Abstract
Identifying differential population structure within metacommunities is key toward describing the mechanisms that maintain biodiversity in natural systems. At both local and regional scales on the North American Atlantic coast, we assessed phylogeographic and genetic diversity patterns of six common salt marsh invertebrates using equivalent sampling schemes and sequence data from the same mitochondrial locus. In general, our results suggest little genetic structure across four previously sampled biogeographic regions and a slight increase in genetic diversity from northern to southern areas; however, two of the species (Geukensia demissa and Uca pugilator) exhibited significant differentiation between the northernmost populations and other regions, consistent with a number of previous studies. Although the minimal genetic structure recovered in this community is consistent with expectations based on the larval life history of the species examined, confirmation of this result suggests that latitudinal shifts in ecological interactions in salt marsh systems are environmentally driven, rather than due to heritable adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Comparative phylogeography strengthens our inferences about impacts of evolutionary and environmental forces on the structure of natural communities (Bermingham and Moritz 1998). This structure is often associated with population connectivity patterns that can be used to design effective management and conservation plans (Moritz and Faith 1998) and can illuminate how communities are assembled and how biological associations are developed in time and space (Avise 2000; Wares and Cunningham 2001). For example, temporal and spatial concordance in phylogeographic patterns may indicate that the same forces are at work regulating both species geographic ranges and community dynamics (Paulay and Meyer 2006).
Concordance of phylogeographic and biogeographic patterns has been found in coastal rocky intertidal faunas on the southwest and northeast coastlines of North America (Dawson 2001; Wares 2002). On the east coast, research in rocky intertidal and seagrass communities indicates that complex factors, such as habitat distribution, ocean currents, and historical events (i.e., glaciation), are involved in the connectivity and phylogeographic patterns of coastal animal communities. Wares (2002) synthesized data from 18 species to show that there is a strong tendency for species to exhibit some form of genetic discontinuity in population structure along Long Island Sound (LIS). This transition is concordant with biogeographic transitions identified previously, particularly the border between Upper (UV) and Lower Virginian (LV) provinces defined by estuarine species distributions (Engle and Summers 1999; Wares 2002).
Description of species distributional patterns has shown that biodiversity tends to increase from the poles to the tropics (Chek et al. 2003; Martin and McKay 2004; Kelly and Eernisse 2007; Eo et al. 2008). This latitudinal variation is possibly responding to historical climate shifts (Taberlet 1998; Hewitt 1996, 2000) and driven by increasingly stable and warm temperatures and suitable habitat toward the equator that promote speciation and divergence (Gillooly et al. 2005; Eo et al. 2008). The pattern for higher diversity in tropical areas is seen not only in the numbers of species inhabiting an area but also in the genetic diversity of species. Regression analysis of phylogeographic data for 42 species in North America showed a significant increase of nucleotide diversity from the north to the south (Taberlet 1998). Similarities between the distribution of species and genetic diversity are not limited to comparisons across latitudes. Recently, both empirical and theoretical research (Vellend 2003, 2005) has shown that similar forces may shape these two levels of biodiversity in natural ecosystems, even across relatively small spatial scales (see Robinson et al. 2009).
Attempting to elucidate the general mechanisms for maintenance of biodiversity is another important goal of comparative phylogeography (Kelly and Eernisse 2007). In marine systems, one factor that is likely important in generating such patterns is the nearshore physical oceanography. Certainly, upwelling and coastal currents drive patterns of intraspecific diversity and abundance, as well as species range limits, along the Pacific coast of North America (Connolly et al. 2001; Byers and Pringle 2006). The dynamics are more complicated on the Atlantic coast of North America, as there is a strong southwestward flow across southern New England and the Mid-Atlantic Bight, with considerable variance caused by seasonal and climatic shifts (Lentz 2008). Further south, a northward flow driven by the Gulf Stream dominates (Domeier 2002); these circulation patterns could be responsible for establishing stable population and community structure (Hare et al. 2005). Despite this oceanographically driven structure, the prevailing belief is that most North American Atlantic coast marine invertebrates with broadly dispersing larvae form panmictic populations that are demographically connected across the entire species range. However, the upstream–downstream spatial relationship of populations can mask the fact that not all regions contribute equally to the long-term diversity of the species, and this needs to be investigated on the Atlantic coast (Pringle and Wares 2007; Wares and Pringle 2008).
Salt marsh communities on the east coast of North America are model systems for studying ecosystem and community ecology (Bruno and Bertness 2001). Over the last few decades, for example, research has revealed that a number of processes that vary latitudinally in other marine communities (i.e., rocky intertidal and seagrass) can also affect the diversity and abundance of salt marsh ecosystems. Northern marshes suffer from strong annual erosion due to winter ice, while southern marsh populations must endure stronger predatory and physiological transitions (Bertness 1999). Ecological interactions, such as facilitation and herbivory (Pennings et al. 2001; Salgado and Pennings 2005), may also vary latitudinally, with stressors increasing in a southerly direction (Bortolus and Iribarne 1999; Bruno and Bertness 2001; Silliman and Bertness 2002).
In addition to being model systems for the study of community and ecosystem ecology, salt marshes are well suited to test the effects of historical vicariant events and gene flow on phylogeographic structure and diversity patterns (Bilton et al. 2002; Haney et al. 2007). Theoretical models of community assembly and diversity (e.g., Hubbell 2001) require an understanding of whether a community is structured by barriers or limits to immigration. Marsh ecosystems can be evaluated on broad biogeographic scales as well as local scales, since hydrologic and physiological barriers may exist over small distances in such habitats (Marko and Barr 2007). Moreover, marshes are ideal systems to detect clines of gene flow created by physiological barriers, variable coastal circulation, and organism life cycles (Kelly et al. 2006; Siegel et al. 2003). Despite all of this potential for marshes as good study systems for latitudinal and ecotypic variation, there has been relatively little research comparing genetic diversity and structure patterns in salt marsh communities. It is not clear, for example, whether the relatively uniform break between the UV and LV provinces discussed above (Wares 2002) influences salt marsh species. If so, considerations of management and ecology might be necessarily different between the northernmost Atlantic salt marshes and those along the southeastern coast of the USA.
For several salt marsh and intertidal species, the typical Gulf–Atlantic disjunction (Avise 1992) has been recovered through phylogeographic and population genetic studies (i.e., Spartina alterniflora, Blum et al. 2007; Brachidontes exustus, Lee and O’ Foighil 2005; and Crassostrea virginica, Hare and Avise 1996). However, little intraspecific divergence has generally been recovered along the Atlantic coast in salt marsh animals, such as Uca minax (Felder and Staton 1994) and (at a local scale) Uca pugnax (Sanford et al. 2006). The few examples of strong genetic structure in this community along this coast tend to be associated with the major biogeographic transitions at Cape Canaveral (Reeb and Avise 1990) and Cape Cod (Haney et al. 2007), leaving a span of nearly 2,000 km of the Atlantic coast with little apparent structure despite the ecological variation noted above.
However, it is often difficult to compare results of species sampled from different locations or with different larval durations (Marko 2004). Furthermore, sampling variances among studies also make the integration of this information challenging for a community (Wares 2002). Here, we simultaneously assess the phylogeographic structure and genetic diversity patterns of six common salt marsh invertebrate species (Geukensia demissa, Ilyanassa obsoleta, Littoraria irrorata, U. pugnax, U. pugilator, and C. virginica) by looking at two geographic scales: a local scale, using sites located within the Georgia Coastal Ecosystems (GCE) Long Term Ecological Research (LTER) domain at Sapelo Island, Georgia and a regional/latitudinal scale, spanning the Atlantic coast from Massachusetts to Florida, sampling across four defined biogeographic regions (Engle and Summers 1999). This stratified sampling allowed us to investigate not only whether there are latitudinal genetic diversity patterns in this community (including concordance with the northern break noted in other fauna) but also whether patterns of genetic differentiation are affected by the physiological gradients and complex coastlines inherent to the estuarine habitats of the North American Atlantic coast. The results of our inquiry are intended to establish a baseline understanding of the geographic breadth of the propagule pool that is involved in assembly of the coastal salt marsh ecosystem and whether we expect adaptation or isolation to play a role in generating structure on local or biogeographic scales.
Materials and Methods
Study System
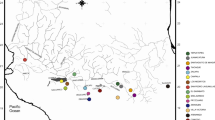
The salt marsh habitat of the eastern USA has a distribution from Maine to Florida, with increasing abundance and size of habitat toward the south (up to 200,000 ha of marsh habitat in Georgia; Bertness 1999). Figure 1 illustrates the scale of our study and separates marsh habitats along the US Atlantic coast into four regions based on the biogeographic survey and synthesis of Engle and Summers (1999). These regions include the UV and LV provinces (separated at 39° N latitude), which are also often differentiated in phylogeographic surveys of other fauna (Wares 2002), the Georgia–South Carolina province (labeled Georgia in Fig. 1), and the Florida province.
Map of sampled locations for this study of six salt marsh invertebrate species. Regional delineations based on biogeographic results of Engle and Summers (1999). Some species are only sampled from a subset of indicated sites (see Appendix); for example, L. irrorata was only recovered from Corson Inlet, NJ in the UV province and is also the only species included from Cedar Key, FL (on the Gulf Coast). Samples from the GCE LTER site are grouped into upstream, midmarsh, and coastal locations as shown
Sampling
Figure 1 also illustrates the sampled marsh habitats (circles) for our study from each of the four provinces. Locations sampled in each province include UV (Wellfleet, MA, 41.903° N, 70.080° W; Manchester, MA, 43.575° N, 70.715° W; Corson’s Inlet, NJ, 39.211° N, 74.486° W), LV (Hooper Island, MD, 38.160° N, 76.110° W), and FL (St. Augustine, FL, 29.880° N, 81.330° W; Cedar Key, FL, 29.140° N, 83.030° W). Details of the Georgia sampling sites are in Robinson et al. (2009). Because of physiological and/or sampling constraints, not all species were available from the same sample locations within each province. For the purpose of broad-scale phylogeographic analysis, samples of a given species from each province are grouped together and not analyzed at the local scale, with the exception of the eight sites from the GCE-LTER site at Sapelo Island, Georgia, which are instead grouped by their hydrological position in the LTER into upstream, midmarsh, and coastal GCE samples.
At each site, we collected up to 20 specimens from each location for each of six species: bivalves G. demissa (average sample n = 15 per location) and C. virginica (average n = 20), gastropods I. obsoleta (average n = 17) and L. irrorata (average n = 21), and decapod crabs U. pugnax (average n = 19) and U. pugilator (average n = 18). The majority of these species are intimately associated with the salt marsh habitat, although some (C. virginica for example) are ubiquitous members of the nearshore intertidal community in general. These species were chosen primarily based on their abundance across this large geographic range. Sample sizes (in numbers of sequences) for each species are provided in Table 1. Tissues were individually labeled and preserved in 95% ethanol.
Data Collection
DNA was extracted using the Puregene DNA Purification Kit (Gentra Systems) or a modified cetyltrimethylammonium bromide isolation protocol (Doyle and Doyle 1987) and quantified using a Nanodrop spectrophotometer. Polymerase chain reaction amplifications were performed on the mitochondrial cytochrome c oxidase I (mtCOI) using primers from Folmer et al. (1994) and standard conditions as in Wares and Cunningham (2005) with an annealing temperature of 40°C. Amplified products were prepared and sequenced using an ABI 3730xl automated sequencer and edited using CodonCode Aligner 2.0.4. Sites with a PHRED score (Ewing and Green 1998) <20 were considered ambiguous and coded as such. Best-fit substitution models were determined for each data set using PAUP* 4.0b10 (Swofford 2002) and Modeltest 3.7 (Posada and Crandall 1998).
Data Analysis
Summary statistics, including the number of alleles, nucleotide diversity (π), and the standard neutrality test Tajima’s D, hereafter D T (Tajima 1989), were calculated using DNAsp v.5.0 (Librado and Rozas 2009) for each species at the regional and site level. It is typically assumed that under selective neutrality (the gene region has not been affected by a selective sweep or has other fitness consequences) and demographic equilibrium (population has had a stable size), D T will be approximately zero. Significant deviations from neutral expectations were confirmed using standard coalescent simulation methods, also implemented in DNAsp (1,000 replicates). Population differentiation statistics were calculated across all sites and among contiguous biogeographic provinces. In this case, the S nn “nearest neighbor” statistic of Hudson (2000) is used because of the high locus variability and modest sample size of each species data set. S nn is a sequence-based statistic that measures how often “nearest neighbor” sequences (haplotypes that are most similar to one another) are found in the same geographic location. Significance of the nearest-neighbor statistic was determined via permutational test (1,000 replicates).
Phylogeographic structure within each species was assessed via analysis of molecular variance (AMOVA), a nonparametric test that quantifies variation in and among populations as well as in and among regional groups of populations (Excoffier et al. 1992). This approach generates pairwise estimates of genetic variance among populations (F st) as well. Group structure for AMOVA was based on biogeographic regions established by Engle and Summers (1999) and implemented in Arlequin 3.11 (Excoffier et al. 2005). All calculations used an appropriate mutational model to correct for substitution saturation. Serial analysis of large-scale structure was performed as in Dupanloup et al. (2002), with each of the three possible province-level structures imposed on the analysis and the grouping that resulted in the highest levels of genetic variance among regions (F ct) chosen for further AMOVA calculations. As described above, differences among sites within biogeographic provinces are only analyzed within the GCE region comparing upstream, midmarsh, and coastal GCE sites via pairwise F st.
Results
A total of 687 COI sequences were analyzed from six invertebrate species from coastal salt marshes (Table 1). Using likelihood ratio tests to distinguish the best-fit substitutional models—essential to ensure that mutational saturation (i.e., at third codon positions) is not masking the divergence of populations—indicated that four of the six species were fit by an HKY + Γ model (Hasegawa et al. 1985). The other two species (U. pugnax and I. obsoleta) were fit by similar models with only small additional likelihood ratio penalties relative to HKY + Γ, so we chose this uniform model (with species-specific α parameters) for tractable analysis in both Arlequin 3.11 and DNAsp v.5.0. Basic diversity measures (π, D T, S nn) for each species (including data from all regions) are indicated in Table 1.
Atlantic Salt Marsh Genetic Diversity and Structure
The highest median community-wide diversity was found in the GCE samples (Fig. 2), while the lowest values that were from the UV region. Overall, there is a modest increase in genetic diversity from north to south at the community level in three out of six species (G. demissa, C. virginica, and U. pugnax); however, no trend is observed in the remaining species (Fig. 2; Table 2).
Provincial differentiation at mitochondrial COI gene region for six marsh invertebrate species (Gd G. demissa, Cv C. virginica, Io I. obsoleta, Li L. irrorata, Ug U. pugilator, Up U. pugnax). For each regional pairwise comparison, S nn is shown with across-species mean indicated with dashed line (global S nn for each species in Table 1). Asterisks denote significant differentiation based on permutational test of the S nn statistic. In the case of U. pugilator, populations in Virginia, Georgia, and Florida were monomorphic and so no statistic was calculated (marked with an a; the expectation of S nn = 0.50 is shown with a dashed bar). In the case of U. pugnax, insufficient data remained after quality control from the Florida site and so the statistic was not calculated (marked with a b). Inset box plot is of nucleotide diversity (π) across all six species by province (see Table 4)
Phylogeographic structure in this salt marsh community is minimal. However, the regional pairwise measure of differentiation S nn increased from north to south (Fig. 2; Table 3), but with no clear trend when individual species are considered. The only significant pairwise S nn contrasts are between the UV and LV regions in G. demissa and U. pugilator (noting that populations of U. pugilator other than in the UV region appear to be monomorphic at this locus), suggesting some concordance with previous phylogeography in this region; but the mean across-species S nn only increases in more southern contrasts if the taxa with monomorphic data are not included in the average (Fig. 2).
AMOVA was applied to each species data set, to determine in part whether there is significant structure at either local or regional levels and to identify what regional barriers might play the most important roles. Table 4 shows that only U. pugilator exhibits significant regional structure (F ct of 0.91, p < 0.05), while both U. pugilator and G. demissa have elevated overall genetic structure (F st for G. demissa of 0.10, p < 0.05; F st for U. pugilator of 0.90, p < 0.01). Spatial AMOVA (grouping contiguous sets of populations to find the strongest value of F ct) suggested no significant pattern when the FL region was compared to all other regions; when the GA/FL regions were compared to the UV/LV regions, only G. demissa and U. pugilator had positive F ct values (F ct for G. demissa of 0.15, n.s.; F ct for U. pugilator 0.14, n.s.); when all regions were compared to UV, again only G. demissa and U. pugilator had positive F ct values, with the only significant result being for U. pugilator (F ct for G. demissa of 0.20, n.s.; F ct for U. pugilator of 0.91, p < 0.05). No significant structure was found within the GCE domain except for a single moderate pairwise F st (0.11) in U. pugnax between the GCE coastal and midmarsh sites (Appendix; Robinson et al. 2009). Some significant pairwise F st values are also reported for C. virginica between the FL population and more northern populations (Appendix).
Demographic patterns in each species were also assessed using values of D T to test for population expansion and/or deviations from neutral expectations. Across the entire sampled range, four of six species presented significant negative values of D T (Table 1), although at the regional level, many of these samples were not significantly different from neutral expectations (Table 2). Samples from the GCE-LTER locations tended to show more negative D T values than other regional samples in four of six species (Table 2).
Discussion
Structure and Community Assembly in the Atlantic Salt Marsh
Although our results tend to corroborate earlier studies and biological expectations in showing only limited genetic structure for these six salt marsh invertebrates, there is nevertheless substantial deviation from panmixia in this community. First, there is some indication that at least two species may exhibit phylogeographic differentiation that is concordant with previous results spanning the UV/LV boundary around LIS and along the New Jersey coast (reviewed in Wares 2002; Haney et al. 2007). Both the bivalve G. demissa and the fiddler crab U. pugilator appear to harbor strong allele frequency shifts in this region corroborated with higher and significant values of S nn, F st, and F ct (Tables 3 and 4). In the case of G. demissa, there are a few groups of divergent haplotypes found in the UV samples that are likely to be driving the signal of divergence; in the U. pugilator samples, typical haplotype diversity is found in the UV region (Table 1) but no haplotype diversity in more southern populations, and the single haplotype present in the LV, GCE, and FL regions is not found in our UV sample.
The key feature of these observations is their concordance with so much previous evidence (Wares 2002) for some sort of deep differentiation across the LIS region. Genetic differentiation between the UV and LV regions has been detected in tidal salt marsh fish species (Cyprinodon variegatus, Haney et al. 2007), estuarine fish species (Fundulus heteroclitus, Smith et al. 1998; Paralichthys dentatus, Jones and Quattro 1999), and marine intertidal species like Marenzellaria viridis (Bastrop et al. 1998), Idotea baltica (Wares 2001), Amphithoe longimana (Sotka et al. 2003), and Gammarus tigrinus (Kelly et al. 2006). This consistent genetic pattern suggests some ecological relevance (Schmidt et al. 2008); though the defining environmental factors that are restricting gene flow may not yet be clear (Wares 2002), there are too many species and genetic transitions in this region to ignore as a component of community assembly. For example, one species with a very similar phylogeographic pattern to U. pugilator may be the sheepshead minnow (C. variegatus; Haney et al. 2007). In this species, there are “northern” haplotypes (north of LIS) with extremely limited geographic distributions (only found in the UV region of Engle and Summers 1999), even though many haplotypes are quite uniformly distributed across populations to the south. While historical isolation caused by climate change (i.e., glaciation) may be responsible for such patterns in part (Maggs et al. 2008), strong environmental gradients associated with the LIS region (Wares 2002; Schmidt et al. 2008) indicate that local adaptation—with the potential for strong effects on metacommunity dynamics (Leibold et al. 2004)—could be important in the northernmost portions of the Atlantic salt marsh ecosystem, at least for G. demissa and U. pugilator.
In addition to these two examples, the structure of the salt marsh community overall suggests minor spatial limitations to gene flow, as the overall nearest-neighbor statistic S nn deviates significantly from panmixia in three species (Table 1). Across all six species, the average S nn is 0.33 ± 0.10 (meaning that most closely related haplotypes have ∼1/3 probability of being recovered from the same sample site), with the expectation for panmixia across six collection sites of 0.17 (0.25 if you pool the GCE samples; Robertson and French 2007). The concordance of significant results between traditional AMOVA/F st-based approaches and Hudson’s (2000) S nn statistic indicates, however, that future work should be focused on two “significantly structured” invertebrate species and that overall the community south of LIS (across the LV, GCE, and FL regions) seems to behave as a single unit with respect to community assembly.
Overall, there is a slight increase in divergence measures (S nn) from north to south (Fig. 2), with significant divergence between the UV and LV regions for two species. While the isolation between the UV and LV regions may be expected from previous studies (as discussed above), the latitudinal trend of increasing structure toward southern areas also reflects a consistent latitudinal diversification pattern that other studies have recovered (Martin and McKay 2004; Chek et al. 2003; Kelly and Eernisse 2007; Eo et al. 2008). Other than the UV/LV split, the only significant pairwise F st was detected in C. virginica between Floridian and UV sites (Appendix) and for some comparisons in the two Uca species (Appendix). Previous studies on C. virginica have shown strong differentiation across Cape Canaveral (Reeb and Avise 1990; Hare and Avise 1996, 1998; Hellberg et al. 2002), while the rest of the Atlantic coast exhibits little structure (Buroker 1983; Reeb and Avise 1990; Hoover and Gaffney 2005).
We established sampling at both broad regional scales, discussed above, and among local sites within the GCE domain, to ensure that local population structure associated with adaptation to inland habitats (lower salinity), increased retention for inland populations, or other mechanisms would not influence our overall assessment of connectivity in this community. Essentially, no significant patterns were recovered among the upstream, midmarsh, and coastal GCE sites (Robinson et al. 2009; Appendix), suggesting that hydrologic and physiological barriers do not strongly influence the diversity patterns of the community at larger scales.
Genetic Diversity in the Atlantic Salt Marsh
North American Atlantic salt marshes bear an interesting pattern of genetic diversity at the regional level, with the lowest average community-wide diversity found in the UV samples (Fig. 2); however, some species did not show any latitudinal pattern in genetic diversity. This latitudinal pattern could be a sampling effect, or could be related to predicted latitudinal effects on genetic diversity—several authors have proposed an increase in metabolism and, thus, the mutation rate, in populations at increased temperature exposure (e.g., latitudinal shifts; Rand 1994; Martin and Palumbi 1993; Gillooly et al. 2005). However, the diversity increase from north to south indicated across these six taxa is slight and inconsistent, with only two of six species showing what could be considered a linear increase (Table 2). This observation coincides with previous work investigating latitudinal patterns of genetic diversity (Taberlet 1998). Latitudinal gradients in genetic diversity have been observed in other US Atlantic coastal species. Bernatchez and Wilson (1998) assessed the influence of Pleistocene glaciations on genetic diversity by combining phylogeographic data of freshwater and anadromous fishes and found a significant increase in nucleotide diversity from north to south. This common pattern has also been recovered for other diverse taxa including Mercenaria mercenaria (Dillon and Manzi 1992), Alosa sapidissima (Bentzen et al. 1989; Nolan et al. 1991; Waldman et al. 1996), F. heteroclitus (Smith et al. 1998), Callinectes sapidus (McMillen-Jackson and Bert 2004), and C. variegatus (Haney et al. 2007). The lower diversity in the UV region could be attributed to several factors. First, the UV region was glaciated (Wares 2002), and postglacial recolonization is often considered to reduce genetic diversity (Hewitt 1996). Postglacial expansion into new territory was previously indicated to be an important driving force in patterns of reduced genetic diversity at the edges of species ranges in other coastal systems as well as along the east coast of the USA (Taberlet 1998; Hewitt 2000; Haney et al. 2007). However, this region does not exhibit the lowest (nor any significant) regional values of Tajima’s D (Table 2), and values of D T for the UV region are not significantly lower than mean observed values for these taxa (Wares, unpublished data), indicating little additional support for population expansion as a cause of lower diversity.
Habitat availability and population density may also be important for shaping latitudinal patterns of genetic diversity. The pattern of latitudinal variation is only consistent for G. demissa (Table 2) for which recruitment success is related to available Spartina substrate (Bertness and Grosholz 1985; Silliman and Bertness 2002). Salt marsh habitat, often dominated by Spartina, increases dramatically from north to south along the US Atlantic coast (Bertness 1999); this increase in available habitat could directly affect population sizes in species that depend on Spartina as substrate for recruitment and an energy source (Kreeger et al. 1988; Silliman and Bertness 2002). In addition, positive relationships between community diversity and genetic diversity have been observed for several systems (Vellend 2003; Cleary et al. 2006), including the salt marsh communities in Georgia (Robinson et al. 2009).
Analysis of polymorphism data also provides insight into the demographic histories of these species. Four of the six species studied here exhibit consistently negative (and often significant) values for D T (Table 1). Negative values of D T indicate an excess of rare alleles relative to the expectation under neutrality and demographic stability (Tajima 1989). The most negative values were found in the Georgia samples, which pooled a larger set of samples, perhaps illustrating the effect of increased sampling on recovery of rare (perhaps transient) alleles (Wakeley and Takahashi 2003). Our polymorphism data were objectively evaluated using standard quality scores (Ewing and Green 1998), so it is unlikely that polymerase error could contribute significantly to this pattern. Nevertheless, for all species except for L. irrorata, the effect is not as extreme in the subpopulations of the Georgia region. Increased sample size may be more likely to recover additional rare haplotypes in general, but the consistent significantly negative pattern across subsamples as well as pooled samples in the Georgia populations of L. irrorata are consistent with a population expansion (Pyhäjärvi et al 2007), perhaps associated with the extraordinary density of habitat in this region (Bertness 1999) and other changes in the historical ecology of the region.
A Genealogical Perspective on the Atlantic Salt Marsh and Implications for Policy and Management
The salt marsh community along the US Atlantic coast overall has only modest phylogeographic structure, with some important caveats. This suggests that propagules may recruit to most sites from diverse sources, and thus, the marsh inhabitants south of LIS behave as a single community. However, it is important to recognize that in the face of probable asymmetric dispersal of larvae (Lentz 2008), a number of factors may influence the diversity and divergence of populations. What appears to be panmixia may in fact be the effect of a metapopulation dynamic in which only “upstream” populations truly drive the diversity of the ‘downstream’ sites (Pringle and Wares 2007; Wares and Pringle 2008), and larval behavior may play a more significant role in maintaining self-sustainable populations (Sanford et al. 2006).
Given the concordant divergence of two of our six species with previous assessments of isolation across the LIS region, there is more work needed to determine whether latitudinal effects such as that of water temperature on metabolism play a role in latitudinal patterns of diversity and divergence (Sanford et al. 2006; T.M. Bell and J.P. Wares, unpublished data). The only way to appropriately control among multiple species for comparisons under such hypotheses is to ensure that they are collected, evaluated, and analyzed in comparable ways (Bermingham and Moritz 1998). For species in this community that have already been analyzed genetically, our work has confirmed those results and placed them in the context of the broader invertebrate community. Knowing that the allelic composition of these six species is similar across a large geographic range leads to questions of whether all sites act equally as larval retentive source populations and whether variation in ecological interactions—including recruitment patterns, competition vs facilitation, and overall abundance—is then due to locally adaptive or other abiotic features (Haydon et al. 1994; Bruno et al. 2003). These influences may vary in strength latitudinally, as expected for a community with such tight species interactions (Bertness and Callaway 1994; Bruno et al. 2003; Silliman and Bertness 2002).
Overall, the minimal genetic structure of this sample of the salt marsh community might appear a disappointing result. However, the elements that make the Atlantic salt marsh community of great interest to researchers evaluating latitudinal and stress-related changes in species interactions across distant study sites (Bortolus and Iribarne 1999; Bruno and Bertness 2001; Pennings et al. 2001; Silliman and Bertness 2002; Salgado and Pennings 2005) make it important that we assess the degree to which these changes are due to the interaction of regionally distinct gene pools or are driven by species–species and species–environment interactions. As an example, the intertidal habitats of the northwestern Pacific Ocean also exhibit differing ecological dynamics among species at different latitudes, with Pisaster ochraceus acting as keystone predator (Paine 1974) north of Cape Blanco (42° N) and interactions limited by recruit density south of this region (Connolly et al. 2001). The shift in density is oceanographically driven, with profound effects on the ecological interactions among species, and there is little to no evidence for genetic differences among populations north and south of Cape Blanco (e.g., Sanford et al. 2003; Sotka et al. 2004, Wares and Castañeda 2005; Harley et al. 2006; Ort and Pogson 2007). While our assessment of the potential for local adaptation in this study is not complete, our results suggest a similar situation to that seen on the Pacific coast for Atlantic salt marshes. Any latitudinal variation in interactions that exist may be driven by the environment rather than the genetic composition of populations. It is important to note that gene flow among populations does not preclude adaptation to local conditions; however, isolation, either in time or space, greatly facilitates the process. At least two species show support for a well-recognized pattern (Wares 2002; Haney et al. 2007) of phylogeographic discontinuity between the UV and LV biogeographic regions, indicating that the interaction between evolutionary and ecological dynamics across this coastal habitat is nevertheless complex. Future work should continue to focus on the mechanisms that maintain the genetic differentiation across Long Island Sound (Wares 2002) and the impact of abiotic gradients on ecological interactions in this model system.
References
Avise, J. 1992. Molecular population structure and the biogeographic history of a regional fauna: A case history with lessons for conservation biology. Oikos 63: 62–76.
Avise, J. 2000. Phylogeography: the history and the formation of the species. Cambridge: Harvard University Press.
Bastrop, R. K. Jurss, and C. Sturmbauer. 1998. Cryptic species in a marine polychaete and their independent introduction from North America to Europe. Molecular Biology and Evolution 15: 97–103.
Bentzen, P. G.C. Brown, and W.C. Leggett. 1989. Mitochondrial DNA polymorphism, population structure, and life history variation in American shad (Alosa sapidissima). Canadian Journal of Fisheries and Aquatic Sciences 46: 1446–1454.
Bermingham, E. and C. Moritz. 1998. Comparative phylogeography: Applications and concepts. Molecular Ecology 7: 367–369.
Bernatchez, L. and C. Wilson. 1998. Comparative phylogeography of Neartic and Paleartic fishes. Molecular Ecology 7: 431–452.
Bertness, M. 1999. The ecology of the Atlantic shorelines. Sunderland: Sinauer Associates. 417p.
Bertness, M. and E. Grosholz. 1985. Population dynamics of the ribbed mussel, Geukensia demissa: The cost and benefits of an aggregated distribution. Oecologia 67: 192–204.
Bertness, M.D. and R.M. Callaway. 1994. Positive interactions in communities. Trends in Ecology and Evolution 9: 191–193.
Bilton, D.T. J. Paula, and D.D. Bishop. 2002. Dispersal, genetic differentiation, and speciation in estuarine organisms. Estuarine Coast and Shelf Science 55: 937–952.
Blum, M. J. Bando, M. Katz, and D. Strong. 2007. Geographic structure, genetic diversity and source of tracking of Spartina alterniflora. Journal of Biogeography 34: 2055–2069.
Bortolus, A. and O. Iribarne. 1999. Effects of the SW Atlantic burrowing crab Chasmagnatus granulata on a Spartina salt marsh. Marine Ecology Progress Series 178: 78–88.
Bruno, J. and M. Bertness. 2001. Habitat modification and facilitation in benthic marine communities. In Marine community ecology, ed. M. Bertness, S. Gaines, and M. Hay, 201–218. Sunderland: Sinauer.
Bruno, J. J. Stachowicz, and M. Bertness. 2003. Inclusion of facilitation into ecological theory. Trends in Ecology and Evolution 18: 119–125.
Buroker, N. 1983. Population genetics of the American oyster Crassostrea virginica along the Atlantic coast and the Gulf of Mexico. Marine Biology 75: 99–112.
Byers, J.E. and J.M. Pringle. 2006. Going against the flow: Retention, range limits and invasions in advective environments. Marine Ecology Progress Series 313: 27–41.
Chek, A. J. Austin, and S. Lougheed. 2003. Why is there a tropical–temperate disparity in the genetic diversity and taxonomy of species?. Evolutionary Ecology Research 5: 69–77.
Christy, J. 2003. Reproductive timing and larval dispersal on intertidal crabs: The predator avoidance hypothesis. Revista Chilena de Historia Natural 76: 177–185.
Cleary, D.F.R. C. Fauvelot, M.J. Genner, S.B.J. Menken, and A.O. Mooers. 2006. Parallel responses of species and genetic diversity to El Niño Southern Oscillation-induced environmental destruction. Ecology Letters 9: 304–310.
Connolly, S. B. Menge, and J. Roughgarden. 2001. A latitudinal gradient in recruitment of intertidal invertebrates in the Northeast Pacific ocean. Ecology 82: 1789–1813.
Dawson, M. 2001. Phylogeography in coastal marine animals: A solution from California? Journal of Biogeography 28: 723–736.
Dillon, R. and J. Manzi. 1992. Population genetics of the hard clam (Mercenaria mercenaria), at the northern limit of its range. Canadian Journal of Fisheries and Aquatic Sciences 49: 2574–2578.
Domeier, M. 2002. A potential larval recruitment pathway originating from Florida marine protected area. Fisheries Oceanography 13: 287–294.
Doyle, J. and J. Doyle. 1987. Genomic plant DNA preparation from freshwater tissue—CTAB method. Phytochemical Bulletin 19: 11.
Dupanloup, I. S. Schneider, and L. Excoffier. 2002. A simulated annealing approach to define the genetic structure of populations. Molecular Ecology 11: 2571–2581.
Engle, V. and J. Summers. 1999. Latitudinal gradients in benthic community composition in western Atlantic estuaries. Journal of Biogeography 26: 1007–1023.
Eo, S. J. Wares, and J. Carroll. 2008. Population divergence in plant species reflects latitudinal biodiversity gradients. Biology Letters 4: 382–384.
Ewing, B. and P. Green. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Research 8: 186–194.
Excoffier, L. P.E. Smouse, and J.M. Quattro. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131: 479–491.
Excoffier, L. G. Laval, and S. Schneider. 2005. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1: 47–50.
Felder, D. and J. Staton. 1994. Genetic differentiation in Trans-Floridian species complexes of Sesarma and Uca (Decapoda: Brachyura). Journal of Crustacean Biology 14: 191–209.
Folmer, O. M. Black, W. Hoeh, R. Lutz, and R. Vrijenhoek. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Gallagher, S. and G. Reid. 1974. Reproductive behavior and early development in Littorina scabra angulifera and Littorina irrorata (Gastropoda:Prosobranchia) in the Tampa Bay region of Florida. Malacological Review 7: 105–125.
Gillooly, J. A. Allen, G. West, and J. Brown. 2005. The rate of DNA evolution: Effects of body size and temperature on the molecular clock. Proceedings of the Natural Academy of Sciences USA 102: 141–145.
Haney, R.A. B.R. Silliman, A.J. Fry, C.A. Layman, and D.M. Rand. 2007. The Pleistocene history of the sheepshead minnow (Cyprinodon variegatus): Non-equilibrium evolutionary dynamics within a diversifying species complex. Molecular Phylogenetics and Evolution 43: 743–754.
Hare, M. and J. Avise. 1996. Molecular genetic analysis of a stepped multilocus cline in the American oyster (Crassostrea virginica). Evolution 50: 2305–2315.
Hare, M. and J. Avise. 1998. Population structure in the American oyster as inferred by nuclear gene genealogies. Molecular Biology and Evolution 15: 119–128.
Hare, M. C. Guenter, and W.F. Fagan. 2005. Nonrandom larval dispersal can steepen marine clines. Evolution 59: 2509–2517.
Harley, C.D.G. M.S. Pankey, J.P. Wares, R.K. Grosberg, and M.J. Wonham. 2006. Color polymorphism and genetic structure in the sea star Pisaster ochraceus. Biological Bulletin 211: 248–262.
Hasegawa, M. K. Kishino, and T. Yano. 1985. Dating the human–ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22: 160–174.
Haydon, D. B. Crother, and E. Pianka. 1994. New directions in biogeography? Trends in Ecology and Evolution 9: 403–406.
Hellberg, M. R. Burton, J. Niegel, and S. Palumbi. 2002. Genetic assessment of connectivity among marine populations. Bulletin of Marine Science 70: 273–290.
Hewitt, G. 1996. Some genetic consequences of ice ages, and their role in divergence and speciation. Biological Journal of the Linnean Society 58: 247–276.
Hewitt, G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405: 907–913.
Hoover, C. and P. Gaffney. 2005. Geographic variation in nuclear genes of the eastern oyster, Crassostrea virginica Gmelin. Journal of Shellfish Research 24: 103–112.
Hubbell, S.P. 2001. The unified neutral theory of biodiversity and biogeography. Princeton: Princeton University Press. 375pp.
Hudson, R. 2000. Snn, a new statistic to detect genetic differentiation. Genetics 155: 2011–2014.
Jones, W.J. and J.M. Quattro. 1999. Genetic structure of summer flounder (Paralichthys dentatus) populations north and south of Cape Hatteras. Marine Biology 133: 129–135.
Kelly, R. and D. Eernisse. 2007. Southern hospitality: A latitudinal gradient in gene flow in the marine environment. Evolution 61: 700–707.
Kelly, D. H. MacIsaac, and D. Heath. 2006. Vicariance and dispersal effects on phylogeographic structure and speciation in a widespread estuarine invertebrate. Evolution 60: 257–267.
Kreeger, D. C. Langdon, and R. Newell. 1988. Utilization of refractory cellulosic carbon derived from Spartina alterniflora by the ribbed mussel Geukensia demissa. Marine Ecology Progress Series 42: 171–179.
Lee, T. and D. O’ Foighil. 2005. Placing the Floridian marine genetic disjunction into a regional evolutionary context using the scorched mussel Brachidontes exustus, species complex. Evolution 59: 2139–2158.
Leibold, M.A. M. Holyoak, M. Mouquet, P. Amarasekare, J.M. Chase, M.F. Hoopes, R.D. Holt, J.B. Shurin, R. Law, D. Tilman, M. Loreau, and A. Gonzalez. 2004. The metacommunity concept: A framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Lentz, S. 2008. Observations and a model of the mean circulation over the middle Atlantic bight continental shelf. Journal of Physical Oceanography 38: 1203–1221.
Librado, P. and J. Rozas. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452.
Maggs, C.A. R. Castilho, D. Foltz, C. Henzler, M.T. Jolly, J. Kelly, J. Olsen, K.E. Perez, W. Stam, R. Vainola, F. Viard, and J. Wares. 2008. Evaluating signatures of glacial refugia for North Atlantic benthic marine taxa. Ecology 89: S108–S122.
Marko, P. 2004. ‘What’s larvae got to do with it?’ Disparate patterns of post-glacial population structure in two benthic marine gastropods with identical dispersal potential. Molecular Ecology 13: 597–611.
Marko, P.B. and K.R. Barr. 2007. Basin-scale patterns of mtDNA differentiation and gene flow in the bay scallop Argopecten irradians concentricus. Marine Ecology Progress Series 349: 139–150.
Martin, D. and J. McKay. 2004. Latitudinal variation in genetic divergence of population and the potential for future speciation. Evolution 58: 938–945.
Martin, A. and S. Palumbi. 1993. Body size, metabolic rate, generation time and the molecular clock. Proceedings of the National Academy of Science USA 90: 4087–4091.
McMillen-Jackson, A. and T. Bert. 2004. Mitochondrial variation and population structure of the blue crab Callinectes sapidus. Marine Biology 145: 769–777.
Menzel, W. 1991. Estuarine and marine bivalve mollusk culture, 362pp. Boca Raton: CRC.
Moritz, C. and D.P. Faith. 1998. Comparative phylogeography and the identification of genetically divergent areas for conservation. Molecular Ecology 7: 419–429.
Nolan, K. J. Grossfield, and I. Wirgin. 1991. Discrimination among Atlantic coast populations of American shad (Alossa sapidissima) using mitochondrial DNA. Canadian Journal of Fisheries and Aquatic Sciences 48: 1724–1734.
Ort, B.S. and G.H. Pogson. 2007. Molecular population genetics of the male and female mitochondrial DNA molecules of the California sea mussel, Mytilus californianus. Genetics 177: 1087–1099.
Paine, R.T. 1974. Intertidal community structure: Experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15: 93–120.
Paulay, G. and C. Meyer. 2006. Dispersal and divergence across the greatest ocean region: Do larvae matter? Integrative and Comparative Biology 46: 269–281.
Pennings, S. E. Siska, and M. Bertness. 2001. Latitudinal differences in plant palatability in Atlantic coast salt marshes. Ecology 82: 1344–1359.
Posada, D. and K. Crandall. 1998. Modeltest: Testing the model of DNA substitution. Bioinformatics 14: 817–818.
Pringle, J. and J. Wares. 2007. The maintenance of alongshore variation in allele frequency in a coastal ocean. Marine Ecology Progress Series 335: 69–84.
Pyhäjärvi, T. M. del Rosario Garcia-Hill, T. Knurr, M. Mikkonen, W. Wachowiac, and O. Savolainen. 2007. Demographic history has influenced nucleotide diversity in European Pinus silvestris populations. Genetics 177: 1713–1724.
Rand, D. 1994. Thermal habitat, metabolic rate and evolution of mtDNA. Trends in Ecology and Evolution 9: 125–131.
Reeb, C. and J. Avise. 1990. A genetic discontinuity in a continuously distributed species: Mitochondrial DNA in the American oyster, Crassostrea virginica. Genetics 124: 397–406.
Richmond, C. and S. Woodin. 1996. Short-term fluctuations in salinity: Effects on planktonic invertebrate larvae. Marine Ecology Progress Series 133: 167–177.
Robertson, N.L. and R. French. 2007. Genetic structure in natural populations of barley/cereal yellow dwarf virus isolates from Alaska. Archives of Virology 152: 891–902.
Robinson, J.D., E. Díaz-Ferguson, M. Poelchau, S. Pennings, T.D. Bishop, and J.P.Wares. 2009. Multiscale diversity in the marshes of the Georgia Coastal Ecosystems LTER. Estuaries and Coasts. doi:10.1007/s12237-009-9188-2.
Salgado, C. and S. Pennings. 2005. Latitudinal variation in palatability of salt-marsh plants: Are differences constitutive? Ecology 86: 1571–1579.
Sanford, E. M.S. Roth, G.C. Johns, J.P. Wares, and G.N. Somero. 2003. Local selection and latitudinal variation in a marine predator–prey interaction. Science 300: 1135–1137.
Sanford, E. S. Holzman, R. Haney, D. Rand, and M. Bertness. 2006. Larval tolerance, gene flow, and the northern geographic range limit of fiddler crabs. Ecology 87: 2882–2894.
Schmidt, P. E. Serrao, G. Pearson, C. Riginos, P. Rawson, T. Hilbish, S. Brawley, G. Trussell, E. Carrington, D. Whetey, J. Grahame, F. Bonhome, and D. Rand. 2008. Ecological genetics in the North Atlantic: Environmental gradients and adaptation at specific loci. Ecology 89: 91–107.
Siegel, D.A. B.P. Kinlan, B. Gaylord, and S. Gaines. 2003. Lagrangian descriptions of marine larval dispersion. Marine Ecology Progress Series 260: 83–96.
Silliman, B. and M. Bertness. 2002. A trophic cascade regulates salt marsh primary production. Proceedings of the National Academy of Sciences USA 99: 10500–10505.
Smith, M.W. R.W. Chapman, and D.A. Powers. 1998. Mitochondrial DNA analysis of Atlantic coast, Chesapeake Bay, and Delaware Bay populations of the teleost Fundulus heteroclitus indicates temporally unstable distributions over geologic time. Molecular Marine Biology and Biotechnology 7: 79–87.
Sotka, E.E. J.P. Wares, and M.E. Hay. 2003. Geographic and genetic variation in feeding preference for chemically defended seaweeds. Evolution 57: 2262–2276.
Sotka, E.E. J.P. Wares, J.A. Barth, R.K. Grosberg, and S.R. Palumbi. 2004. Strong genetic clines and geographical variation in gene flow in the rocky intertidal barnacle Balanus glandula. Molecular Ecology 13: 2143–2156.
Swofford, D. 2002. Phylogenetic analysis using Parsimony (PAUP) v. 4.0b10. Sunderland: Sinauer Associates.
Taberlet, P. 1998. Biodiversity at the intraspecific level: The comparative phylogeographic approach. Journal of Biotechnology 64: 91–100.
Tajima, F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595.
Vellend, M. 2003. Island biogeography of genes and species. American Naturalist 162: 358–365.
Vellend, M. 2005. Species diversity and genetic diversity: Parallel processes and correlated patterns. American Naturalist 166: 199–215.
Wakeley, J. and T. Takahashi. 2003. Gene genealogies when sample size exceeds the effective size of a population. Molecular Biology and Evolution 20: 208–213.
Waldman, J.R. K. Nolan, J. Hart, and I.I. Wirgin. 1996. Genetic differentiation of three key anadromous fish populations of the Hudson River. Estuaries 19: 759–768.
Wares, J.P. 2001. Intraspecific variation and geographic isolation in Idotea balthica (Isopoda: Valvifera). Journal of Crustacean Biology 21: 1007–1013.
Wares, J.P. 2002. Community genetics in the Northwestern Atlantic intertidal. Molecular Ecology 11: 1131–1144.
Wares, J. and C. Cunningham. 2001. Phylogeography and historical ecology of the North Atlantic intertidal. Evolution 55: 2455–2469.
Wares, J.P. and A.E. Castañeda. 2005. Geographic range in Chthamalus along the west coast of North America. J Mar Biol Ass UK 85: 327–331.
Wares, J.P. and C. Cunningham. 2005. Isolation before glaciation in Balanus glandula. Biological Bulletin 208: 60–68.
Wares, J. and J. Pringle. 2008. Drift by drift: Effective population size is limited by advection. BMC Evolutionary Biology 8: 235.
Acknowledgments
The authors want to thank M. Cozad., S. Pankey., S. Pennings., D. Bishop., T. Bell., C. Zakas, and K. Dyer for technical and intellectual assistance during this research. C. Embach aided tremendously with technical editing and composition. We also thank G. Moyer at USFW Fish Technology Center for logistical support provided to E. Díaz-Ferguson. Two anonymous reviewers greatly improved the manuscript through their helpful comments. We also thank the University of Georgia Research Foundation (Wares), the National Science Foundation (DEB-054822, Wares and Silliman), the National Geographic Society Committee for Research and Exploration (Grant #8351-07, Wares), and also SEAGEP—Young Investigator Grant from the Mellon Foundation (Silliman) for funding this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edgardo Díaz-Ferguson and John D. Robinson have equal authorship responsibilities.
Appendix. Pairwise F st Values by Species
Appendix. Pairwise F st Values by Species
Triangular matrix of pairwise F st values for COI in C. virginica populations. Significant p values (p < 0.05 and p < 0.01) are indicated with one or two asterisks, respectively.
Population | Massachusetts (UV) | Virginia (LV) | GCE coastal | GCE midmarsh | GCE upstream |
|---|---|---|---|---|---|
Virginia | 0.02 | ||||
GCE coastal | −0.024 | 0.018 | |||
GCE midmarsh | −0.04 | −0.011 | 0.0053 | ||
GCE upstream | −0.026 | 0.02 | 0.0072 | 0.008 | |
Florida | 0.07* | 0.06 | 0.021 | −0.05 | −0.005 |
Triangular matrix of pairwise F st values for COI in G. demissa populations. Significant p values (p < 0.05 and p < 0.01) are indicated with one or two asterisks, respectively.
Population | Massachusetts (UV) | Virginia (LV) | GCE coastal | GCE midmarsh | GCE upstream |
|---|---|---|---|---|---|
Virginia | 0.05 | ||||
GCE coastal | 0.21** | −0.073 | |||
GCE midmarsh | 0.16** | −0.1 | −0.018 | ||
GCE upstream | 0.28** | −0.03 | 0.0008 | 0.0013 | |
Florida | 0.19* | −0.1 | −0.051 | −0.04 | −0.026 |
Triangular matrix of pairwise F st values for COI in I. obsoleta populations. Significant p values (p < 0.05 and p < 0.01) are indicated with one or two asterisks, respectively.
Population | New Jersey (UV) | Virginia (LV) | GCE coastal | GCE midmarsh | GCE upstream |
|---|---|---|---|---|---|
Virginia | 0.05 | ||||
GCE coastal | −0.135 | −0.058 | |||
GCE midmarsh | −0.158 | −0.048 | 0.0047 | ||
GCE upstream | −0.06 | 0.0054 | −0.035 | −0.02 | |
Florida | −0.03 | 0.0048 | −0.083 | −0.09 | −0.038 |
Triangular matrix of pairwise F st values for COI in L. irrorata populations. Significant p values (p < 0.05 and p < 0.01) are indicated with one or two asterisks, respectively.
Population | New Jersey (UV) | Virginia (LV) | GCE coastal | GCE midmarsh | GCE upstream |
|---|---|---|---|---|---|
Virginia | 0.04 | ||||
GCE coastal | −0.12 | −0.053 | |||
GCE midmarsh | −0.014 | −0.039 | 0.0032 | ||
GCE upstream | −0.059 | 0.0058 | −0.033 | −0.019 | |
Florida | −0.037 | 0.0077 | −0.076 | −0.089 | −0.034 |
Triangular matrix of pairwise F st values for COI in U. pugnax populations. Significant p values (p < 0.05 and p < 0.01) are indicated with one or two asterisks, respectively.
Population | Massachusetts (UV) | Virginia (LV) | GCE coastal | GCE midmarsh | GCE upstream |
|---|---|---|---|---|---|
Virginia | −0.04 | ||||
GCE coastal | −0.01 | 0.083 | |||
GCE midmarsh | −0.13 | −0.03 | 0.11** | ||
GCE upstream | 0.0036 | −0.0059 | 0.02 | 0.05 | |
Florida | N/A | N/A | N/A | N/A | N/A |
Triangular matrix of pairwise F st values for COI in U. pugilator populations. Only a single haplotype was found in all samples south of LIS, so F st is not calculated. Significant p values (p < 0.05 and p < 0.01) are indicated with one or two asterisks, respectively.
Population | Massachusetts (UV) | Virginia (LV) | GCE coastal | GCE midmarsh | GCE upstream |
|---|---|---|---|---|---|
Virginia | 0.85** | ||||
GCE coastal | 0.88** | – | |||
GCE midmarsh | 0.88** | – | – | ||
GCE upstream | 0.9** | – | – | – | |
Florida | 0.83** | – | – | – | – |
Rights and permissions
About this article
Cite this article
Díaz-Ferguson, E., Robinson, J.D., Silliman, B. et al. Comparative Phylogeography of North American Atlantic Salt Marsh Communities. Estuaries and Coasts 33, 828–839 (2010). https://doi.org/10.1007/s12237-009-9220-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-009-9220-6