export shed leaves, and phytoplankton moves at the mercy of the water and has a higher export potential than loose macroalgae (Bach et al. 1986; Cebrian 2002; Flindt et al. 2004).
Along with detritus decomposition rates, grazing rates could also increase as dominant producers shift from seagrasses to loose macroalgae/phytoplankton to phytoplankton. The percentage of primary production consumed by grazers is typically higher in phytoplankton than loose macroalgae than seagrasses, in agreement with higher producer nutritional quality (higher nutrient contents and lower contents of structural carbon compounds) in phytoplankton than in loose macroalgae than in seagrasses (Cebrian 1999; Cebrian et al. 1998). If both the percentage of primary production consumed by herbivores and the percentage consumed by detritivores/decomposers (as indicated by the decomposition rate) increase, we could also expect higher levels of secondary productivity with the shift from seagrasses to loose macroalgae/phytoplankton to phytoplankton as long as the total system's primary productivity is not significantly reduced, which may well be the case in shallow coastal systems (Borum and Sand-Jensen 1996; Nixon et al. 2001). However, changes in the quality and quantity of biotic structural complexity and potential shelter for organisms that would occur with this shift in dominant producer assemblages could certainly influence secondary productivity in the system. Perhaps such changes would not be too dramatic when loose macroalgae replace seagrasses as dominant producers, since both seagrass and macroalgal canopies are structurally complex and can provide shelter to many organisms (Norkko et al. 2000; Österling and Pihl 2001; Hughes et al. 2002; Råberg and Kautsky 2007), but large declines in loose macroalgal abundance under intense eutrophication would likely reduce biotic structural complexity and potential shelter for organisms in the system.
At any rate, the impacts of eutrophication-induced shifts in producer assemblages on grazing rates, quality of biotic structure, and secondary productivity should depend on whether anoxic/hypoxic conditions co-occur with the producer shift. Here we present evidence for this hypothesis focusing on blooms of loose macroalgae. Using published data we show that, when the blooms develop pervasive hypoxic/anoxic conditions within the algal canopy, epifaunal abundance is reduced in relation to the epifaunal abundance found for surrounding substrate uncovered by the bloom (i.e., bare sediment or seagrass beds), but the blooms harbor larger epifaunal abundances than surrounding substrate when they maintain well-oxygenated conditions. These results provide support to the hypothesis that functional changes in shallow coastal systems as a result of eutrophication-induced shifts in producer assemblages are contingent upon the co-occurrence of anoxic/hypoxic conditions. Namely, as seagrasses are replaced by loose macroalgae during incipient and moderate eutrophication, total secondary productivity may increase owing to higher levels of herbivory and detritivory (i.e., higher consumption rates on the macroalgae than on the seagrass) in conjunction with little change in biotic structural complexity (i.e., the amount of quality shelter offered to organisms) as long as the macroalgal canopies remain well oxygenated, but this would not be the case if pervasive hypoxia/anoxia occurs in the canopy. The results also point to two mechanisms that can keep loose macroalgal blooms well oxygenated. Our comparison suggests that blooms with relatively low biomass (<100 g DW m−2) remain well oxygenated, even though they are stagnant. As bloom macroalgal biomass becomes large (>100 g DW m−2), only drifting blooms remain well oxygenated, whereas stagnant blooms develop recurrent or permanent hypoxic/anoxic conditions. Therefore, the mobility of macroalgal blooms seems to be influential for oxygen conditions within the canopy mostly when blooms accumulate large levels of macroalgal biomass.
In conclusion, our results help implement our current model of eutrophication of shallow coastal systems by providing additional support for poorly documented predictions of the model (Valiela et al. 2000a; McGlathery et al. 2007). We first provide new evidence that loose macroalgal blooms that are formed at incipient/moderate eutrophication can decline at intense eutrophication due to severe water column shading (Fig. 7). This process seems widespread, based on the diversity of approaches used in this paper. However, we also show that severe water column shading and macroalgal decline do not necessarily always occur at high eutrophication levels. Short water residence time, grazing on phytoplankton, and light limitation of phytoplankton could account for this, although our work does not permit close examination of these possibilities. We also illustrate how the occurrence of pervasive hypoxia/anoxia can condition functional change in the system that results from eutrophication-driven shifts in dominant primary producers. We focus on the development of macroalgal blooms and associated seagrass decline that frequently occurs under incipient to moderate eutrophication and show positive changes in epifaunal abundance from the seagrass to macroalgal-dominated stage if the bloom maintains well-oxygenated conditions, but negative changes under pervasive hypoxic/anoxic conditions. We show that bloom macroalgal biomass and degree of mobility affect oxygen conditions in the bloom. In light of our findings, we suggest that future work to further improve our current model of eutrophication of shallow coastal systems could focus on ascertaining the mechanisms that can prevent severe water column shading and macroalgal decline at high eutrophication intensity, as well as thorough documentation of how the development of anoxic/hypoxic conditions influences system functionality across different stages of eutrophication.
Eutrophication-driven shifts in primary producer assemblages in shallow coastal systems. In shallow (a few meters deep), well-lit systems dominated by seagrasses, incipient to moderate eutrophication favors growth of macroalgae and phytoplankton in detriment of the seagrasses. These newly formed stands of loose macroalgae may enhance secondary productivity in the system if they remain well oxygenated, but depress it if they exhibit persistent anoxia/hypoxia. As the system now co-dominated by loose macroalgae and phytoplankton is further eutrophied to intense levels, larger phytoplankton stocks will accumulate in the water column and, through severe shading, induce macroalgal decline. In some instances, however, factors such as fast flushing (low water residence time) and elevated grazing on phytoplankton may prevent large phytoplankton accumulation and macroalgal decline
References
Arnold, K.E., and S.N. Murray. 1980. Relationships between irradiance and photosynthesis for marine benthic green algae (Chlorophyta) of differing morphologies. Journal of Experimental Marine Biology and Ecology 43: 183–192.
Arroyo, N.L., K. Aarnio, M. Mäensivu, and E. Bonsdorff. 2012. Drifting filamentous algal mats disturb sediment fauna: Impacts on macro-meiofaunal interactions. Journal of Experimental Marine Biology and Ecology 420–421: 77–90.
Bach, S., G. Thayer, and M. LaCroix. 1986. Export of detritus from eelgrass (Zostera marina) beds near Beaufort, North Carolina, USA. Marine Ecology Progress Series 28: 265–278.
Banta, G.T., M.F. Pedersen, and S.L. Nielsen. 2004. Decomposition of marine primary producers: Consequences for nutrient recycling and retention in coastal ecosystems. In Estuarine nutrient cycling: The influence of primary producers, ed. S.L. Nielsen, G.T. Banta, and M.F. Pedersen, 187–216. Dordrecht: Kluwer Academic.
Beach, K.S., C.M. Smith, T. Michael, and H.W. Shin. 1995. Photosynthesis in reproductive unicells of Ulva fasciata and Enteromorpha flexuosa: Implications for ecological success. Marine Ecology Progress Series 125: 229–237.
Borum, J., and K. Sand-Jensen. 1996. Is total primary production in shallow coastal marine waters stimulated by nitrogen loading? Oikos 76: 406–410.
Boström, C., and E. Bonsdorff. 1997. Community structure and spatial variation of benthic invertebrates associated with Zostera marina (L.) beds in the northern Baltic Sea. Journal of Sea Research 37: 153–166.
Boynton, W.R., L. Murray, J.D. Hagy, C. Stokes, and W.M. Kemp. 1996. A comparative analysis of eutrophication patterns in a temperate coastal lagoon. Estuaries 19: 408–421.
Bricker, S.B., B. Longstaff, W. Dennison, A. Jones, K. Boicourt, C. Wicks, and J. Woerner. 2008. Effects of nutrient enrichment in the nation’s estuaries: A decade of change. Harmful Algae 8: 21–32.
Bruschetti, M., T. Luppi, E. Fanjul, A. Rosenthal, and O. Iribarne. 2008. Grazing effect of the invasive reef-forming polychaete Ficopomatus enigmaticus (Fauvel) on phytoplankton biomass in a SW Atlantic coastal lagoon. Journal of Experimental Marine Biology and Ecology 354: 212–219.
Burkholder, J.M., K.M. Mason, and H.B. Glasgow. 1992. Water-column nitrate enrichment promotes decline of eelgrass Zostera marina: Evidence from seasonal mesocosm experiments. Marine Ecology Progress Series 81: 163–178.
Burkholder, J.M., H.B. Glasgow, and J.E. Cooke. 1994. Comparative effects of water-column nitrate enrichment on eelgrass Zostera marina, shoalgrass Halodule wrightii, and widgeongrass Ruppia maritima. Marine Ecology Progress Series 105: 121–138.
Burkholder, J.M., D.A. Tomasko, and B.W. Touchette. 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology 350: 46–72.
Cebrian, J. 1999. Patterns in the fate of production in plant communities. The American Naturalist 154: 449–468.
Cebrian, J. 2002. Variability and control of carbon consumption, export and accumulation in marine communities. Limnology and Oceanography 47: 11–22.
Cebrian, J., and J. Lartigue. 2004. Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecological Monographs 74: 237–259.
Cebrian, J., M. Williams, J. McClelland, and I. Valiela. 1998. The dependence of heterotrophic consumption and C accumulation on autotrophic nutrient content in ecosystems. Ecology Letters 1: 165–170.
Cebrian, J., J.P. Stutes, and B. Christiaen. 2013. Effects of grazing and fertilization on epiphyte growth dynamics under moderately eutrophic conditions: Implications for grazing rate estimates. Marine Ecology Progress Series 474: 121–133.
Chisholm, J.R.M., and J.M. Jaubert. 1997. Photoautotrophic metabolism of Caulerpa taxifolia (Chlorophyta) in the NW Mediterranean. Marine Ecology Progress Series 153: 113–123.
Chisholm, J.R.M., M. Marchioretti, and J.M. Jaubert. 2000. Effect of low water temperature on metabolism and growth of a subtropical strain of Caulerpa taxifolia (Chlorophyta). Marine Ecology Progress Series 201: 189–198.
Cloern, J.E. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Cole, M.L., K.D. Kroeger, J.W. McClelland, and I. Valiela. 2006. Effects of watershed land use on nitrogen concentration and δ15nitrogen in groundwater. Biogeochemistry 77: 199–215.
Collado-Vides, L., V.G. Gaccia, J.N. Boyer, and J.W. Fourqurean. 2007. Tropical seagrass-associated macroalgae distributions and trends relative to water quality. Estuarine, Coastal and Shelf Science 73: 680–694.
D’Avanzo, C., and J.N. Kremer. 1994. Diel oxygen dynamics and anoxic events in an eutrophic estuary of Waquoit Bay, Massachusetts. Estuaries 17: 131–139.
Dawes, C.J., and E.W. Koch. 1990. Physiological responses of the red algae Gracilaria verrucosa and G. tikvahiae before and after nutrient enrichment. Bulletin of Marine Science 46: 335–344.
Dawes, C.J., J. Orduña-Rojas, and D. Robledo. 1999. Response of the tropical red seaweed Gracilaria cornea to temperature, salinity and irradiance. Journal of Applied Phycology 10: 419–425.
De Casabianca, M.L. 1996. France—the Mediterranean lagoons. In Marine benthic vegetation: Recent changes and the effects of eutrophication, ed. W. Schramm and P.H. Nienhuis, 307–329. Berlin: Springer.
De Vries, I., C.J.M. Philippart, E.G. De Groot, and M.W.M. van der Tol. 1996. Coastal eutrophication and marine benthic vegetation: A model analysis. In Marine benthic vegetation: Recent changes and the effects of eutrophication, ed. W. Schramm and P.H. Nienhuis, 79–113. Berlin: Springer.
Duarte, C.M. 1995. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41: 87–112.
Engelsen, A., K. Sundbäck, and S. Hulth. 2010. Links between bottom-water anoxia, the polychaete Nereis diversicolor, and the growth of green-algal mats. Estuaries and Coasts 33: 1365–1376.
Enriquez, S., C.M. Duarte, and K. Sand-Jensen. 1993. Patterns in decomposition rates among photosynthetic organisms: The importance of detritus C:N:P content. Oecologia 94: 457–471.
Flindt, M.R., L. Kamp-Nielsen, J.C. Marques, M.A. Pardal, M. Bocci, G. Bendoricchio, J. Salomonsen, S.N. Nielsen, and S.E. Jorgensen. 1997. Description of the three shallow estuaries: Mondego River (Portugal), Roskilde Fjord (Denmark) and the Lagoon of Venice (Italy). Ecological Modeling 102: 17–31.
Flindt, M.R., J. Neto, C.L. Amos, M.A. Pardal, A. Bergamasco, C.B. Pedersen, and F.Ø. Andersen. 2004. Plant bound nutrient transport: Mass transport in estuaries and lagoons. In Estuarine nutrient cycling: The influence of primary producers, ed. S.L. Nielsen, G.T. Banta, and M.F. Pedersen, 93–128. Dordrecht: Kluwer Academic.
Fox, S.E., E. Stieve, I. Valiela, J. Hauxwell, and J. McClelland. 2008. Macrophyte abundance in Waquoit Bay: Effects of land-derived nitrogen loads on seasonal and multi-year biomass patterns. Estuaries and Coasts 31: 532–541.
Franz, D.R., and I. Friedman. 2002. Effects of macroalgal mat (Ulva lactuca) on estuarine sand flat copepods: An experimental study. Journal of Experimental Marine Biology and Ecology 271: 209–226.
Gacia, E., M.M. Littler, and D.S. Littler. 1996a. The relationships between morphology and photosynthetic parameters within the polymorphic genus Caulerpa. Journal of Experimental Marine Biology and Ecology 204: 209–224.
Gacia, E., C. Rodriguez-Prieto, O. Delgado, and E. Ballesteros. 1996b. Seasonal light and temperature responses of Caulerpa taxifolia from the northwestern Mediterranean. Aquatic Botany 53: 215–225.
Gallegos, C.L., P.J. Werdell, and C.R. McClain. 2011. Long-term changes in light scattering in Chesapeake Bay inferred from Secchi depth, light attenuation, and remote sensing measurements. Journal of Geophysical Research 16: C00H08. doi:10.1029/2011JC007160.
Gayol, P., C. Falconetti, J.R.M. Chisholm, and J.M. Jaubert. 1995. Metabolic responses of low-temperature-acclimated Caulerpa taxifolia (Chlorophyta) to rapidly elevated temperature. Botanica Marina 38: 61–67.
Gordon, D.M., P.B. Bird, and A.J. McComb. 1980. The effect of light, temperature and salinity on photosynthesis rates of an estuarine Cladophora. Botanica Marina 23: 749–755.
Gray, J.S., R.S. Wu, and Y.Y. Or. 2002. Effects of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series 238: 249–279.
Grillini, C.L., and L. Lazzara. 1978. Ciclo annuale del fitoplancton nelle acque costiere del Parco Naturale della Maremma. I. Variazioni quantitive. Giornale Botanico Italiano 112: 157–173.
Haritonidis, S. 1996. Greece. In Marine benthic vegetation: Recent changes and the effects of eutrophication, ed. W. Schramm and P.H. Nienhuis, 403–420. Berlin: Springer.
Hauxwell, J., and I. Valiela. 2004. Effects of nutrient loading on shallow seagrass-dominated coastal systems: Patterns and processes. In Estuarine nutrient cycling: The influence of primary producers, ed. S.L. Nielsen, G.T. Banta, and M.F. Pedersen, 59–92. Dordrecht: Kluwer Academic.
Hauxwell, J., J. McClelland, P.J. Behr, and I. Valiela. 1998. Relative importance of grazing and nutrient controls of macroalgal biomass in three temperate shallow estuaries. Estuaries 21: 347–360.
Hauxwell, J., J. Cebrian, C. Furlong, and I. Valiela. 2001. Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology 82: 1007–1022.
Hauxwell, J., J. Cebrian, and I. Valiela. 2003. Eelgrass Zostera marina loss in temperate estuaries: Relationship to land-derived nitrogen loads and effect of light limitation imposed by algae. Marine Ecology Progress Series 247: 59–73.
Heck, K.L., J.R. Pennock, J.F. Valentine, L.D. Coen, and S.A. Sklenar. 2000. Effects of nutrient enrichment and small predator density on seagrass ecosystems. An experimental assessment. Limnology and Oceanography 45: 1041–1057.
Heck, K.L., J.F. Valentine, J.R. Pennock, G. Chaplin, and P.M. Spitzer. 2006. Effects of nutrient enrichment and grazing on shoalgrass (Halodule wrightii) and its epiphytes: Results of a field experiment. Marine Ecology Progress Series 326: 145–156.
Henley, W.J. 1992. Growth and photosynthesis of Ulva rotundata (Chlorophyta) as a function of temperature and square wave irradiance in indoor culture. Journal of Phycology 28: 625–634.
Henley, W.J., G. Levavasseur, L.A. Franklin, S.T. Lindley, J. Ramus, and C.B. Osmond. 1991. Diurnal responses of photosynthesis and fluorescence in Ulva rotundata acclimated to sun and shade in outdoor culture. Marine Ecology Progress Series 75: 19–28.
Hessing-Lewis, M.L., S.D. Hacker, B.A. Menge, and S.S. Rumrill. 2011. Context-dependent eelgrass–macroalgae interactions along an estuarine gradient in the Pacific Northwest, USA. Estuaries and Coasts 34: 1169–1181.
Höffle, H., M.S. Thomsen, and M. Holmer. 2011. High mortality of Zostera marina under high temperature regimes but minor effects of the invasive macroalgae Gracilaria vermiculophylla. Estuarine, Coastal and Shelf Science 92: 35–46.
Holmer, M., and R.M. Nielsen. 2007. Effects of filamentous algal mats on sulfide invasion in eelgrass (Zostera marina). Journal of Experimental Biology and Ecology 353: 245–252.
Hughes, J.E., L.A. Deegan, J.C. Wyda, M.J. Weaver, and A. Wright. 2002. The effects of eelgrass habitat loss on estuarine fish communities of Southern New England. Estuaries 25: 235–249.
Hughes, A.R., K.J. Bando, L.F. Rodriguez, and S.L. Williams. 2004. Relative effects of grazers and nutrients on seagrasses: A meta-analysis approach. Marine Ecology Progress Series 282: 87–99.
Iizumi, H., and A. Hattori. 1982. Growth and organic production of eelgrass (Zostera marina L.) in temperate waters of the Pacific coast of Japan. III: The kinetics of nitrogen uptake. Aquatic Botany 12: 245–256.
Israel, A.A., M. Friedlander, and A. Neori. 1995. Biomass yield, photosynthesis and morphological expression of Ulva lactuca. Botanica Marina 38: 297–302.
Johansson, G., and P. Snoeijs. 2002. Macroalgal photosynthetic responses to light in relation to thallus morphology and depth zonation. Marine Ecology Progress Series 244: 63–72.
Koenings, J.P., and J.A. Edmundson. 1991. Secchi disk and photometer estimates of light regimes in Alaskan lakes: Effects of yellow color and turbidity. Limnology and Oceanography 36: 91–105.
Krause-Jensen, D., A.L. Middelboe, J. Carstensen, and K. Dahl. 2007a. Spatial patterns of macroalgal abundance in relation to eutrophication. Marine Biology 152: 25–36.
Krause-Jensen, D., J. Carstensen, and K. Dahl. 2007b. Total and opportunistic algal cover in relation to environmental variables. Marine Pollution Bulletin 55: 114–125.
Krause-Jensen, D., S. Sagert, H. Schubert, and C. Boström. 2008. Empirical relationships linking distribution and abundance of marine vegetation to eutrophication. Ecological Indicators 8: 515–529.
Kristensen, E. 2000. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 426: 1–24.
Kristensen, E., S.I. Ahmed, and A.H. Devol. 1995. Aerobic and anaerobic decomposition of organic matter in marine sediment: Which is fastest? Limnology and Oceanography 40: 1430–1437.
Lavery, P.S., R.J. Lukatelich, and A.J. McComb. 1991. Changes in the biomass and species composition of macroalgae in a eutrophic estuary. Estuarine, Coastal and Shelf Science 33: 1–22.
Lee, V., and S. Olsen. 1985. Eutrophication and management initiatives for the control of nutrient inputs to Rhode Island coastal lagoons. Estuaries 8: 191–202.
Levavasseur, G., G.E. Edwards, C.B. Osmond, and J. Ramus. 1991. Inorganic carbon limitation of photosynthesis in Ulva rotundata (Chlorophyta). Journal of Phycology 27: 667–672.
Liu, S.L., W.L. Wang, D.T. Dy, and C.C. Fu. 2005. The effect of ulvoid macroalgae on the inorganic carbon utilization by an intertidal seagrass Thalassia hemprichii. Botanical Bulletin of Academia Sinica 46: 197–203.
Malone, T.C. 1977. Environmental regulation of phytoplankton productivity in the lower Hudson Estuary. Estuarine and Coastal Marine Science 5: 157–171.
Malta, E., and J.M. Verschuure. 1997. Effects of environmental variables on between-year variation of Ulva growth and biomass in a eutrophic brackish lake. Journal of Sea Research 38: 71–84.
McComb, A.J., and R. Humphries. 1992. Loss of nutrients from catchments and their ecological impacts in the Peel-Harvey estuarine system, Western Australia. Estuaries 15: 529–537.
McGlathery, K.J., K. Sundbäck, and I.C. Anderson. 2007. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Marine Ecology Progress Series 348: 1–18.
Middelburg, J.J., K. Soetaert, P.M.J. Herman, and H.T.S. Boschker. 2004. Burial of nutrient in coastal sediments: The role of primary producers. In Estuarine nutrient cycling: The influence of primary producers, ed. S.L. Nielsen, G.T. Banta, and M.F. Pedersen, 217–230. Dordrecht: Kluwer Academic.
Monbet, Y. 1992. Control of phytoplankton biomass in estuaries: A comparative analysis of microtidal and macrotidal estuaries. Estuaries 15: 563–571.
Mvungi, E.F., T.J. Lyimo, and M. Björk. 2012. When Zostera marina is intermixed with Ulva, its photosynthesis is reduced by increased pH and lower light, but not by changes in light quality. Aquatic Botany 102: 44–49.
Nicholls, R.J., and C. Small. 2002. Improved estimates of coastal population and exposure to hazards released. Eos 83: 301–305.
Nielsen, S.L., K. Sand-Jensen, J. Borum, and O. Geertz-Hansen. 2002. Depth colonization of eelgrass (Zostera marina) and macroalgae as determined by water transparency in Danish coastal waters. Estuaries 25: 1025–1032.
Nixon, S.W., B. Buckley, S. Granger, and J. Bintz. 2001. Responses of very shallow marine ecosystems to nutrient enrichment. Human Ecological Risk Assessment 7: 1457–1481.
Norkko, J., E. Bonsdorff, and A. Norkko. 2000. Drifting algal mats as an alternative habitat for benthic invertebrates: Species-specific responses to a transient resource. Journal of Experimental Marine Biology and Ecology 248: 79–104.
Oberg, J. 2005. Model simulations of conditions suitable for the establishment of Enteromorpha sp. (Chlorophyta) macroalgal mats. Marine Biological Research 1: 97–106.
Orduña-Rojas, J., D. Robledo, and C.J. Dawes. 2002. Studies on the tropical agarophyte Gracilaria cornea J. Agardh (Rhodophyta, Gracilariales) from Yucatan Mexico. I. Seasonal physiological and biochemical responses. Botanica Marina 45: 453–458.
Österling, M., and L. Pihl. 2001. Effects of filamentous green algal mats on benthic macrofaunal functional feeding groups. Journal of Experimental Marine Biology and Ecology 263: 159–183.
Paalme, T., J. Kotta, P. Kersen, G. Martin, H. Kukk, and K. Torn. 2011. Inter-annual variations in biomass of loose lying algae Furcellaria–Coccotylus community: The relative importance of local versus regional environmental factors in the West Estonian Archipelago. Aquatic Botany 95: 146–152.
Peckol, P., and J.S. Rivers. 1995. Physiological responses of the opportunistic macroalgae Cladophora vagabunda (L.) van den Hoek and Gracilaria tikvahiae (McLachlan) to environmental disturbances associated with eutrophication. Journal of Experimental Marine Biology and Ecology 190: 1–16.
Pedersen, M.F., and J. Borum. 1996. Phosphorus recycling in eelgrass (Zostera marina L.). In Biology and ecology of shallow coastal waters, ed. A. Eleftheriou, 45–50. Chicago: Olsen og Olsen.
Pedersen, M.F., and J. Borum. 1997. Nutrient control of estuarine macroalgae: Growth strategy and the balance between nitrogen requirements and uptake. Marine Ecology Progress Series 161: 155–163.
Pedersen, M.F., J. Borum, and F.L. Fotel. 2010. Phosphorus dynamics and limitation of fast- and slow-growing temperate seaweeds in Oslofjord, Norway. Marine Ecology Progress Series 399: 103–115.
Pierson, D.C., S. Kratzer, N. Strömbeck, and B. Håkansson. 2008. Relationship between the attenuation of downwelling irradiance at 490 nm with the attenuation of PAR (400 nm–700 nm) in the Baltic Sea. Remote Sensing of Environment 112: 668–680.
Piñon-Gimate, A., F. Paez-Osuna, E. Serviere-Zaragoza, and M. Casas-Valdez. 2012. Macroalgal blooms in coastal lagoons of the Gulf of California eco-region: A summary of current knowledge. Botanica Marina 55: 129–142.
Råberg, S., and L. Kautsky. 2007. A comparative biodiversity study of the associated fauna of perennial fucoids and filamentous algae. Estuarine, Coastal and Shelf Science 73: 249–258.
Rasmussen, J.R., B. Olesen, and D. Krause-Jensen. 2012. Effects of filamentous macroalgae mats on growth and survival of eelgrass, Zostera marina, seedlings. Aquatic Botany 99: 41–48.
Rasmussen, J.R., M.F. Pedersen, B. Olesen, S.L. Nielsen, and T.M. Pedersen. 2013. Temporal and spatial dynamics of ephemeral drift-algae in eelgrass, Zostera marina, beds. Estuarine, Coastal and Shelf Science 119: 167–175.
Rinne, H., S. Salovius-Lauren, and J. Mattila. 2011. The occurrence and depth penetration of macroalgae along environmental gradients in the northern Baltic Sea. Estuarine, Coastal and Shelf Science 94: 182–191.
Romero, J., F.X. Neill, A. Martinez-Arroyo, M. Perez, and J. Camp. 1996. The Spanish Mediterranean Coasts. In Marine benthic vegetation: Recent changes and the effects of eutrophication, ed. W. Schramm and P.H. Nienhuis, 295–304. Berlin: Springer.
Rosenberg, G., and J. Ramus. 1982. Ecological growth strategies in the seaweeds Gracilaria foliifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): Photosynthesis and antenna composition. Marine Ecology Progress Series 8: 233–241.
Salomonsen, J., M.R. Flindt, and O. Geertz-Hansen. 1997. Significance of advective transport of Ulva lactuca for a biomass budget on a shallow water location. Ecological Modeling 102: 129–132.
Sand-Jensen, K. 1988. Photosynthetic responses of Ulva lactuca at very low light. Marine Ecology Progress Series 50: 195–201.
Sand-Jensen, K., and J. Borum. 1991. Interactions among phytoplankton, periphyton, and macrophytes in temperate freshwaters and estuaries. Aquatic Botany 41: 137–175.
Sfriso, A., B. Pavoni, A. Marcomini, and A.A. Orio. 1992. Macroalgae, nutrient cycles and pollutants in the Lagoon of Venice. Estuaries 15: 517–528.
Sfriso, A., C. Facca, and P.F. Ghetti. 2003a. Temporal and spatial changes of macroalgae and phytoplankton in a Mediterranean coastal area: The Venice lagoon as a case study. Marine Environmental Research 56: 617–636.
Sfriso, A., C. Facca, S. Ceoldo, S. Silvestri, and P.F. Ghetti. 2003b. Role of macroalgal biomass and clam fishing on spatial and temporal changes in N and P sedimentary pools in the central part of the Venice lagoon. Oceanologica Acta 26: 3–13.
Shepherd, S.A., J.E. Watson, H.B.S. Womersley, and J.M. Carey. 2009. Long-term changes in macroalgal assemblages after increased sedimentation and turbidity in Western Port, Victoria, Australia. Botanica Marina 52: 195–206.
Smith, S.V. 1979. Responses of Kaneohe Bay, Hawaii, to relaxation of sewage stress. In Estuaries and nutrients, ed. B.J. Neilson and L.E. Cronin, 391–410. Clifton: Humana.
Strickland, J.D.H., and T.R. Parsons. 1972. A practical handbook of seawater analysis, 2nd edn. Bulletin of the Fisheries Research Board of Canada 167:1–310.
Tagliapietra, D., M. Pavan, and C. Wagner. 1998. Macrobenthic community changes related to eutrophication in Palude della Rosa (Venetian Lagoon, Italy). Estuarine, Coastal and Shelf Science 47: 217–226.
Taylor, D.I., S.W. Nixon, S. Granger, and B. Buckley. 1995a. Nutrient limitation and the eutrophication of coastal lagoons. Marine Ecology Progress Series 127: 235–244.
Taylor, D.I., S.W. Nixon, S.L. Granger, B.A. Buckley, J.P. McMahon, and H.J. Lin. 1995b. Responses of coastal lagoon plant communities to different forms of nutrient enrichment—a mesocosm experiment. Aquatic Botany 52: 19–34.
Taylor, D.I., S.W. Nixon, S.L. Granger, and B.A. Buckley. 1999. Responses of coastal lagoon plant communities to levels of nutrient enrichment: A mesocosm study. Estuaries 22: 1041–1056.
Teichberg, M., S.E. Fox, Y.S. Olsen, I. Valiela, P. Martinetto, O. Iribarne, E. Yuriko Muto, M.A.V. Petti, T.N. Corbisier, M. Soto-Jimenez, F. Paez-Osuna, P. Castro, H. Freitas, A. Zitelli, M. Cardinaletti, and D. Tagliapietra. 2010. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Global Change Biology 16: 2624–2637.
Tomasky, G., J. Barak, I. Valiela, P. Behr, L. Soucy, and K. Foreman. 1999. Nutrient limitation of phytoplankton growth in Waquoit Bay, Massachusetts, USA: A nutrient enrichment study. Aquatic Ecology 33: 147–155.
Trimmer, M., D.B. Nedwell, D.B. Sivyer, and S.J. Malcolm. 2000. Seasonal organic mineralization and denitrification in intertidal sediments and their relationship to the abundance of Enteromorpha sp. and Ulva sp. Marine Ecology Progress Series 203: 67–80.
Valiela, I. 2006. Global coastal change. Malden, MA, USA: Blackwell Publishing.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Valiela, I., G. Tomasky, J. Hauxwell, M.L. Cole, J. Cebrian, and K.D. Kroeger. 2000a. Operationalizing sustainability: Management and risk assessment of land-derived nitrogen loads to estuaries. Ecological Applications 10: 1006–1023.
Valiela, I., M. Geist, J. McClelland, and G. Tomasky. 2000b. Nitrogen loading from watersheds to estuaries: Verification of the Waquoit Bay nitrogen loading model. Biogeochemistry 49: 277–293.
Valiela, I., S. Mazzilli, J.L. Bowen, K.D. Kroeger, M.L. Cole, G. Tomasky, and T. Isaji. 2004. ELM, an estuarine nitrogen loading model: Formulation and verification of predicted concentrations of dissolved inorganic nitrogen. Water, Air, and Soil Pollution 157: 365–391.
Vergara, J.J., J.L. Perez-Llorens, G. Peralta, I. Hernandez, and F.X. Niell. 1997. Seasonal variation of photosynthetic performance and light attenuation in Ulva canopies from Palmones River Estuary. Journal of Phycology 33: 773–779.
Vergara, J.J., M. Sebastian, J. Lucas Perez-Llorens, and I. Hernandez. 1998. Photoacclimation of Ulva rigida and U. rotundata (Chlorophyta) arranged in canopies. Marine Ecology Progress Series 165: 283–292.
Wall, C.C., B.J. Peterson, and C.J. Gobler. 2011. The growth of estuarine resources (Zostera marina, Mercenaria mercenaria, Crassostrea virginica, Argopecten irradians, Cyprinodon variegatus) in response to nutrient loading and enhanced suspension feeding by adult shellfish. Estuaries and Coasts 34: 1262–1277.
Xu, J., K. Yin, J.H.W. Lee, H. Liu, A.Y.T. Ho, X. Yuan, and P.J. Harrison. 2010. Long-term and seasonal changes in nutrients, phytoplankton biomass, and dissolved oxygen in Deep Bay, Hong Kong. Estuaries and Coasts 33: 399–416.
Zar, J.H. 1984. Biostatistical analysis, 2nd ed. Englewood Cliffs: Prentice-Hall.
Acknowledgments
We thank Jennifer Bowen and Marci Cole for their help and insights with the work related to the Cape Cod estuaries. This paper has benefited from discussions and insights provided by several colleagues, in particular Ivan Valiela, Jennifer Hauxwell, Michael Kemp, and Andrea Anton. We also thank two reviewers for their useful comments. Josh Goff provided technical assistance with figures and tables. This publication was supported by NOAA (NA86RG0039 and NA16RG2258), MASGC (5-21838), and ACES (5-21871) grants to J. Cebrian.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Wayne S. Gardner
Rights and permissions
About this article
Cite this article
Cebrian, J., Corcoran, D. & Lartigue, J. Eutrophication-Driven Shifts in Primary Producers in Shallow Coastal Systems: Implications for System Functional Change. Estuaries and Coasts 37 (Suppl 1), 180–197 (2014). https://doi.org/10.1007/s12237-013-9689-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9689-x