Abstract
Sediment and porewater samples (1997–1999) were collected in the Northern Reach of the San Francisco Bay and Sacramento–San Joaquin Delta for determinations of sedimentary selenium and its chemical speciation. Total sedimentary selenium increased with depth, with approximately 50% of the sedimentary selenium as elemental selenium and 35% as organic selenide. Porewater total dissolved selenium increased with depth in the estuary and Delta, and fluxes out of the sediments were calculated at 0.01 and 0.06 nmol cm−2 year−1 for the estuary and Delta, respectively. Present-day sediment–water exchange of dissolved selenium and internal transformations cannot explain the observed increase in total sedimentary selenium with depth. However, mass balance calculations demonstrate that the increase in total selenium with depth may be linked to higher dissolved selenium concentrations in the water column in the 1980s, suggesting that the sediments could be used as historical recorders of selenium in the estuary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The biogeochemical cycle of selenium in aquatic systems is receiving considerable attention because it can either be essential or toxic to organisms depending on its concentration and chemical speciation (Wrench and Measures 1982; Cutter and Bruland 1984; Lemly 1996). In this respect, selenium can exist in four oxidation states (−II, selenide; O, elemental selenium; IV, selenite; and VI, selenate), in different chemical forms (i.e., organic and inorganic) within these oxidation states, and in different phases (i.e., particulate and dissolved). Agricultural (e.g., irrigation) and industrial (e.g., petroleum refining, power production) practices are increasing fluxes of selenium to aquatic systems (Elrashidi et al. 1987; Engber 1997; Huang et al. 2009), and causing elevated concentrations in waterfowl, fish, and bivalves of some estuaries (Ohlendorf et al. 1986; Skorupa 1998; Linville et al. 2002; Lee et al. 2006).
Due to the biological effects of dissolved selenium as it is transferred into the food web, extensive research examined dissolved selenium and its speciation in estuaries like the San Francisco Bay (Cutter 1989; Cutter and San Diego-McGlone 1990; Cutter and Cutter 2004), Kaoping estuary (Hung and Shy 1995), St. Lawrence estuary (Takayanagi and Cossa 1985), Zhujiang estuary (Yao et al. 2006), and Bohai Bay (Duan et al. 2010). Some research has focused on selenium in marine sediments (Johns et al. 1988; Shumilin et al. 2001; Miao et al. 2001), however, only a few researchers have examined the depth distributions of selenium in estuarine sediments (Takayanagi and Belzile 1988; Hu et al. 1996; Peters et al. 1999). Diagenetic processes can change the speciation or phase (dissolved/particulate) of a trace element like selenium, in estuarine sediments, and thus cause sediments to act as an important source or sink to an estuary. For example, dissimilatory reduction of selenite or selenate to elemental selenium may be a mechanism by which selenium is incorporated and retained in sediments (Elrashidi et al. 1987; Oremland et al. 1989). Other possible reactions include the release or oxidation of particulate organic selenide, the oxidation of elemental selenium to dissolved selenite, adsorption of dissolved selenite and selenate to iron or manganese oxides, and the formation of solid phase selenium minerals such as achavalite (FeSe) or ferroselite (FeSe2; Velinsky and Cutter 1991; Belzile et al. 2000). Bacterial methylation can form various volatile selenium species, including dimethyl selenide, dimethyl selenyl sulfide, and dimethyl diselenide (Amouroux and Donard 1997; Amouroux et al. 2000) and thus serve as a loss of selenium to estuaries and their sediments. Takayanagi and Belzile (1988) found that total selenium in sediments from the St. Lawrence estuary remained constant with depth, but there was an upward flux of porewater selenium (0.11 nmol cm−2 year−1) that was balanced by a loss of oxalic acid-leachable (i.e., associated with iron oxides) selenium in the sedimentary phase. In Jiulong Estuary, China, an upward flux of dissolved inorganic selenium was estimated at 0.20 nmol cm−2 year−1 (Hu et al. 1996). In freshwater sediments, Martin et al. (2011) found that elemental and organic selenium were sinks for dissolved selenium in a marsh. These findings in marine and freshwater sediments suggest that the flux across the sediment–water interface might be quantitatively important in the transfer of sedimentary selenium to the overlying water.
Concerns over inputs of selenium to the San Francisco Bay resulted in numerous studies to determine dissolved selenium in the San Francisco Bay estuary in the mid-1980s (Cutter 1989; Cutter and San Diego-McGlone 1990). The San Francisco Bay has a total surface area of 1,240 km2, with an average depth of 6.1 m (Conomos et al. 1985), and is divided into what is known as the “Northern Reach” and South Bay. Sampling in the mid-1980s found high concentrations of dissolved selenite from oil refineries in the Northern Reach, which includes Central, San Pablo and Suisun Bays (Fig. 1), which could account for 50–90% of the total inputs of selenium. Elevated concentrations of selenium in the clam Macoma balthica (e.g., Johns et al. 1988) and Potamocorbula amurensis (Linville et al. 2002; Lee et al. 2006) were also reported. These findings led to a subsequent study that examined estuarine selenium cycling from 1997 to 1999 as mandated reductions in selenium discharges from the refineries occurred. Cutter and Cutter (2004) found that there had been an 82% decrease in selenite and total dissolved selenium concentration from 1986 to 1999 which could be linked to the decrease in refinery fluxes (Cutter and Cutter 2004). Doblin et al. (2006) reported that changes in speciation and concentration of suspended particulate selenium in the water column over the same 1986–1999 period had been relatively small due to “buffering” by estuarine phytoplankton that accumulate dissolved selenium. To date, the concentrations and speciation of dissolved and particulate selenium in sediments, and sediment–water exchange, in San Francisco Bay are largely unknown. This paper describes and quantifies the cycling of sedimentary selenium in this estuary.
Methods
Sampling Methods
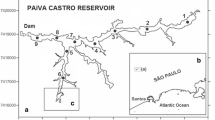
Sediment samples were taken between 1997 and 1999 in San Pablo Bay, Suisun Bay, the Sacramento–San Joaquin watershed area (also referred to as the “Delta”), and a mudflat and marsh located in Martinez (Fig. 1). A total of 24 sites were sampled with the criteria for sediment sampling including areas where high selenium concentrations were reported in clams (e.g., M. balthica and Corbicula sp.; Johns et al. 1988), areas of net fine-grained sediment accumulation, and sites close to refinery effluent discharges. Sediment and porewater samples were collected on 7 November 1997, 18 June 1998, 7–8 October 1998, and 4–5 November 1999 using a box corer aboard the R.V. David Johnson. From each box core, one subcore was taken for sediment sectioning and two subcores were taken to obtain porewater samples. Solid phase sediment subcores used an acrylic core tube (5.7 cm O.D., 30 cm length). These were immediately sectioned on board at 1–5 cm intervals, and at 2 cm intervals thereafter. A portion of this material was used to determine sediment porosity in order to convert the volume of porewater collected to depth intervals (Bender et al. 1987). Sediment samples were placed in polyethylene bags and immediately frozen until processing in the laboratory.
Whole-core squeezing (Bender et al. 1987) was used to obtain the maximum amount of porewater at millimeter-scale resolution and was easily performed in the field. Moreover, the extracted porewater did not come in contact with the atmosphere (kept anoxic). Schults et al. (1992) found that different methods of interstitial water collection (including centrifugation and porewater squeezing) resulted in similar recoveries for trace metals. The acrylic subcore tube (7.7 cm O.D., 20 cm length) was inserted into the box core sediments, and the top piston, fitted with a 3.5 cm porous polyethylene disk on the bottom and three-way valve to remove air bubbles during porewater extraction, was placed in the top of the subcore tube. Upon removal of the capped subcore from the box corer, a movable bottom piston was inserted and the entire assembly placed in an aluminum rack with hydraulic jack pushing the bottom piston upwards. When the top of the sediment reached the top piston/porous disk, a gas tight syringe with Teflon fittings was attached onto a three-way valve. After 10 mL of porewater was pushed into the syringe, the three-way valve was closed, another syringe attached, and squeezing continued at 10 mL intervals until no more porewater could be extracted. To minimize reactions of dissolved selenium species with the solid phase, we performed the entire porewater extraction in less than 15 min. Each sample in a syringe was directly filtered through a 0.4 μm membrane filter into precleaned borosilicate glass vials. Due to low concentrations of dissolved selenium, porewaters from two subcores were combined in the vials. Porewater samples were immediately acidified to pH 1.5 with hydrochloric acid and refrigerated until analyses.
Analytical Methods
A portion of the sediment samples was dried at 50°C, ground with an agate mortar and pestle, and sieved through a 150 μm nylon mesh screen; these sediments were stored in polyethylene bottles. These processed samples were used to determine total sedimentary selenium (as below), and organic carbon, organic nitrogen, and sulfur (CNS) using a Carlo Erba 1500 Elemental Analyzer (Cutter and Radford-Knoery 1991).
Total sedimentary selenium was determined using a three-step nitric–perchloric acid digestion on dried sediment as described by Cutter (1985). The method was modified so that after the final digestion and addition of hydrochloric acid, the sample solutions were passed through Bio-Rad AG 1 × 8 anion exchange resin (chloride form, 100–200 mesh) to remove any iron that might interfere in the subsequent determinations, and the eluent was collected. The resin was rinsed with 10 mL of 4 mol L−1 hydrochloric acid (HCl) and this eluent was combined with the previous one in 30 mL polyethylene bottles. To measure digestion accuracy, the National Institute of Standards and Technology (NIST) standard (SRM 2704, Buffalo River Sediment) was digested with the samples. We obtained a concentration of 1.09 ± 0.06 μg g−1 total selenium (n = 13) for the NIST standard, in agreement with the reported value of 1.1 μg g−1.
Elemental selenium was determined on immediately thawed (i.e., not processed to minimize speciation changes) sediments using a sodium sulfite extraction and oxidation of the sulfite solution with nitric acid as described by Velinsky and Cutter (1990). Sedimentary selenite + selenate was determined by a sodium hydroxide leaching technique on freshly thawed sediments (Cutter 1985). The eluant for each was stored in 30 mL polyethylene bottles until analysis. For sediment samples, particulate organic selenium was calculated as the difference between total sedimentary selenium and the sum of elemental selenium and particulate selenite + selenate.
Both the digested sediment solutions and porewater samples were analyzed for selenium as described by Cutter (1978, 1982, 1983) and Velinsky and Cutter (1990) using selective hydride generation. Briefly, this involves the generation of hydrogen selenide from dissolved selenite via acidification and sodium borohydride addition, liquid nitrogen-cooled trapping, and atomic absorption detection using a quartz tube burner with an air hydrogen flame. Total dissolved selenium in porewater samples was determined by boiling a sample adjusted to 4 mol L−1 HCl with potassium persulfate for 30 min, and then the method for generating hydrogen selenide was used. For dissolved selenite + selenate determination, a 4 mol L−1 HCl-acidified sample was boiled for 15 min, then subjected to the method of generating hydrogen selenide briefly described above. Total dissolved selenium was determined in all porewater samples, and selenite + selenate when adequate volumes were available. Dissolved organic selenide + elemental selenium (the colloidal fraction that may pass through a 0.4 μm filter) was calculated as the difference between total dissolved selenium and selenite + selenate. However, many studies (e.g., Cutter 1982; Cutter and Bruland 1984; Cutter and Cutter 1995) found that colloidal elemental Se is undetectable, and therefore this difference value will be referred to as “dissolved organic selenide.” This fraction does not include any volatile dimethyl selenides as the samples were not analyzed immediately or stored in a fashion to preserve these unstable forms.
The standard additions method of calibration was used to ensure accuracy (in addition to the analyses of SRMs) and all digests were done in duplicate, with determinations made in triplicate. The detection limit for porewater dissolved selenium forms was 0.06 nmol L−1 and the precision was generally better than 15% (relative standard deviation; RSD). Total sedimentary selenium and its speciation had a detection limit of 0.01 nmol g−1 when 0.3 g was processed, with the precision better than 10% (RSD).
Sediment Dating
The activity of 210Pb was determined by total sediment digestion, ion exchange and subsequent precipitation, and anticoincidence beta counting (Alexander et al. 1993).
Since there were no grain size variations in the sediments examined, 210Pb depth profiles were fitted to the simple exponential decay equation: \( {A_z} = {A_0}{e^{{ - \left( {0.0{311}/w} \right)\, \times \,z}}} \), where A z is the activity at any depth z (cm), A 0 is the activity just below the bioturbated, mixed layer (typically 2 cm as noted below), 0.0311 is the decay constant for 210Pb, and w is the sedimentation rate (cm/year). For the seven sites dated, r 2 values for the fits ranged from a low of 0.3 to a high of 0.8.
Results
General Sediment Characteristics
Estuarine sites (stations 1–7, 18–20, and 23; Fig. 1) were predominately fine grain silt and clay, similar to Conomos and Peterson’s (1977) report that the channels of the estuary were composed of poorly sorted silty clay, clayey silt, and sand–silt–clay. Surface sediment, where bioturbation was observed (i.e., the active layer with clams), was defined as the depth from 0 to 2 cm for all sites The porosity and dry sediment density for surface sediments between estuarine sites were similar and averaged 0.66 ± 0.06 (n = 88) and 2.15 ± 0.10 g cm−3 (n = 88), respectively, which agrees with values reported by van Geen and Luoma (1999). The sedimentation rate using 210Pb profiles at station 19 in Suisun Bay was 0.01 cm year−1, consistent with reports that this embayment is receiving reduced sediment inputs due to upstream impoundments (McKee et al. 2006). Organic carbon, nitrogen, and sulfur in the surface estuarine sediments were 1.35 ± 0.50%, 0.11 ± 0.03%, and 0.11 ± 0.01% (n = 88), respectively.
The mudflat and salt marsh sites were located in the Martinez salt marsh (stations 24 and 25; Fig. 1). Approximately 200 m of the mudflat site is exposed during low tide. The salt marsh flora was predominantly Spartina alterniflora. Both the mud flat and the salt marsh stations had fine grain, silty clay compositions. The average surface sediment porosity at the mud flat site was 0.68 ± 0.04, with a dry density of 2.21 ± 0.08 g cm−3 (n = 4). The sedimentation rates were not measured at these sites. The composition of these sediments averaged 1.25 ± 0.02% organic carbon, 0.10 ± 0.02% organic nitrogen, and 0.14 ± 0.04% sulfur.
The Delta stations (stations 8 and 10–17; Fig. 1) had a clay and silt composition, with a 0.5 cm flocculent layer on top. As with the estuarine sites, there were clams in the surface 0–2 cm sediments. The average surface sediment porosity was 0.74 ± 0.09, with a dry density of 2.21 ± 0.08 g cm−3 (n = 99). The 210 Pb sedimentation rates in the Delta were measured at stations 12, 13, 15, 16, and 22. Sedimentation rates were lower near where the Delta flows into the Northern Reach of the Bay and varied from 0.14 to 0.56 cm year−1. Stations 12 and 22 had a sedimentation rate of 0.14 cm year−1; station 13, 0.56 cm year−1; station 16, 0.27 cm year−1; and station 15, 0.25 cm year−1. Organic carbon, nitrogen, and sulfur concentrations for the surface sediments averaged 6.09 ± 0.11% (n = 99), 0.43 ± 0.01% (n = 99), and 0.12 ± 0.02% (n = 99), respectively.
Overall Sedimentary Selenium Characteristics
Total sedimentary selenium for surface sediments at all the stations ranged between 2.00 and 12.40 nmol g−1, with concentrations in the Delta higher than those in the Bay and the salt marsh (Fig. 1). Johns et al. (1988) reported total sedimentary selenium concentrations of 1–6 nmol g−1 from six stations in Suisun Bay, which are consistent with our results. In comparison to other locations, total selenium concentrations in the Northern Reach were half those reported by Takayanagi and Belzile (1988) for the St. Lawrence estuarine sediments, and Velinsky and Cutter (1991) for salt marsh sediments. An analysis of variance of the surface concentrations of total sedimentary selenium shows that there were significant differences (p < 0.01) between concentrations in the Delta (6.51 ± 1.00 nmol g−1) and the estuary (3.37 ± 0.29 nmol g−1), and between the Delta and salt marsh (2.28 ± 0.24 nmol g−1, p = 0.02). There was little statistical difference in surface sedimentary selenium concentrations between the estuarine and salt marsh sites (p = 0.16).
The concentration of elemental selenium at all sites ranged from 0.29 to 9.56 nmol g−1. The estuarine stations had an average elemental concentration in the surface sediments of 1.65 ± 0.45 nmol g−1 (n = 34), or 52% of the total. The surface concentrations of elemental selenium in the Delta were greater than in the estuary (3.19 ± 0.53 nmol g−1, n = 24), but the percentage of elemental selenium relative to the total was similar to the estuarine sites (52% of the total). The surface elemental selenium concentration in the salt marsh was 0.92 ± 0.02 nmol g−1 (n = 4), or 47% of the total. There was no statistical difference in the percentage of elemental selenium between the Delta, the estuarine, or salt marsh sites (p = 0.317). For all sediment sites, the percentage of elemental selenium was consistent with previous measurements of salt marsh (49–68%; Velinsky and Cutter 1991) and freshwater sediments (40–95%; Belzile et al. 2000).
Sedimentary selenite + selenate, which would be adsorbed to sediment particles, ranged from 0.03 to 1.99 nmol g−1 for all the sediment sites. The Delta sediments had higher surface concentrations of selenite + selenate (0.98 ± 0.37 nmol g−1) than the estuarine (0.41 ± 0.09 nmol g−1) and salt marsh sites (0.16 ± 0.04 nmol g−1) in the upper 2 cm, but once normalized to total selenium, the percentages of selenite + selenate in the Delta were similar to those in the estuary (16 ± 6%, n = 22, for the Delta and 13 ± 3%, n = 9, for the estuary). The salt marsh had the lowest percentage of selenite + selenate for surface sediments (8 ± 2% of the total, n = 8). As with elemental selenium, there was little statistical difference in the percentage of selenite + selenate between the estuarine, salt marsh, and Delta sites (p = 0.12).
For the entire data set, surface organic selenide varied from 0.07 to 8.07 nmol g−1. In the Delta, the average surface organic selenide concentration of 1.96 ± 0.31 nmol g−1 (n = 9), 1.08 ± 0.35 nmol g−1 (n = 22) for the estuarine sites, and 0.91 ± 0.14 nmol g−1 (n = 4) for the salt marsh. When normalized to total selenium, organic selenide for surface sediments was 34 ± 11% of the total selenium for the estuarine sites and 32 ± 5% in the Delta, but there was little statistical difference (p = 0.69) between the estuarine and Delta sites. The salt marsh had the highest percentage of organic selenide compared to the other stations (46 ± 7%), but it was not statistically significant compared to the other sites (p = 0.11).
Depth Profiles of Sedimentary Selenium
Due to the number of stations, it is unfeasible to show the selenium depth distributions for each station, and thus representative depth profiles from a station in San Pablo Bay (station 1), Suisun Bay (station 19), and the Delta (station 12) are presented in Fig. 2. For estuarine and Delta sites, there was a slight increase in total sedimentary selenium with depth (Fig. 2). These observed depth distributions are contrary to what has been observed in most coastal marine sediments, where total sedimentary selenium is either constant or decreases with depth (Takayanagi and Belzile 1988; Velinsky and Cutter 1991). However, increases of total selenium with depth have been observed consistently in freshwater sediments (Belzile et al. 2000; Martin et al. 2011).
Depth distributions of total sedimentary selenium for stations 1 (San Pablo Bay), 19 (Suisun Bay), and 12 (Delta). Sediments were sectioned in 1 or 2 cm intervals and data are plotted versus the mean depth of each section. Error bars represent the total error of duplicate sediment digest and triplicate sample analyses
The vertical distributions of elemental selenium as a percentage of total sedimentary selenium for Suisun Bay and the Delta show little statistically significant variations with depth, while that in San Pablo Bay increases with depth (Fig. 3). A decrease with depth has been observed in salt marsh sediments from Delaware (Velinsky and Cutter 1991), although an increase with depth was reported in freshwater sediments (Belzile et al. 2000; Martin et al. 2011).
Depth distributions of elemental Se (filled squares), Se IV + VI (filled circles), and organic Se-II (open triangles) normalized to total sedimentary selenium. Sediments were sectioned in 1 or 2 cm intervals and data are plotted versus the mean depth of each section. Error bars represent the total error of duplicate sediment digest and triplicate sample analyses
Selenite + selenate vertical distributions as a percentage of total sedimentary selenium were relatively constant for stations 1, 19, and 12 for the upper 5 cm. There was a slight increase observed in sedimentary selenite + selenate at 4.5–6.5 cm, followed by a decrease. This type of profile was consistent with sediments from a salt marsh in Delaware (Velinsky and Cutter 1991).
The depth distributions of organic selenide expressed as a percentage of total sedimentary selenium varied from 20% to 40% with depth (Fig. 3). A minimum percentage of sedimentary organic selenide was found at 2.5 cm for station 19, while at the same depth for stations 1 and 12 maxima were observed (Fig. 3). A slight increase with depth for stations 19 and 12 was detected. In comparison, Martin et al. (2011) found 30–35% of sedimentary selenium in freshwater sediments from western Canada was in the organic form, while Velinsky and Cutter (1991) reported sedimentary organic selenide concentrations in a Delaware salt marsh that were 47 ± 11% of the total sedimentary selenium and had no consistent trend with depth.
Dissolved Selenium in Porewaters
Total dissolved selenium in porewaters ranged from 1 to 6 nmol L−1 in the upper 2 cm of San Francisco Bay sediments. The average porewater concentration for the Delta was 2.3 ± 0.2 nmol L−1 (n = 24), with the estuarine sites having similar concentrations (2.7 ± 0.3 nmol L−1, n = 56). The average concentration for the salt marsh was slightly higher at 3.4 ± 0.2 nmol L−1 (n = 7), but not statistically different from the other sites (p = 0.09). Concentrations of total dissolved selenium in porewaters increased with depth for San Pablo Bay and the Delta, and remained constant in Suisun Bay (Fig. 4). In general, total dissolved selenium concentrations in porewater were similar to those reported by Takayanagi and Belzile (1988) in the St. Lawrence Estuary (approximately 2.1 nmol L−1). Zawislanski and McGrath (1998) reported a total dissolved selenium porewater concentration of approximately 12 nmol L−1 for the Martinez mudflat in the San Francisco Bay in 1995, which is four times higher found in this study. The difference between their porewater concentrations and those reported here could be due to different extraction techniques (they centrifuged), sampling sites, and sampling period (1995) when there were higher total dissolved selenium concentrations in the estuary (Cutter and Cutter 2004).
Total dissolved selenium in porewater samples from three stations in the San Francisco Bay estuary. Depth intervals were determined using porosity data and the volume sampled (Bender et al. 1987). Error bars represent the total error of duplicate sediment digest and triplicate sample analyses
For all stations, interstitial dissolved selenite + selenate was 60–80% of the total dissolved selenium in the top 1 cm, but decreased with depth (Fig. 5). Organic selenide was approximately 20–40% of the total dissolved selenium in the top 1 cm and increased with depth (Fig. 5). Velinsky and Cutter (1991) found similar results for porewater selenite + selenate and organic selenide. Belzile et al. (2000) and Martin et al. (2011) also reported net increases of dissolved organic selenide with depth in porewaters from freshwater sediments. Additionally, Belzile et al. (2000) found that selenite was the major fraction of dissolved selenium in porewaters, but there was insufficient volume of porewater in our study to allow separate determinations of selenite and selenate.
Discussion
Solid Phase Selenium and Carbon Relationship
Even though the concentrations of solid phase total selenium were different between the estuarine, salt marsh, and the Delta sites of SF Bay, the total selenium to organic carbon atomic ratios at the surface of the sediments were not significantly different (p = 0.07, n = 59). The linear regression using all stations resulted in a strong positive correlation between total particulate selenium and organic carbon (Fig. 6, PSe = 1.5 ± 0.1 × 10−6 × C + 1.9 × 10−9, r = 0.91). In comparison, estuarine sediments from the mid-Chesapeake Bay (Cutter, unpublished data) have an average total selenium concentration of 17.4 ± 2.0 nmol g−1, which is five times greater than those in San Francisco Bay. However, the total selenium to carbon atomic ratio of 5.0 ± 0.5 × 10−6 in the Chesapeake Bay (Cutter, unpublished data) is similar to those in the San Francisco Bay sediments. Thus, organic carbon appears to be an important carrier phase for particulate selenium in aquatic environments.
Positive correlations between solid phase total selenium and organic carbon are normally found in marine sediments (Sokolova and Pilipchuk 1973; Belzile and Lebel 1988; Wen and Carignan 2011). These positive correlations can be explained by the actual incorporation of selenium into organic matter (e.g., seleno amino acids in proteins of phytoplankton; Wrench 1978; Fan et al. 2002) and subsequent flux to underlying sediments. Indeed, the sediment ΣSe/C ratios in SF Bay (1.5 ± 0.2 × 10−6) are within the range of those found in estuarine phytoplankton cultures (3.2 ± 6.2 × 10−6; Baines et al. 2001; Doblin et al. 2006). Thus, organic selenide can be delivered to sediments by phytoplankton detritus much like particle fluxes to the deep ocean (e.g., Cutter and Bruland 1984). An additional source of selenium to San Francisco Bay sediments, downward diffusion of water column dissolved selenium and subsequent conversion to the particulate phase (e.g., in situ reduction of selenite + selenate to elemental selenium), is discussed below.
Porewater Selenium Diagensis
The internal cycle of sedimentary selenium in the San Francisco Bay includes: the oxidation of elemental (Geering et al. 1968; Oremland et al. 1989; Matamoros-Veloza et al. 2011) and organic selenide (Velinsky and Cutter 1991); the microbial dissimilatory reduction of dissolved selenite and selenate to elemental selenium (Oremland et al. 1989; Dowdle and Oremland 1998; Stolz et al. 2002); and biotic conversion to volatile selenium (Reamer and Zoller 1980; Velinsky and Cutter 1991; Amouroux and Donard 1997). This cycle also includes changes between the solid and dissolved phases (i.e., porewater intermediates), and is therefore intimately linked with the flux of porewater selenium.
Porewater dissolved selenium exchange with the overlying water can be quantified using a modified form of Fick’s first law:
where, J is the flux, ϕ is the porosity, m has a value of 3 for surface sediments (Ullman and Aller 1982), D o is the effective diffusion coefficient of SeO 24 −(4.87 × 10−6 cm2 s−1 at 4.5°C , Li and Gregory 1974), and ∂Se/∂z is the observed concentration gradient of porewater selenium. The selenium concentration at z = 0 cm, Seo, in water overlying the core was used as the first point in concentration gradient. A negative J indicates that the dissolved selenium is fluxing out of the sediments, while a positive J results from dissolved selenium fluxing into the sediments.
Using Eq. 1, the fluxes of dissolved selenite + selenate were calculated for those stations where there was enough sample to do speciation analyses (n = 4 sites in the estuary, n = 4 sites in the Delta). For both the estuary and Delta, dissolved selenite + selenate were fluxing into the sediments (Fig. 5). The average porewater flux of selenite + selenate was +0.02 ± 0.01 and +0.06 ± 0.02 nmol cm−2 year−1 in the estuary and Delta, respectively. These fluxes are being driven by the microbial reduction of dissolved selenate (and selenite) to particulate elemental selenium (Oremland et al. 1989), as shown by Velinsky and Cutter (1991) for salt marshes, and Belzile et al. (2000) and Martin et al. (2011) for freshwater sediments. In contrast to Se IV + VI, the total dissolved selenium and organic selenide porewater profiles show that these forms were fluxing out of the sediments (Figs. 4 and 5). Because there is no diffusion coefficient for organic selenide, this flux can only be estimated by using the diffusion coefficient of SeO 2−4 (Li and Gregory 1974). Calculations demonstrate that there can be a −0.03 ± 0.01 and −0.12 ± 0.02 nmol cm−2 year−1 of organic selenide fluxing out of the estuarine and Delta sediments, respectively. This means for the estuarine sites the total dissolved selenium flux was −0.01 ± 0.02 nmol cm−2 year−1, while for the Delta it was −0.06 ± 0.02 nmol cm−2 year−1. These total dissolved selenium fluxes out of San Francisco Bay sediments to the overlying water column are comparable to fluxes from other marine and freshwater sediments (−0.01 to −0.11 nmol cm−2 year−1; Takayanagi and Belzile 1988; Velinsky and Cutter 1991; Belzile et al. 2000). Porewater speciation data for other estuaries are unavailable and thus only freshwater results from Belzile et al. (2000) and Martin et al. (2011) can be used for comparison. Based on porewater profiles at Clearwater Lake, Canada (Belzile et al. 2000), selenite + selenate were fluxing into the sediments, while organic selenide was fluxing out, in agreement with the San Francisco Bay results. At two western Canadian freshwater sites (Martin et al. 2011), dissolved organic selenide was also fluxing out of sediments and selenate fluxing in, but selenite was found to be fluxing out of sediments as well. In should be noted that these SF Bay flux calculations do not consider advection by bioturbation, which could be significant in the Bay due to the large number of bivalves found in the sediments (e.g., P. amurensis; Thompson 2000). Moreover, fluxes of volatile methyl selenides from these sediments are not included and thus sediment water fluxes given here are conservative underestimates.
By knowing the area of Suisun Bay and San Pablo Bay (443 km2), an estimate of total dissolved organic selenide flux from the sediments to the estuarine water column was calculated. The dissolved organic selenide flux from the sediments to the overlying water is 36.4 mol day−1, which is three times greater than the reported organic selenide flux from the refineries (11.8 mol day−1, Cutter and Cutter 2004). Depending on riverine flow, the sediments could have a greater flux of dissolved organic selenide to the estuary than the Sacramento–San Joaquin River (inputs varied from 1.2 to 82.2 mol day−1, Cutter and Cutter 2004). Therefore, estuarine sediments are a significant source of dissolved organic selenium to the Northern Reach of San Francisco Bay and can affect selenium speciation in the estuary.
Solid-Phase Selenium Diagensis
In most marine studies to date (e.g., Takayanagi and Belzile 1988; Velinsky and Cutter 1991), solid phase selenium is found to decrease with depth in sediments, indicating net remobilization. In order for selenium to accumulate in San Francisco Bay sediments as observed, its inputs via sedimentation or porewater influx must be greater than its loss via diagenetic remobilization and porewater efflux. For example, in several lake sediments, Belzile et al. (2000) found an increase in total selenium with depth and concluded this was due to in situ fixation of dissolved selenate to solid phase elemental selenium. For most of the stations sampled in the San Francisco Bay system (e.g., stations 1, 12, and 19; Fig. 2), selenium increased with sediment depth, consistent with net input or historical changes in the fluxes. To calculate the depth-integrated gain or loss of total sedimentary selenium, the equation from Berner (1980) was used:
where, R is the flux, ω is the sedimentation rate, ρ is the dry sediment density, ϕ is the porosity, and ΔSe is the change in selenium. The speciation data (see above) indicate that elemental selenium was increasing in the sediments. Using the data reported here, the average accumulation of elemental selenium in the sediments was 0.05 ± 0.01 in the estuary and 0.07 ± 0.02 nmol cm−2 year−1 in the Delta.
Currently, dissolved selenite + selenate are fluxing into the sediments at a rate of +0.02 ± 0.01 nmol cm−2 year−1, therefore in situ fixation via reduction to elemental selenium can only account for 40% of the accumulation of sedimentary selenium in the estuary. The only other possible explanation for increasing sedimentary selenium with depth is a historical change in the inputs. These changes could be from a greater flux of particulate selenium or inputs of dissolved selenium from the overlying water via porewater exchange. Doblin et al. (2006) showed that from 1986 to 1999, the concentration of suspended particulate selenium has remained relatively constant; changes in particulate selenium inputs thus seem unlikely. However, Cutter and Cutter (2004) have shown a substantial decrease in estuarine dissolved selenium of 2 nmol L−1 from 1986 to 1999, suggesting that porewater fluxes in the past may have been greater. The change in dissolved selenium in the estuary over this period was due to decreased inputs of dissolved selenite + selenate from the refineries that are located in Suisun Bay and Carquinez Strait (Cutter and Cutter 2004). Dissolved selenite + selenate is currently fluxing into the sediments (see above), and this flux would have been greater in the past when the overlying water concentration of selenite + selenate were higher. Dissolved selenite + selenate concentrations were as high as 2.8 nmol L−1 in 1986 (Cutter 1989; Cutter and San Diego-McGlone 1990). Assuming that only the overlying water concentration of selenium changed from 1986 to 1999 (porewater concentrations remained the same), the estimated flux into the sediments could have been as high as 0.12 nmol cm−2 year−1 for selenite + selenate and 0.03 nmol cm−2 year−1 of total dissolved selenium for the estuary; only 0.05 nmol cm−2 year−1 is required to explain the increase of total sedimentary selenium with depth. This calculation suggests that in the 1980s, the sediments would have been a sink instead of a source of selenium (as it was in this study) to the estuary. As an example, for San Pablo Bay, the increase in selenium is first observed at 4 cm depth (a 0.1 nmol g−1), which corresponds to approximately 13 years based on the 0.3 cm year−1 sedimentation rate reported by van Geen and Luoma (1999). This sedimentary selenium increase then corresponds to the mid-1980s (sampling in 1997 minus 13 years), when higher concentrations of dissolved selenium were recorded in the water column (Cutter 1989; Cutter and San Diego-McGlone 1990). Based on these simple calculations, it is possible that due to the higher total dissolved selenium concentrations in waters overlying these sediments in the 1980s, sediments have recorded historical changes in the inputs of selenium to the estuary. Martin et al. (2011) made the same conclusion about sediments recording historical porewater fluxes for the freshwater sites they examined in western Canada. In contrast to the estuarine sediments, the increase in elemental selenium observed in the Delta sediments (+0.07 ± 0.02 nmol cm−2 year−1) can be fully explained by in situ fixation of dissolved selenite + selenate (flux of +0.06 ± 0.02 nmol cm−2 year−1) without any historical changes.
Summary
The primary sources of selenium to SF Bay sediments are particulate selenium from the rivers (biogenic and mineral detritus), biogenic particles produced in the water column (organic phytoplankton detritus), and diffusion of dissolved selenite + selenate from the water column followed by in situ reduction to insoluble elemental selenium. The Se/organic C ratios in these sediments suggest that phytoplankton detritus is a primary source. Unlike other marine sediments, the San Francisco Bay sedimentary selenium increased with depth. Simple porewater flux calculations indicate that this increase may be due to historical changes in the overlying water concentrations of selenite + selenate (i.e., a change in the porewater flux and in situ fixation). Thus, in situ fixation may supplement inputs from biogenic detritus. However, deeper sediment cores (i.e., >50 cm) would provide confirmation of this hypothesis by sampling sediments that predate oil refineries in the Northern Reach that affected the abundance and speciation of water column dissolved selenium.
References
Alexander, Clark R., Ralph G. Smith, Steven Schropp, Fred D. Calder, and Herbert L. Windom. 1993. The historical record of metal enrichment in two Florida estuaries. Estuaries 16: 627–637.
Amouroux, David and Olivier F.X. Donard. 1997. Evasion of selenium to the atmosphere via biomethylation processes in the Gironde estuary, France. Marine Chemistry 58: 173–188.
Amouroux, David, Christophe Pécheyran, and Olivier F.X. Donard. 2000. Formation of volatile selenium species in synthetic seawater under light and dark experimental conditions. Applied Organometallic Chemistry 14: 236–244.
Baines, Stephen B., Nicholas S. Fisher, Martina A. Doblin, and Gregory A. Cutter. 2001. Uptake of dissolved organic selenides by marine phytoplankton. Limnology and Oceanography 46: 1936–1944.
Belzile, Nelson, and Jean Lebel. 1988. Selenium profiles in the sediments of the Laurentian trough (Northwest North Atlantic). Chemical Geology 68: 99–103.
Belzile, Nelson, Yu-Wei Chen, and Rongrong Xu. 2000. Early diagenetic behaviour of selenium in freshwater sediments. Applied Geochemistry 15: 1439–1454.
Bender, Michael, William Martin, Jennifer Hess, Fred Sayles, Larry Ball, and Claude Lambert. 1987. A whole core squeezer for interfacial pore-water sampling. Limnology and Oceanography 32: 1214–1225.
Berner, Robert A. 1980. Early diagenesis: A theoretical approach. Princeton: Princeton University Press.
Conomos, T. John, and David H. Peterson. 1977. Suspended-particle transport and circulation in San Francisco Bay: an overview. Estuarine Processes Vol. II; Circulation, Sediments, and Transfer of Material in the Estuary. New York: Academic Press, Inc.
Conomos, T. John, Richard E. Smith, and Jeffrey W. Gartner. 1985. Environmental setting of San Francisco Bay. Hydrobiologia 129: 1–12.
Cutter, Gregory A. 1978. Species determination of selenium in natural waters. Analytica Chimica Acta 98: 59–66.
Cutter, Gregory A. 1982. Selenium in reducing waters. Science 217: 829–831.
Cutter, Gregory A. 1983. Elimination of nitrite interferences in the determination of selenium by hydride generation. Analytica Chimica Acta 149: 391–394.
Cutter, Gregory A. 1985. Determination of selenium speciation in biogenic particulate material and sediments. Analytical Chemistry 57: 2951–2955.
Cutter, Gregory A. 1989. The estuarine behaviour of selenium in San Francisco Bay. Estuarine, Coastal and Shelf Science 28: 13–34.
Cutter, Gregory A., and Kenneth W. Bruland. 1984. The marine biogeochemistry of selenium in a simple system. Geochimica et Cosmochimica Acta 48: 1417–1433.
Cutter, Gregory A., and Lynda Cutter. 1995. Behavior of dissolved antimony, arsenic, and selenium in the Atlantic Ocean. Marine Chemistry 49: 295–306.
Cutter, Gregory A., and Lynda Cutter. 2004. The biogeochemistry of selenium in the San Francisco Bay estuary: changes in water column behavior. Estuarine, Coastal and Shelf Science 61: 463–476.
Cutter, Gregory A., and Joël Radford-Knoery. 1991. Determination of carbon, nitrogen, sulfur, and inorganic sulfur species in marine particles. In Marine particles: analysis and characterization, eds. Derek W. Spencer and David C. Hurd, 57–63. Washington, DC: American Geophysical Union.
Cutter, Gregory A., and Maria L.C. San Diego-McGlone. 1990. Temporal variability of selenium fluxes in the San Francisco Bay. Science of the Total Environment 97: 235–250.
Doblin, Martina A., Stephen B. Baines, Lynda S. Cutter, and Gregory A. Cutter. 2006. Sources and biochemical cycling of particulate selenium in the San Francisco Bay estuary. Estuarine and Coastal Shelf Science 67: 681–694.
Dowdle, Philip R., and Ronald S. Oremland. 1998. Microbial oxidation of elemental selenium in soil slurries and bacterial cultures. Environmental Science and Technology 32: 3749–3755.
Duan, Liqin, Jinming Song, Xuegang Li, Huamao Yuan, and Sisi Xu. 2010. Distribution of selenium and its relationship to the eco-environment in Bohai Bay seawater. Marine Chemistry 121: 87–99.
Elrashidi, M.A., D.C. Adriano, S.M. Workman, and W.L. Lindsay. 1987. Chemical equilibrium of selenium in soils: a theoretical development. Soil Science 144: 141–152.
Engber, R.A. 1997. Remediation of irrigation-related contamination at department of the interior project area in the western United States. In Agroecosystems and the environment: sources, control and remediation of potentially toxic, trace element oxyanions, ed. L.M. Dudley and J.C. Guitjens, 57–76. San Francisco: American Association Advancement Science-Pacific Division.
Fan, Teresa W.M., Swee J. Teh, David E. Hinton, and Richard M. Higashi. 2002. Selenium biotransformations in to proteinaceous forms by foodweb organisms of selenium laden drainage waters in California. Aquatic Toxicology 57: 65–84.
Geering, Harold R., Earle E. Cary, L.H.P. Jones, and W.H. Allaway. 1968. Solubility and redox criteria for the possible forms of selenium in soils. Soil Science of America Journal 32: 35–40.
Hu, Minghui, Yiping Yang, Genfang Wang, and J.M. Martin. 1996. Chemical behaviour of selenium in Jiulong Estuary. Journal of Oceanography in Taiwan Strait/Taiwan Haixia 15: 41–47.
Huang, Shunsheng, Ming Hua, Jinshun Feng, Xinyong Zhong, Yang Jin, Baiwan Zhu, and Hua Lu. 2009. Assessment of selenium pollution in agricultural soils in the Xuzhou District, Northwest Jiangsu, China. Journal of Environmental Sciences 21: 481–487.
Hung, J.J. and C.P. Shy. 1995. Speciation of dissolved selenium in the Kaoping and Erhjen Rivers and Estuaries, Southwestern Taiwan. Estuaries 18: 234–240.
Johns, Carolyn, Samuel N. Luoma, and Virginia Elrod. 1988. Selenium accumulation in benthic bivalves and fine sediments of San Francisco Bay, the Sacramento-San Joaquin Delta, and selected tributaries. Estuarine, Coastal and Shelf Science 27: 381–396.
Lee, Byeong-Gweon, Jung-Suk Lee, and Samuel N. Luoma. 2006. Comparison of selenium bioaccumulation in the clams Corbicula fluminea and Potamocorbula amurensis: A bioenergetic modeling approach. Environmental Toxicology and Chemistry 25: 1933–1940.
Lemly, A. Dennis. 1996. Assessing the toxic threat of selenium to fish and aquatic birds. Environmental Monitoring Assessments 43: 19–35.
Li, Yuan-Hui, and Sandra Gregory. 1974. Diffusion of ions in sea water and in deep sea sediments. Geochimica et Cosmochimica Acta 38: 703–714.
Linville, Regina G., Samuel N. Luoma, Lynda S. Cutter, and Gregory A. Cutter. 2002. Increased selenium threat as a result of invasion of the exotic bivalve Potamocorbula amurensis into the San Francisco Bay Delta. Aquatic Toxicology 57: 51–64.
Martin, Alan J., Stephanie Simpson, Skya Fawcett, Cheryl I.E. Wiramanaden, Ingrid J. Pickering, Nelson Belzile, Y.W. Chen, Jacqueline London, and Dirk Wallschläger. 2011. Biogeochemical mechanisms of selenium exchange between water and sediments in two contrasting lentic environments. Environmental Science and Technology 45: 2605–2612.
Matamoros-Veloza, Adriana , Robert J. Newton, and Liane G. Benning. 2011. What controls selenium release during shale weathering? Applied Geochemistry 26: S222-S226.
McKee, Lester, Neil Ganju, and David Shoellhammer. 2006. Estimates of suspended sediment entering San Francisco Bay from the Sacramento and San Joaquin Delta, San Francisco Bay, California. Journal of Hydrology 323: 335–352.
Miao, Xiu-Sheng, Lee A. Woodward, Chris Swenson, and Qing X. Li. 2001. Comparative concentrations of metals in marine species from French Frigate Shoals, North Pacific Ocean. Marine Pollution Bulletin 42: 1049–1054.
Ohlendorf, Harry M., Roy W. Lowe, Paul R. Kelly, and Thomas E. Harvey. 1986. Selenium and heavy metals in San Francisco diving ducks. Journal of Wildlife Management 50: 64–71.
Oremland, Ronald S., James T. Hollibaugh, Ann S. Maest, Theresa S. Presser, Laurence G. Miller, and Charles W. Culbertson. 1989. Selenate reduction to elemental selenium, by anaerobic bacteria in sediments and cultures: biogeochemical significance of a novel, sulfate-independent respiration. Applied Environmental Microbiology 55: 2333–2343.
Peters, G.M., W.A. Maher, F. Krikowa, A.C. Roach, H.K. Jeswani, J.P. Barford, V.G. Gomes, and D.D. Reible. 1999. Selenium in sediments, pore waters and benthic infauna of Lake Macquarie, New South Wales, Australia. Marine Environmental Research 47: 491–508.
Reamer, D.C., and W.H. Zoller. 1980. Selenium biomethylation products from soil and sewage sludge. Science 208: 500–502.
Schults, Donald W., Steven P. Ferraro, Lawrence M. Smith, Fredrick A. Roberts, and Carolyn K. Poindexter. 1992. A comparison of methods for collecting interstitial water for trace organic compounds and metals analyses. Water Research 26: 989–995.
Shumilin, Evgueni, Federico Páez-Osuna, Carlos Green-Ruiz, Dmitry Sapozhnikov, Guadalupe D. Rodriquez-Meza, and Lucio Godinez-Orta. 2001. Arsenic, antimony, selenium and other trace elements in sediments of the La Paz Lagoon, Peninsula of Baja California, Mexico. Marine Pollution Bulletin 42: 174–178.
Skorupa, Joseph P. 1998. Selenium poisoning of fish and wildlife in nature: lessons from twelve real-world examples. In Environmental chemistry of selenium, eds. William T. Frankenberger Jr, and Richard A. Engberg, 315–354. New York: Marcel Dekker Inc.
Sokolova, Y.G., and M.D. Pilipchuk. 1973. Geochemistry of selenium in sediments in the N.W. part of the Pacific Ocean. Geochemistry International 10: 1537–1546.
Stolz, J.P., P. Basu, and R. Oremland. 2002. Microbial transformation of elements: the case of arsenic and selenium. International Microbiology 5: 201–207.
Takayanagi, Kazufumi, and Nelson Belzile. 1988. Profiles of dissolved and acid-leachable selenium in a sediment core from the lower St. Lawrence estuary. Marine Chemistry 24: 307–314.
Takayanagi, Kazufumi, and D. Cossa. 1985. Speciation of dissolved selenium in the upper St. Lawrence Estuary. In Marine and estuarine geochemistry, eds. Anne C. Siglo and Akihiko Hattori, 275–284. Michigan: Lewis Publishers, Inc.
Thompson, J.K. 2000. Two stories of phytoplankton control by bivalves in San Francisco Bay: the importance of spatial and temporal distribution of bivalves. Journal of Shellfish Research 19: 612.
Ullman, William J., and Robert C. Aller. 1982. Diffusion coefficients in nearshore marine sediments. Limnology and Oceanography 27: 552–556.
van Geen, Alexander, and Samuel N. Luoma. 1999. The impact of human activities on sediments of San Francisco Bay, California: an overview. Marine Chemistry 64: 1–6.
Velinsky, David J., and Gregory A. Cutter. 1990. Determination of elemental selenium and pyrite-selenium in sediments. Analytica Chimica Acta 235: 419–425.
Velinsky, David J., and Gregory A. Cutter. 1991. Geochemistry of selenium in a coastal salt marsh. Geochimica et Cosmochimica Acta 55: 179–191.
Wen, Hanjie, and Jean Carignan. 2011. Selenium isotopes trace the source and redox processes in the black shale-hosted Se-rich deposits in China. Geochimica et Cosmochimica Acta 75: 1411–1427.
Wrench, J.J. 1978. Selenium metabolism in the marine phytoplankters Tetraselmis tetrathele and Dunaliella minuta. Marine Biology 49: 231–236.
Wrench, J.J., and C.I. Measures. 1982. Temporal variations in dissolved selenium in a coastal ecosystem. Nature 299: 431–433.
Yao, Qing-Zheng, Jing Zhang, Xiao-Guang Qin, Hui Xiong, and Li-Xian Dong. 2006. The behavior of selenium and arsenic in the Zhujiang (Pearl River) Estuary, South China Sea. Estuarine, Coastal, and Shelf Science 67: 170–180.
Zawislanski, Peter T., and Angus E. McGrath. 1998. Selenium cycling in estuarine wetlands: overview and new results from the San Francisco Bay. In Environmental chemistry of selenium, eds. William T. Frankenberger, Jr., and Richard A. Engberg, 223–242. New York: Marcel Dekker, Inc.
Acknowledgments
We thank Jason Beck and Paul Richardson for ancillary analyses. We also thank the captain of the Research Vessel David Johnson, Robin Stewart, and Sam Luoma for their help in obtaining the sediment cores. Furthermore, we thank Martina Doblin and Lynda Cutter for their invaluable assistance in the field and for generating many of the ancillary data. This project was supported by funds from NSF (grant OCE-9707946) to G. Cutter.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meseck, S., Cutter, G. Selenium Behavior in San Francisco Bay Sediments. Estuaries and Coasts 35, 646–657 (2012). https://doi.org/10.1007/s12237-011-9444-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-011-9444-0