Abstract
River flow variability is known to influence estuarine production, yet knowledge on its effect upon estuarine food webs dynamics is still scarce. Stable carbon and nitrogen isotopes were used to assess the effect of river flow in the connectivity and food web interactions between the two main fish nursery areas of the Tagus estuary. The aims of the present work were to investigate the seasonal variation in food web structure and the exchange rate of individuals of marine juvenile fish among estuarine nurseries, to compare the spring of a rainy year (2001) with that of an average year (2000), and to investigate the impact of the winter floods of 2001. A low level of connectivity was observed for the fish species that use these areas as nurseries. In low river flow conditions, two isotopically distinct food webs were established in each nursery area. These food webs were very sensitive to small variations in the freshwater input. Winter floods seem to disrupt the localized food webs that are established in low river flow periods, leading to the re-establishment of a wider food web. While in rainy years this wide food web is maintained until spring, in average years the food web undergoes fragmentation into two localized and isotopically distinctive food webs. The increase in frequency of droughts due to climate change should lower the connectivity of the estuarine fish nurseries food webs, causing habitat fragmentation and consequent loss in complexity and resilience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries are important transition zones, where energy from terrestrial origin mixes with that from marine origin. A gradient in terrestrial-derived material is usually established in the organisms feeding in different parts of the estuary, depending on the distance to the freshwater source, on the river flow input, and on the estuarine hydrology (Fry 1999, 2002; Vizzini et al. 2005; Wissel and Fry 2005; Zeug and Winemiller 2008). Estuarine food webs generally rely on a high level of primary production (e.g., from salt marshes, mangroves, seagrasses, etc.), which is associated with a detritus food chain that often supports complex trophic webs (Simenstad and Wissmar 1985; Pasquaud et al. 2008). Although a considerable amount of work has been done on estuarine food web dynamics (Michener and Schnell 1994; Peterson 1999; Hadwen and Arthingtona 2007; Carlier et al. 2008; Choya et al. 2008), less attention has been given to the important issue of the influence of river flow upon estuarine food webs. River flow alterations are known to affect downstream estuarine production (Nixon 1997; Loneragan and Bunn 1999; Chícharo et al. 2006a, b), as well as coastal fisheries (Deegan et al. 1986; Sklar and Browder 1998; Darnaude 2005; Darnaude et al. 2004; Erzini 2005).
Such observations are particularly interesting in areas where precipitation patterns are highly variable, such as south-west Europe. Rain in this region is highly influenced by the strength and position of high pressure cells created by the Azores anticyclone that shifts toward the pole in the summer and toward the equator in the winter (Miranda et al. 2006). This is particularly important since regional climatic models foresee higher variability in this system in the future, due to climate change (Miranda et al. 2006).
The Tagus estuary, Portugal, is an estuarine system of great interest because it is one of the largest estuaries in Europe (325 km2) and includes two nurseries inside the estuary (Costa and Bruxelas 1989). This system is highly productive and has a complex hydrology, since 40% of all estuarine area is intertidal leading to the formation of intertidal mudflats and islets that change according to river flow (Brotas et al. 1995). A preliminary stable isotope study, which focused on sole juveniles, Solea solea and Solea senegalensis, and its prey, indicated that these two nursery areas presented a low level of connectivity—defined as the rate of exchange of individuals of the same species among spatial units (Polis et al. 1997)—and different levels of dependence upon the freshwater and marine energy pathways (Vinagre et al. 2008). Yet, since these areas are subjected to such variable inputs of freshwater inter-annually and throughout the year, a deeper investigation is needed to assess the dynamics of these food webs in a broader temporal scale, taking into account more species and under different river flow conditions.
The aim of the present work was (1) to investigate the seasonal variation in food web structure and habitat connectivity for marine juvenile fish in estuarine nurseries, (2) to compare the spring of a rainy year (2001) with that of an average year (2000), and (3) to look at the impact of the winter floods of 2001. For this, we studied intra and inter-annual variations in the stable isotope signatures (δ13C and δ15N) of the main food web elements in the Tagus estuary (NE Atlantic, Portugal).
A stable isotope approach was chosen in the present study, because this technique has proven to be powerful in describing the relative consumption of materials with different sources, as well as the trophic level of organisms. Terrestrial primary producers generally have lower δ13C than marine producers (Haines and Montague 1979; Riera and Richard 1996; Bouillon et al. 2000), and the increase in δ13C from prey to predator is of 0.4‰ (±1.3‰; De Niro and Epstein 1978; Peterson and Fry 1987, Post 2002), making this isotope particularly useful in estuarine systems, since it allows the identification of the primary source of organic carbon in the diet of organisms and also the evaluation of its dependence on the freshwater and marine energy pathways (Simenstad and Wissmar 1985; Paterson and Whitfield 1997; Darnaude et al. 2004). The nitrogen isotope is useful as a marker of trophic position, since δ15N increases by 3.4‰ (±1%) from prey to predator (Owens 1987; Peterson and Fry 1987; Post 2002).
To the best of our knowledge, there are no previous studies that follow a similar approach to the present work. Fry (1999) observed how the carbon isotopic composition of clams responded to riverine inputs, providing an index of hydrological mixing across the estuarine system. Harrod et al. (2005) described the isotopic signatures of eels at different salinities. While Fry (1999) observed the response in a spatial continuum and Harrod et al. (2005) in a salinity continuum, here we observed the response of a much broader food web taking into account a spatial and a temporal gradient and investigating both intra- and inter-annual variability in food web structure.
Methods
Study Area
The Tagus estuary is located in south-west Europe, in a climatic transition zone, between a subtropical anticyclone, the Azores anticyclone, and sub-polar depressions. It is also strongly influenced by the proximity to the Atlantic Ocean (Miranda et al. 2006). Precipitation is highly variable inter-annually and occurs mainly in winter, accounting for 42% of the total precipitation. The driest season is summer, with only 6% of the precipitation. The remaining 52% of precipitation occurs in spring and autumn (Miranda et al. 2006).
The Tagus estuary (Fig. 1), with an area of 325 km2, is a partially mixed estuary with a tidal range of circa 4 m. Mean depth is less than 10 m, and about 40% of the estuarine area is composed of intertidal mudflats (Cabral and Costa 1999) fringed by extensive areas of salt marshes dominated by Spartina maritima, Halimione portulacoides, and Sarcocornia fruticosa (Caçador et al. 1996). Although its bottom is composed of a heterogeneous assortment of substrates, its prevalent sediment is muddy sand in the upper and middle estuary and sand in the low estuary and adjoining coastal area (Cabral and Costa 1999). Mean flow of the Tagus river is 400 m3 s−1, though it is highly variable both seasonally and inter-annually (data obtained from the Portuguese Water Institute—INAG). Salinity in the estuary varies from 0, 50 km upstream from the mouth, to 35 at the mouth of the estuary (Cabral et al. 2001). Water temperature ranges from 8°C to 26°C (Cabral et al. 2001).
Two important fish nurseries were identified in the Tagus estuary in previous studies (Costa and Bruxelas 1989; Cabral and Costa 1999). They differ in terms of depth, sediment composition, and salinity (Cabral and Costa 1999). Hence, nursery A (Fig. 1) is located in a sandy area of 4.4 m mean depth, characterized by low and highly variable salinities; nursery B (Fig. 1) is located in shallower (1.9 m mean depth) muddy bottoms and exhibits more saline and stable salinities.
The fish community of these nursery areas is composed of marine juvenile species and estuarine residents. Several fish species, among which the most abundant and commercially important are the sea bass Dicentrarchus labrax and the soles S. solea and S. senegalensis, use these areas as nurseries (although, S. solea is only present at nursery A; Costa and Bruxelas 1989; Cabral and Costa 1999). The 0-group fish arrive in spring and stay until early autumn.
Mullets are important components of the resident community, especially the grey mullet Liza ramada because of its abundance and wide distribution throughout the estuary (Moreira et al. 1992). The gobies Pomatoschistus microps and Pomatoschistus minutus are important resident species and benthic predators, being P. microps the most abundant fish species in the Tagus estuary (Costa and Bruxelas 1989). Among the many epibenthic crustaceans, there is the crab Carcinus maenas and various shrimps such as Palaemonetes varians and Crangon crangon (Moreira et al. 1992). The food web of these areas relies heavily on the annelid Nereis diversicolor, as well as in the amphipod Corophium spp. and the bivalve Scrobicularia plana (Moreira et al. 1992).
Sampling
Sampling surveys were conducted in both nursery areas in the spring (May) of 2000 and winter (January), spring (May), summer (July). and autumn (September) of 2001. Samples (three replicates per date/site) of water, zooplankton, sediment (collected with a van Veen grab and the superficial first 1 cm, selected for analysis), salt marsh plants, benthic microalgae, fishes’ main prey species, fish, and epibenthic crustaceans were collected. Water samples for particulate organic matter (POM) analysis (2 l per replicate) were collected at high tide in the two nurseries, in salt marsh tidal creeks, and in the subtidal area. At low tide, the main freshwater sources, the Tagus river (source 1; Fig. 1), the Sorraia river (source 2; Fig. 1), and the Ribeira das Enguias (source 3; Fig. 1), were also sampled for POM analysis. The waters in the middle of the estuary were sampled at high tide in order to analyze the marine water coming into the nurseries with the flooding tide (source 4; Fig. 1).
In order to collect zooplankton samples, three trawls were conducted with zooplankton trawling nets in each nursery area. Tissues of salt marsh plants, S. maritima, H. portulacoides, and S. fruticosa, were cleaned of mud and when present, epiphytes were removed by scraping with a razor blade. Pools of ten plants of the same species were used to produce replicate samples for each salt marsh.
Benthic microalgae samples were collected in nursery A and B, in the intertidal mudflats at low tide. Textile panels of 20 cm by 20 cm were laid in the sediment surface in order to collect the benthic microalgae that concentrate in the surface during low tide. The panels were rinsed with distilled water that was later decanted in order to separate the microalgae from the sediment that was also attached to the panels. The supernatant was then filtered onto precombusted filters. Phytoplankton was not sampled in the water since they occurred in low numbers in the upper Tagus estuary due to the high turbidity of the system. Previous studies have shown that much of the organic matter in the water column is composed of suspended benthic microalgae (Vale and Sundby 1987). Three replicates of sediment were taken in each nursery in order to collect fishes’ main prey, amphipods, isopods, polychaetes, and bivalves.
Beam trawls were carried out in order to capture fish and epibenthic crustaceans (crabs and shrimps). A 4-m beam trawl with one tickler chain and 5-mm stretched mesh at the codend was used. All individuals caught were measured (total length with 1-mm precision). Individuals of P. microps were divided into juveniles (<30 mm) and adults (>30 mm) because this species undergoes a diet shift from meiofauna to macrofauna, which changes its position in the trophic chain (Pihl 1985; Jackson and Rundle 2008). Individuals of L. ramada were divided into three size groups (<55 mm; >55 and <150 mm; >150 mm), this species also goes through important diet shifts throughout its ontogeny (Almeida et al. 1993), which will place it at very different trophic levels. Analyses were done on pools of seven to ten individuals for each size group. For S. solea, S. senegalensis, and D. labrax, analysis was done for each individual.
Stable Isotope Analysis
Water samples for POM analysis were filtered until clogged onto precombusted filters. Sediment samples were dried at 60°C and ground to a fine powder. Water samples for zooplankton analyses were kept frozen, and the lab organisms were identified and separated for further analysis. Samples of salt marsh plants, S. maritima, H. portulacoides, and S. fruticosa, were dried to constant weight at 60°C. The dried tissues were ground to a fine powder. Benthic microalgae were dried at 60°C and ground to a fine powder.
Each sample of macrobenthic species represented a subsample with a minimum of 5 g from a pool of several individuals. While for S. plana, only the valve muscle was used for isotopic analysis; for shrimps, only the internal muscle was used; for amphipods, isopods, oligochaetes, and polychaetes, the whole animals were used. Muscle tissue samples of all fish were dissected and dried at 60°C, for C and N stable isotope analysis. The dried tissues were ground to fine powder with a mortar and a pestle.
In samples suspected of having carbonate contamination, such as those of sediment, zooplankton, plants, benthic microalgae, and macrobenthic species, a subsample was taken and acidified with several drops of 10% HCl while being observed under a dissecting microscope. If bubbling occurred, the whole sample was acidified, rinsed with distilled water, re-dried at 60°C, and stored in glass vials.
13C/12C and 15N/14N ratios in the samples were determined by continuous flow isotope mass spectrometry (Preston and Owens 1983). The standards used were Pee Dee Belemnite for carbon and atmospheric N2 for nitrogen. Precision of the mass spectrometer, calculated using values from duplicate samples of standard material, was ≤0.2‰.
Isotope ratios were expressed as parts per thousand (‰) differences from a standard reference material:
where X is 13C or 15N, R is the ratio of 13C/12C or 15N/14N, and δ is the measure of heavy to light isotopes in the sample.
The acidified subsamples were used for 13C determination, while the remaining sample was used for 15N determination.
Data Analysis
In order to investigate connectivity between nursery areas, from the different sized juveniles that were caught throughout the sampling period, ANOVAs were performed to test differences of isotopic composition of D. labrax and S. senegalensis between nurseries A and B, according to sampling month. The percentage of individuals from the two nurseries with overlapping isotopic values was calculated for each month.
Differences in δ13C and δ15N in the particulate organic matter from the water sources were tested with an ANOVA, taking into account site and month. Whenever the null hypothesis was rejected, Tukey’s post hoc tests were conducted. Differences in δ13C and δ15N in the particulate organic matter from the sediment, salt-marshes, respective salt marsh creeks, microalgae, and the three species of plants were tested with an ANOVA, taking into account site and month, followed by Tukey’s post hoc tests.
In order to investigate food web interactions for consumers, the effect of site and date on the isotopic signatures was tested with a two-way analysis of variance (ANOVA), followed by Tukey’s post hoc tests whenever the null hypotheses were rejected. δ13C and δ15N were tested separately. Every group/species was analyzed based on three replicates of pooled material. In the cases of soles and seabass, whose isotopic values concerned individual fish, isotopic values were randomly pooled into three replicates, in order to analyze them in the same way as for all other species/groups. A significance level of 0.05 was considered in all test procedures. Results were interpreted taking into account mean monthly river drainage (Fig. 2, Table 1).
Results
Connectivity
Distinct isotopic signatures could be observed over the nursery period of 2001 for both D. labrax and S. senegalensis, with the individuals from nursery B being generally more enriched in 13C and 15N (mean δ13C of −19.5‰ in nursery A and −16.5‰ in nursery B and mean δ15N of 15.2‰ in nursery A and 16.2‰ in nursery B, for D. labrax; mean δ13C of −18.1‰ in nursery A and −15.9‰ in nursery B and mean δ15N of 16.6‰ in nursery A and 17.8‰ in nursery B, for S. senegalensis).
For D. labrax, the ANOVAs showed a significant effect of site, month, and the interaction between site and month (Fig. 3, P < 0.05). Post hoc tests revealed differences in isotopic signature both for δ13C and δ15N, in all months (Fig. 3, P < 0.05). No overlap was found in the isotopic values of D. labrax. However, it was observed that the isotopic signatures seem to shift with time, bridging the signature gap between the two nurseries, in September.
For S. senegalensis, the ANOVAs showed a significant effect of site, month, and the interaction between site and month (Fig. 3, P < 0.05). Post hoc tests revealed differences in isotopic signature, both for δ13C and δ15N, in all months (Fig. 3, P < 0.05). There is however isotopic overlap in the spring of 2001, with 35.5% of individuals having intermediate isotopic values in the range of −16.8‰ to 16.2‰ of δ13C. It should be noted that captures of S. senegalensis from the spring of 2001 were composed of individuals from the last cohort from the previous year, while in the following months, all individuals were from the 0-group of 2001, thus much smaller (Table 2). This means that the individuals showing isotopic overlap, in the spring of 2001, are in fact larger, with higher locomotion capabilities and energy demands, than the following ones, from the summer 2001 on, which are 0-groups that have recently colonized the nursery. These larger individuals, from the previous year, left the nursery before summer. For D. labrax, all individuals were 0-group, increasing with length, according to sampling time, as expected (Table 2). Results revealed very low connectivity between the nursery areas, for the species studied.
Food Web Interactions
POM from all freshwater sources (1, 2, 3; Fig. 1) was depleted in δ13C (Figs. 4, 5, and 6), as expected. Tests revealed a significant effect of site, date, and the interaction of site and date (P < 0.05, ANOVA) for δ13C. Post hoc tests showed that POM from the middle estuary (source 4; Fig. 1) that flows into the nurseries at high tide bringing water of marine origin was significantly enriched in δ13C in the spring of 2000 and in the summer and autumn of 2001 (P < 0.05, post hoc), being significantly different from all fresh water sources. This means that the marine isotopic signal could not be detected in the winter and spring of 2001, probably due to the high intensity of river flow. Post hoc tests also show that sources 1 and 2, the two most important freshwater sources of the estuarine system, were always similar (P > 0.05, post hoc), as well as, sources 2 and 3, the two freshwater sources that drain into to nursery B (P > 0.05, post hoc). Sources 1 and 3 were only significantly different in the spring of 2000.
Mean (±SD) δ13C and δ15N of the dominant carbon and nitrogen sources (black triangle as input 1, white square as input 2, white triangle as input 3, and white diamond as input 4, marine influence); POM from each nursery area (POM); POM salt marsh creeks (POM marsh); sediment (Sed); benthic microalgae (Microalg); S. fruticosa (Sarc); H. portulacoides (Hal); S. maritima (Spart); Copepoda (Cop); Oligochaeta (Olig); Corophium spp. (Corop); Sphaeroma serratum (Sph); N. diversicolor (Ner); Streblospio shrubsolii (Sshr); S. plana (Splana); Hydrobia ulvae (Hulv); Mysidacea (Mys); Cyatura carinata (Cy); P. varians (P var); C. crangon (Ccrang); C. maenas (Cm); D. labrax (Dl); S. solea (Ss); S. senegalensis (Sn); Pomatochistus microps (>30 mm; Pmicr-L); P. microps (<30 mm; Pmicr-s); L. ramada (<55 mm; Liza-s); L. ramada (>55 and <150 mm; Liza-m); L. ramada (>150 mm; Liza-L), in the spring of 2001 (a), in the Summer of 2001 (b), and in the autumn of 2001 (c). Black dots stand for nursery A items, white dots stand for nursery B items (some of the SD bars may not be clearly visible because they are very small). Consumers are encircled to show patterns (straight line for nursery A and dotted line for nursery B)
Mean (±SD) δ13C and δ15N of the dominant carbon and nitrogen sources (black triangle as input 1, white square as input 2, white triangle as input 3, and white diamond as input 4, marine influence); POM from each nursery area (POM); POM salt marsh creeks (POM marsh); sediment (Sed); benthic microalgae (Microalg); S. fruticosa (Sarc); H. portulacoides (Hal); S. maritima (Spart); Corophium spp. (Corop); N. diversicolor (Ner); S. shrubsolii (Sshr); S. plana (Splana); H. ulvae (Hulv); Mysidacea (Mys); C. carinata (Cy); P. varians (P var); P. microps (>30 mm; Pmicr-L); P. microps (<30 mm; Pmicr-s); L. ramada (<55 mm; Liza-s); L. ramada (>55 and <150 mm; Liza-m); L. ramada (>150 mm; Liza-L), in the spring of 2000. Black dots stand for nursery A items, white dots stand for nursery B items (some of the SD bars may not be clearly visible because they are very small). Consumers are encircled to show patterns (straight line for nursery A and dotted line for nursery B)
Mean (±SD) δ13C and δ15N of the dominant carbon and nitrogen sources (black triangle as input 1, white square as input 2, white triangle as input 3, and white diamond as input 4, marine influence); POM from each nursery area (POM); POM salt marsh creeks (POM marsh); sediment (Sed); benthic microalgae (Microalg); S. fruticosa (Sarc); H. portulacoides (Hal); S. maritima (Spart); Oligochaeta (Olig); Corophium spp. (Corop); S. serratum (Sph); N. diversicolor (Ner); S. shrubsolii (Sshr); S. plana (Splana); Hydrobia ulvae (Hulv); Mysidacea (Mys); C. carinata (Cy); P. varians (P var); C. crangon (Ccrang); P. microps (>30 mm; Pmicr-L); P. microps (<30 mm; Pmicr-s); L. ramada (<55 mm; Liza-s); L. ramada (>55 and <150 mm; Liza-m); L. ramada (>150 mm; Liza-L), in the winter of 2001. Black dots stand for nursery A items, white dots stand for nursery B items (some of the SD bars may not be clearly visible because they are very small. Consumers are encircled to show patterns (straight line for nursery A and dotted line for nursery B)
This reveals that the main freshwater source draining to nursery A, source 1, and the main freshwater source draining into nursery B, source 2, were always similar in δ13C during the study period. In nursery B, there is also source 3, yet this was always similar in δ13C to the other source draining into this system, source 2, and only differed from the source draining into the opposite nursery in the spring of 2000. This means that the freshwater sources are generally not contributing to isotopic differences in δ13C between the two nurseries, leaving source 4, the marine contribution into the two nurseries being the major source of isotopic variation in the upper estuary.
Tests revealed a significant effect of date and interaction between site and date for POM from the sources (P < 0.05, ANOVA) for δ15N. Post hoc tests showed that the marine influence, source 4, is generally similar in δ15N to all freshwater sources, with the exception of source 1, in the spring of 2001 (P < 0.05, post hoc). The three freshwater sources were all generally similar in δ15N, with the exception of sources 1 and 2, in the winter of 2001. Results for δ15N show that this isotope is not a major contributor to isotopic variation among nurseries.
A significant effect of site, date, and interaction between site and date (P < 0.05, ANOVA) for δ13C was detected for the elements of the lower part of the food web, composed by particulate organic matter from the sediment, salt-marshes, respective salt marsh creeks, microalgae, and the three species of plants. Post hoc tests showed no significant differences between the nurseries, in the months studied. A significant effect of site, date, and interaction between site and date (P < 0.05, ANOVA) was detected for δ15N in the elements of the lower part of the food web. Post hoc tests showed that the two nurseries were always different in δ15N, except for the spring and autumn of 2001 (P > 0.05, post hoc). This results show that the isotopic variations detected for the water sources are not well reflected into the lower links of the food web.
A significant effect of site, date, and interaction between site and date (P < 0.05, ANOVA) was detected for δ13C in for the consumers. Post hoc tests revealed that the two nurseries were different in the spring of 2000 and in the summer and autumn of 2001 (P < 0.05, post hoc). The nurseries were isotopically similar in the winter and spring of 2001. A significant effect of site, date, and interaction between site and date (P < 0.05, ANOVA) was detected for δ15N in the consumers. Post hoc tests yielded similar results to those of δ13C. These results show that the consumers of nursery A and B are isotopically distinctive in the same time periods when the marine POM influence (source 4) was detected in the system (spring of 2000 and summer and autumn of 2001).
Discussion
Connectivity
Clearly distinct isotopic signatures for the two nurseries were observed both for D. labrax and S. senegalensis; and thus, a low level of connectivity was revealed for these species between these areas, distanced ∼21 km, as had already been concluded for S. senegalensis by Vinagre et al. (2008) using a subset of the data analyzed here. D. labrax have a short range of movement (∼16 km; Kennedy and Fitzmaurice 1972; Holden and Williams 1974). Leakey et al. (2008) stable isotope study, in the Thames estuary, with 0-group fish, observed some distinction between the D. labrax caught inside the estuary and the ones caught in the coastal zone (∼40 km), yet sample size was too small for further conclusions. Thus, this is the first study that reveals a clear isotopic distinction between 0-group D. labrax of adjacent nursery areas and the low level of connectivity between them.
Interestingly, by the end of the nursery period, in autumn, the gap between isotopic signatures of D. labrax had been reduced. Although, turnover rates were not accessed (Herzka 2005; Hoffman et al. 2007) and no telemetry or mark–recapture studies are available (Cunjak et al. 2005), the reduction of the isotopic gap likely occurred because individuals were then larger, had higher locomotion ability and energetic demands, and thus started to broaden their feeding areas.
A clear isotopic distinction could also be observed for the 0-group juveniles of S. senegalensis from summer and autumn 2001. A limited range of movement had already been reported in tagging experiments for other flatfish species juveniles, such as S. solea (Coggan and Dando 1988; Leakey et al. 2008), Pseudopleuronectes americanus (Saucerman and Deegan 1991) and Pleuronectes platessa (Burrows et al. 2004).
The fact that this species’ life cycle includes cohorts that enter the estuary late in the year (from September to November) and stay through winter until the following spring allows the observation of the spring individuals (Cabral and Costa 1999), which came from one of those later cohorts and were larger than the 0-group individuals that could be observed later in the summer of 2001. It is interesting to notice that for these larger individuals, there was some overlap in the isotopic signatures, indicative of connectivity between the two nursery areas. It can be concluded that these nurseries present low connectivity for D. labrax and S. senegalensis, especially for the smaller individuals. There is evidence that with growth, the level of connectivity tends to increase, possibly due to a need to broaden the feeding areas to encompass higher energetic demands.
Food Web Interactions—The Impact of River Flow
Nursery Period
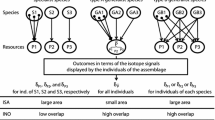
The nursery period, when these areas play an important role for juvenile fish, ranges from spring to early autumn. This study revealed the structuring role of the river flow input on the food web of the upper estuary. As river flow decreases from spring to summer, the structure of two distinct food webs emerges in each nursery (Fig. 4).
The structure of the food webs established in the upper Tagus seems sensitive to even small variations in the freshwater input. This could be observed by the comparative analysis of the consumers’ results from spring to autumn. In spring 2001, nursery B always presented more enriched δ13C values, yet a significant isotopic distinction between the two food webs could not be observed since there was an overlap between the isotopic signatures of the two nurseries. Yet at the peak of summer when river drainage was much lower, not only were the values of each food web link of nursery B more enriched in δ13C, but also a clear and significant isotopic distinction between the two food webs was revealed. In September 2001, although river drainage was still relatively low, the first rains of autumn had started. Distinction between the two food webs was still significant, yet isotopic overlap was higher than in the summer of 2001.
In this study, no difference could be detected in the isotopic signatures of δ13C in the lower food web (local POM, plants, and microalgae) in all dates sampled, yet isotopic differences among the upper food web seem to reflect the differences in the marine POM input. This is probably because the fish analyzed feed on macrobenthic species that rely mainly on detritus and are thus sensitive to changes in the source of POM.
Comparison of an Average Spring with a Rainy Spring
The spring of 2000, an average year in terms of rain and river flow, revealed isotopic distinction between the food webs of the two nurseries (Fig. 5), while in the spring of 2001, an extremely rainy year (Fig. 2, Table 1), no significant isotopic distinction was found. Although the spring of 2000 presented higher river drainage than the spring of 2001, one must observe the river drainage values of the previous months of each of the years (Fig. 2, Table 1); these were much higher in 2001 and have certainly impacted the hydrology of the upper estuary, as well as the incorporation of the isotopic elements into the local food webs.
The earlier establishment of distinct food webs in the upper Tagus, in low river flow conditions, will probably lead to summers where the two nurseries become even more distinct throughout the dry period. They will probably rely more heavily on different energetic pathways, with nursery A depending more on a freshwater pathway and nursery B on a marine pathway, given that the data already indicate more enriched δ13C values for nursery B.
The consequences of such isolation could be negative in terms of resilience—how fast populations recover from disturbances (Ives 1995)—in response to anthropogenic or natural impacts. The loss of interaction between the food webs will lead to a loss of number of links and thus lowered complexity, which can result in increased fragility (McCann 2000; Solé and Montoya 2001).
Having a unique large food web instead of various relatively discrete sub-webs increases the likelihood that there will be species capable of responding in different ways to environmental change (Levin 1999; Naem and Li 1997; McCann 2000). There is also the important issue of functional redundancy; it is more probable that in a larger food web, there will be species capable of replacing the function of others (Levin 1999). However, it should be pointed out that low river flow conditions are natural and seasonal in this area. Thus, fragmentation of these food webs during the dry season and its homogenization by winter floods are part of the normal functioning of this estuarine system.
Effect of Winter Floods
The winter floods of 2001 were of extreme nature, and in January 2001, the river drainage of the Tagus reached a peak (Fig. 2, Table 1). The previously identified local food webs had a different presentation, with high isotopic overlap. Some of the more mobile species presented isotopic values typical of the opposite nursery (e.g., such as L. ramada >150 mm and P. microps >30 mm), thus appearing to have spent the last months in the opposite nursery, having acquired the opposite isotopic composition of the nursery where they were caught in the winter of 2001, as if dragged along a considerable distance by the heavy floods.
Consumers of the two nurseries did not present isotopic distinction, which means that there is only one food web in the upper Tagus estuary. Winter floods seem to disrupt the localized food webs that get established during low river flow levels (Fig. 6). Winter floods possibly lead to the creation of new food web links increasing the interaction between the two food webs to a level where they cease to be distinguishable and become one food web with far reaching links that connect the whole upper estuary community.
The lack of isotopic distinction between the food webs was still patent in the spring of 2001 (Fig. 4). This is evidence of the long-lasting effects of winter floods (Carlier et al. 2007) and of its deep impact on estuarine food webs (Livingston 1997). Malet et al. (2008) showed that the effect of river floods on the incorporation of terrestrial carbon also reaches coastal food webs.
Another important aspect of river floods is the large amount of organic matter that enters the estuarine food web. It should be pointed out that the upper Tagus estuary is an area of sediment deposition and that ca. 40% of all estuarine area is intertidal, composed mainly of mudflats; thus, much of the sediments and POM carried by the river floods get deposited here. Salen-Picard et al. (2002) revealed how floods caused pulses of organic matter in a coastal area of the Gulf of Lions (France) followed, with different time-lags, by peaks of polychaetes which were in turn correlated with sole commercial landings. This correlation between local river flow and abundance of coastal fisheries has also been observed in other Portuguese, European, and North American coasts (Chapman 1966; Moore et al. 1970; Deegan et al. 1986; Chícharo et al. 2006a, b; Wolanski et al. 2006). However, it should be mentioned that high flow periods may simply homogenize the isotopic signatures of the materials consumed by consumers, not necessarily changing feeding relationships or the location where consumers acquire energy. This means that new investigations into the effect of winter floods should be complemented by other tools, such as hydrological models that portray the complex movement of water masses and suspended matter in estuarine systems. Classic mark–recapture and telemetry of nekton species should also elucidate questions on site fidelity under flood conditions.
What to Expect from Climate Change?
Regional climate change models (HadRM3) predict an increase in precipitation variability but most importantly a consistent severe decrease in rainfall throughout the year for the river Tagus watershed (Miranda et al. 2006). This means that important winter floods are still to be expected, due to the higher variability, yet preparations should be undertaken for a climate dominated by droughts.
This work presents evidence of the importance of river flow in the structuring and fragmenting of the estuarine nurseries’ food webs. In the case of the Tagus estuary, the more frequent droughts should lower the connectivity of the estuarine fish nurseries food webs, leading to their fragmentation into sub-webs with consequent loss in complexity and resilience toward natural and anthropogenic impacts. At a time when biological communities will be undergoing adaptation to other alterations induced by climate change, such as temperature rise, sea-level rise, and biological invasions, a decrease in resilience may have important consequences for the balance of this ecosystem and its ability to play its nursery role for fish.
These important consequences of droughts should be taken into account when calculating future ecological river flows allowed by the upstream dams. Management actions should be implemented, such as the maintenance and restoration of natural inundation patterns, adequate access for native fauna to and from the wetlands, and the conservation of multiple suites of intact habitat types concentrated near the important nursery areas.
References
Almeida, P.R., F. Moreira, J.L. Costa, C.A. Assis, and M.J. Costa. 1993. The feeding ecology of Liza ramada (Risso, 1826) in fresh and brackish water in the River Tagus, Portugal. Journal of Fish Biology 42: 95–107.
Bouillon, S., P.C. Mohan, N. Sreenivas, and F. Dehairs. 2000. Sources of suspended organic matter and selective feeding by zooplankton in an estuarine mangrove ecosystem as traced by stable isotopes. Marine Ecology Progress Series 208: 79–92.
Brotas, V., T. Cabrita, A. Portugal, J. Serôdio, and F. Catarino. 1995. Spatio-temporal distribution of the microphytobenthic biomass in intertidal flats of the Tagus estuary. Hydrobiologia 300: 93–104.
Burrows, M.T., R.N. Gibson, L. Robb, and A. MacLean. 2004. Alongshore dispersal and site fidelity of juvenile plaice from tagging and transplants. Journal of Fish Biology 65: 620–634.
Cabral, H.N., and M.J. Costa. 1999. Differential use of nursery areas within the Tagus estuary by sympatric soles, Solea solea and Solea senegalensis. Environmental Biology of Fishes 56: 389–397.
Cabral, H.N., M.J. Costa, and J.P. Salgado. 2001. Does the Tagus estuary fish community reflect environmental changes? Climate Research 18: 119–126.
Caçador, I., C. Vale, and F. Catarino. 1996. Accumulation of Zn, Pb, Cu and Ni in sediments between roots of the Tagus estuary salt marshes, Portugal. Estuarine, Coastal and Shelf Science 42: 393–403.
Carlier, A., P. Riera, J.-M. Amouroux, J.-Y. Bodiou, K. Escoubeyrou, M. Desmalades, J. Caparros, and A. Grémare. 2007. A seasonal survey of the food web in the Lapalme Lagoon (northwestern Mediterranean) assessed by carbon and nitrogen stable isotope analysis. Estuarine, Coastal and Shelf Science 73: 299–315.
Carlier, A., P. Riera, J.-M. Amouroux, J.-Y. Bodiou, M. Desmalades, and A. Grémare. 2008. Food web structure of two Mediterranean lagoons under varying degree of eutrophication. Journal of Sea Research 60: 264–275.
Chícharo, L., M.A. Chícharo, and R. Ben-Hamadou. 2006a. Use of a hydrotechnical infrastructure (Alqueva Dam) to regulate planktonic assemblages in the Guadiana estuary: Basis for sustainable water and ecosystem services management. Estuarine Coastal and Shelf Science 70: 3–18.
Chícharo, M.A., L. Chícharo, and P. Morais. 2006b. Inter-annual differences of ichthyofauna structure of the Guadiana estuary and adjacent coastal area (SE Portugal/SW Spain): Before and after Alqueva dam construction. Estuarine, Coastal and Shelf Science 70: 39–51.
Coggan, R.A., and P.R. Dando. 1988. Movements of juvenile Dover sole, Solea solea (L.), in the Tamar Estuary, South-western England. Journal of Fish Biology 33: 177–184.
Chapman, C.R. 1966. The Texas basins project. In: Smith R., A. Swartz, W. Massman (eds) Symposium on estuarine fisheries. American Fisheries Society Special Publication 3: 83–92.
Choya, E.J., S. Anb, and C.-K. Kang. 2008. Pathways of organic matter through food webs of diverse habitats in the regulated Nakdong River estuary (Korea). Estuarine, Coastal and Shelf Science 78: 215–226.
Costa, M.J., and A. Bruxelas. 1989. The structure of fish communities in the Tagus estuary, Portugal, and its role as a nursery for commercial fish species. Scientia Marina 53: 561–566.
Cunjak, R.A., J.-M. Roussel, M.A. Gray, J.P. Dietrich, D.F. Cartwright, K.R. Munkittrick, and T.D. Jardine. 2005. Using stable isotope analysis with telemetry or mark–recapture data to identify fish movement and foraging. Oecologia 144: 636–646.
Darnaude, A. 2005. Fish ecology and terrestrial carbon use in coastal areas: Implications for marine fish production. The Journal of Animal Ecology 74: 864–876.
Darnaude, A.M., C. Salen-Picard, N.V.C. Polunin, and M.L. Harmelin-Vivien. 2004. Trophodynamic linkage between river runoff and coastal fishery yield elucidated by stable isotope data in the Gulf of Lions (NW Mediterranean). Oecologia 138: 325–332.
De Niro, M.J., and S. Epstein. 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Cosmochimica Acta 42: 495–506.
Deegan, L.A., J.W. Day, J.G. Gosselink Jr., A. Yanez-Arancibia, S. Chavez, and P. Sanchez-Gil. 1986. Relationships among physical characteristics, vegetation, distribution, and fisheries yields in Gulf of Mexico estuaries. In Estuarine Variability, 83–100. New York: Academic.
Erzini, K. 2005. Trends in NE Atlantic landings (southern Portugal): Identifying the relative importance of fisheries and environmental variables. Fisheries Oceanography 14: 195–209.
Fry, B. 1999. Using stable isotopes to monitor watershed influences on aquatic trophodynamics. Canadian Journal of Fisheries and Aquatic Sciences 56: 2167–2171.
Fry, B. 2002. Conservative mixing of stable isotopes across estuarine salinity gradients: A conceptual framework for monitoring watershed influences on downstream fisheries production. Estuaries 25: 264–271.
Jackson, A.C., and S.D. Rundle. 2008. Diet–shifts by an estuarine goby (Pomatoschistus microps) in the face of variable prey availability. Journal of Experimental Marine Biology and Ecology 361: 1–7.
Hadwen, W.L., and A.H. Arthingtona. 2007. Food webs of two intermittently open estuaries receiving 15N-enriched sewage effluent. Estuarine, Coastal and Shelf Science 71: 347–358.
Haines, E.B., and C.I. Montague. 1979. Food sources of estuarine invertebrates analyzed using 13C/12C ratios. Ecology 60: 48–56.
Harrod, C., J. Grey, T. Kieran-McCarthy, and M. Morrissey. 2005. Stable isotope analyses provide new insights into ecological plasticity in a mixohaline population of European eel. Oecologia 144: 673–683.
Herzka, S. 2005. Assessing connectivity of estuarine fishes based on stable isotope ratio analysis. Estuarine, Coastal and Shelf Science 64: 58–69.
Hoffman, J.C., D.A. Bronk, and J.E. Olney. 2007. Tracking nursery habitat use in the York River Estuary, Virginia, by young American shad using stable isotopes. Transactions of the American Fisheries Society 136: 1285–1297.
Holden, M.J., and T. Williams. 1974. The biology, movements and populations dynamics of bass, Dicentrarchus labrax, in English waters. Journal of the Marine Biological Association (United Kingdom) 54: 91–107.
Ives, A.R. 1995. Measuring resilience in stochastic-systems. Ecological Monographies 65: 217–233.
Kennedy, M., and P. Fitzmaurice. 1972. The biology of the bass Dicentrarchus labrax in Irish waters. Journal of the Marine Biological Association (United Kingdom) 52: 557–597.
Leakey, C.D.B., M.J. Attrill, S. Jennings, and M.F. Fitzsimons. 2008. Stable isotopes in juvenile marine fishes and their invertebrate prey from the Thames Estuary, and adjacent coastal regions. Estuarine, Coastal and Shelf Science 77: 513–522.
Levin, S. 1999. Fragile dominion. Reading: Perseus Books. 250 pp.
Livingston, R.J. 1997. Trophic response of estuarine fishes to long-term changes of river runoff. Bulletin of Marine Science 60: 984–1004.
Loneragan, N.R., and S.E. Bunn. 1999. River flows and estuarine ecosystems: Implications for coastal fisheries from a review and a case study of the Logan river, Southeast Queensland. Australian Journal of Ecology 24: 431–440.
Malet, N., P.-G. Sauriau, M. Ryckaert, P. Malestroit, and G. Guillou. 2008. Dynamics and sources of suspended particulate organic matter in the Marennes-Oleron oyster farming bay: Insights from stable isotopes and microalgae ecology. Estuarine, Coastal and Shelf Science 78: 576–586.
McCann, K.S. 2000. The biodiversity-stability debate. Nature 405: 228–233.
Michener, R.H., and D.M. Schnell. 1994. Stable isotope ratios as tracers in marine aquatic food webs. In Stable isotopes in ecology and environmental science, ed. K. Lajtha and R.H. Michener, 138–157. Oxford: Blackwell Scientific Publications.
Miranda, M.A.P., M.A. Valente, A.R. Tomé, R. Trigo, and M.F. Coelho. 2006. O Clima de Portugal nos Séculos XX e XXI. In Alterações Climáticas em Portugal – Cenários, ed. F.D. Santos and P. Miranda, 47–113. Gradiva: Impactos e Medidas de Adaptação.
Moore, D.H., H.A. Brusher, and L. Trent. 1970. Relative abundance, seasonal distribution, and species composition of demersal fishes off Louisiana and Texas, 1962-1964. Contributions in Marine Science 15: 45–70.
Moreira, F., C. Assis, P.R. Almeida, J.L. Costa, and M.J. Costa. 1992. Trophic relationships in the community of the upper Tagus estuary (Portugal): a preliminary approach. Estuarine, Coastal and Shelf Science 34: 617–623.
Naem, S., and S. Li. 1997. Biodiversity enhances ecosystem reliability. Nature 390: 507–509.
Nixon, S.W. 1997. Prehistoric nutrient inputs and productivity in Narrangansett Bay. Estuaries 20: 253–261.
Owens, N.J.P. 1987. Natural variations in 15N in the marine environment. Advances in Marine Biology 24: 389–451.
Pasquaud, S., C. Jeantet, I. Billy, P. Martinez, and M. Girardin. 2008. A preliminary investigation of the fish food web in the Gironde estuary, France, using dietary and stable isotope analyses. Estuarine, Coastal and Shelf Science 78: 267–279.
Paterson, A.W., and A.K. Whitfield. 1997. A stable carbon isotope study of the food-web in a freshwater-deprived South African estuary, with particular emphasis on the ichthyofauna. Estuarine, Coastal and Shelf Science 45: 705–715.
Peterson, B.J. 1999. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oceanologica - International Journal Ecology 20: 479–487.
Peterson, B.J., and B. Fry. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Pihl, L. 1985. Food selection and consumption of mobile epibenthic fauna in shallow marine areas. Marine Ecology Progress Series 22: 169–179.
Polis, G., W. Anderson, and R. Holt. 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28: 289–316.
Post, D.M. 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83: 703–718.
Preston, T., and N.J.P. Owens. 1983. Interfacing an automatic elemental analyser with an isotope ratio mass spectrometer: The potential for fully automated total nitrogen and nitrogen-15 analysis. Analyst 108: 971–977.
Riera, P., and P. Richard. 1996. Isotopic determination of food sources of Crassostrea gigas along a trophic gradient in the estuarine bay of Marennes-Oléron. Estuarine, Coastal and Shelf Science 42: 347–360.
Salen-Picard, C., A.M. Darnaude, D. Arlhac, and M.L. Harmelin-Vivien. 2002. Fluctuations of macrobenthic populations: A link between climate-driven river run-off and sole fishery yields in the Gulf of Lions. Oecologia 133: 380–388.
Saucerman, S.E., and L.A. Deegan. 1991. Lateral and cross-channel movement of young-of-the-year winter flounder (Pseudopleuronectes americanus) in Waiquoit Bay, Massachusetts. Estuaries 14: 440–446.
Simenstad, C.A., and R.C. Wissmar. 1985. δ13C evidence of the origins and fates of organic carbon in estuarine and nearshore food webs. Marine Ecology Progress Series 22: 141–152.
Sklar, F.H., and J.A. Browder. 1998. Coastal environmental impacts brought about by alterations to freshwater flow in the Gulf of Mexico. Environmental Management 22: 547–562.
Solé, R.V., and J.M. Montoya. 2001. Complexity and fragility in ecological networks. Proceedings of the Royal Society of London. Series B 268: 2039–2045.
Vale, C., and B. Sundby. 1987. Suspended sediment fluctuations in the Tagus estuary on semidiurnal and fortnightly time scales. Estuarine, Coastal and Shelf Science 25: 495–508.
Vinagre, C., J. Salgado, M.J. Costa, and H.N. Cabral. 2008. Nursery fidelity, food web interactions and primary sources of nutrition of the juveniles of Solea solea and S. senegalensis in the Tagus estuary (Portugal): A stable isotope approach. Estuarine. Coastal and Shelf Science 76: 255–264.
Vizzini, S., B. Savona, T. Do Chi, and A. Mazzola. 2005. Spatial variability of stable carbon and nitrogen isotope ratios in a Mediterranean coastal lagoon. Hidrobiologia 550: 73–82.
Wissel, B., and B. Fry. 2005. Tracing Mississippi river influences in estuarine food webs of coastal Louisiana. Oecologia 144: 659–672.
Wolanski, E., L. Chicharo, M.A. Chicharo, and P. Morais. 2006. An ecohydrology model of the Guadiana Estuary (South Portugal). Estuarine, Coastal and Shelf Science 70: 132–143.
Zeug, S.C., and K.O. Winemiller. 2008. Evidence supporting the importance of terrestrial carbon in a large-river food web. Ecology 89: 1733–1743.
Acknowledgments
Authors would like to thank the two referees whose contribution greatly improved the manuscript. Authors are also grateful to everyone involved in the field work. This study had the support of the Portuguese Fundação para a Ciência e a Tecnologia (FCT) through the grant SFRH/BPD/34934/2007 awarded to C. Vinagre and the European Union which financed several of the research projects related to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinagre, C., Salgado, J., Cabral, H.N. et al. Food Web Structure and Habitat Connectivity in Fish Estuarine Nurseries—Impact of River Flow. Estuaries and Coasts 34, 663–674 (2011). https://doi.org/10.1007/s12237-010-9315-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-010-9315-0