Abstract
Species of the macroalgae Caulerpa sp. are increasingly being observed in meadows of the endemic Mediterranean seagrass Posidonia oceanica, and in particular Caulerpa taxifolia, has been considered as an invasive species leading to seagrass decline. Studies have so far failed to reveal the underlying mechanisms of the success of the macroalgae, and here, we examine how biogeochemical changes of the environment associated to indigenous (Caulerpa prolifera) and non-indigenous (Caulerpa racemosa and C. taxifolia) species affect the habitat of P. oceanica. Two of the species (C. prolifera and C. racemosa) affect the sediment biogeochemical conditions by increasing organic matter pools, microbial activity, and sulfide pools of the sediments, and limited effects were found for C. taxifolia. Biomass of the macroalgae contributed to the extent of impacts, and high sulfide invasion into the seagrasses and regression of the meadow were pronounced at the location with the highest Caulerpa biomass. This suggests that Caulerpa invasion contributes to seagrass decline probably because Caulerpa thrives better than the seagrasses in the modified environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased nutrient availability in the coastal zones, caused by eutrophication, increased sedimentation, increased rainfall, etc., are almost always followed by loss of seagrass habitat, and the competitive advantage of macroalgae for nutrients is considered a paradigm for seagrass decline (Valiela et al. 1997). The increasing focus on non-indigenous species (NIS) has provided additional evidence of macroalgae as major threats to habitat diversity including loss of seagrass meadows (Jousson et al. 2000; Pedersen et al. 2005). The spread of the green macroalgae, Caulerpa racemosa, invading the Mediterranean Sea from the Red Sea, and Caulerpa taxifolia, originally from Australia and accidentally released into the Mediterranean Sea, are considered to represent detrimental invasive events in the Mediterranean Sea, as widely colonized areas have been found in the whole basin interfering with native communities (Verlaque et al. 2000, 2004).
Although Caulerpa sp. is considered a threat to Posidonia oceanica (Ceccherelli et al. 2000), there are only few direct manipulative studies of interactions between Caulerpa species and P. oceanica and these show mixed results. For instance, C. racemosa negatively affected seagrass growth, whereas C. taxifolia had no measurable impact (Ceccherelli and Campo 2002; Ceccherelli and Sechi 2002; Piazzi et al. 2005; Piazzi and Ceccherelli 2006), and the mechanisms leading to the displacement of P. oceanica under pressure from high abundance of Caulerpa species thus remain unclear. An alternative, yet unexplored, possibility is that Caulerpa species may displace P. oceanica through the modification of sediment properties to generate an adverse environment for growth. Other macroalgae NIS, such as Sargassum muticum and Gracilaria vermiculophylla, have been found to increase the nutrient cycling due to faster decomposition of the detritus compared to native species (Pedersen et al. 2005; Tyler and McGlathery 2006). Chisholm and Moulin (2003) demonstrated that C. taxifolia stimulates N2 fixation due to release of photosynthetic metabolites through plant rhizoids, and these metabolites could also stimulate other microbial processes in the sediments, such as sulfate-reducing bacteria. Indeed, previous studies of sediment biogeochemical cycling in Caulerpa prolifera stands show significantly elevated sulfate reduction rates and increased pools of sulfide compared to those in adjacent seagrass meadows (Holmer et al. 2004). Hydrogen sulfide is highly toxic to plants, and threshold concentrations as low as 10 μM in the porewaters have been associated with the decline of P. oceanica populations (Calleja et al. 2007). Increased sulfide production and possible sulfide intrusion into P. oceanica as a result of Caulerpa sp. abundance in the meadows may thus pose a threat to the growth and survival of the seagrass.
The aim of the present study was to examine the possible effects of ecosystem modifications due to abundance of the macroalgae Caulerpa sp. in seagrass P. oceanica meadows in the Mediterranean. Observations of Caulerpa sp. presence in P. oceanica meadows are increasing around the Balearic Islands (A. Grau, personal communication) where most meadows are currently declining (Marbà et al. 2005). We evaluate the hypothesis that the effects of three different Caulerpa species (two non-indigenous C. racemosa and C. taxifolia and one native C. prolifera) on P. oceanica meadows may be mediated by the development of adverse sediment biogeochemical conditions to support P. oceanica growth. We hypothesize that Caulerpa abundance in seagrass meadows increases sediment pools of organic matter, enhances microbial activity in the sediments, and increases sulfide exposure of P. oceanica.
Materials and Methods
Study Sites
We elected to test the hypothesis through a comparative analysis across adjacent (within 10 m) communities of Caulerpa and Posidonia and corresponding mixing of the two species. The study was conducted in July 2005 in Mallorca (Balearic Island, Spain, Fig. 1) in three subtidal study locations dominated by P. oceanica (L.) Delille, where each of the three Caulerpa species were present. These three study locations are Cala Llonga (39°22.030′ N, 3°13.738′ E) with C. prolifera (Forsskaal) Lamouroux at 3.5-m depth, Cala d’Or (39°22.164′ N, 3°13.887′ E) where C. taxifolia (Vahl.) C. Agardh was merged with P. oceanica meadows at a water depth of 5 m, and Cala Estancia (39°32.17′ N, 2°42.57′ E) at 2.5-m deep with C. racemosa (Forsskaal) J. Agardh. Observations between 2003 and 2005 in Cala Llonga reveal that a nearby meadow of P. oceanica was declining while the C. prolifera cover increased, whereas the sampled P. oceanica meadow in Cala d’Or was in steady state or slightly expanding and the C. taxifolia cover remained constant (data not shown). No such observations are available from Cala Estancia.
Sampling and Handling of Samples
At each location, three 10-m-long transects were established and separated in four sections ranging from 1 to 3 m wide each, representing a different community: bare sediment (Bare), Caulerpa bed (Cau), mixed meadow with Posidonia and Caulerpa (Mix), and P. oceanica meadow (PO). The density of shoots of P. oceanica was obtained using two 400-cm2 quadrats, one on each side of the transect in the vegetated sections. For Caulerpa sp. biomass, SCUBA divers collected all plant material within the quadrat, whereas a subsample of ten P. oceanica shoots was collected from Mix and PO to minimize sampling impact on the seagrass meadows. An exception was at the Mix section at Cala Estància site, where C. racemosa was present at the border and not inside of the P. oceanica meadow, and the biomass was obtained from the border. In addition, five P. oceanica plants (separated into leaves, roots, and rhizome of Posidonia) were collected for analysis of stable sulfur isotopic composition (δ34S). The plants for stable sulfur isotopic composition analyses were rinsed in freshwater to remove salts and freeze-dried immediately after collection.
The algal and seagrass material used to estimate plant biomass was dried at 60°C for 48 h. For Caulerpa, estimates of biomass were based on fronds, and for P. oceanica, aboveground biomass was calculated as the product of P. oceanica shoot density and shoot mass.
One sediment core in acrylic core liners (i.d = 2.6 cm) with pre-drilled silicone-filled holes for each centimeter was collected at each section in each transect for determination of sulfate reduction rates. One additional core (i.d. = 4.3 cm) was collected in Bare, Cau, and PO sections in each transect for determination of sediment characteristics, including δ34S isotopic composition. The depth where the sulfide front was located was determined by inserting silver sticks into each of the sediment cores and leaving them for 6 h. The front was detected where a black precipitate of Ag2S was formed and the sulfide-free zone defined as the distance from the sediment surface to this mark. At each location, two vials with seawater were collected for determination of δ34S of seawater sulfate.
After sampling, SCUBA divers deployed six sediment traps in Bare, Cau, and PO sections with two replicate traps as described by Gacia et al. (1999) for 48 h. Each sediment trap consisted of five cylinders of transparent glass of a diameter of 1.6 cm with an aspect ratio of 5 in a gimbaled frame.
Analysis
Plants
After freeze drying, the plant material was homogenized. Analysis of δ34S was done by elemental analysis where 5 mg of dried plant tissue was added to 9 mg vanadium oxide, packed in tin capsules, and analyzed at Iso-analytical, UK.
SRR and Sulfur Pools
Sulfate reduction rates (SRR) were determined by the core injection technique (Jørgensen 1978) where 2 μl of 35S-sulfate (70 kBq) was injected at 1-cm intervals through predrilled silicone-filled holes within 1 h of collection and incubated for 2 h in darkness at in situ temperature. The incubation was terminated by sectioning the cores into 2-cm intervals down to 10-cm and 5-cm intervals to 15 cm then fixed in 1 M zinc acetate and frozen immediately. Sulfate reduction rates were obtained by the two-step distillation method by Fossing and Jørgensen (1989), which separates sulfides into acid volatile (AVS: porewater H2S and iron monosulfides) and chromium reducible sulfides (CRS: elemental sulfur and pyrite) pools. Radioactivity was counted on a Packard TriCarb 2000 scintillation counter and sulfide concentrations were determined spectrophotometrically according to Cline (1969).
Sediment Characteristics and Porewater
The top 0–2 cm of sediments were analyzed for particulate organic carbon (POC) and nitrogen (PON), whereas water content, density, and porewater were analyzed down to 15 cm. Sediment density was obtained by weight of a known volume, and the water content was obtained after drying overnight at 105°C. Porosity was calculated from sediment density and water content and used for calculation of sulfate reduction rates. Sediment POC and PON contents were determined by elemental analysis according to Kristensen and Andersen (1987). Porewater was extracted by centrifugation of a sediment pellet in double centrifuging tubes (1,500 rpm, 5–10 min) and stored frozen until analysis. The porewater concentration of sulfate was determined by a Dionex autosuppressed ion chromatograph equipped with a conductivity detector (ICS-2500) and used for calculation of sulfate reduction rates.
δ34S in Sediments and Seawater
Sedimentary sulfides for analysis of δ34S were extracted as described for the sulfate reduction rates, except for a change in the traps which contained AgNO3. Ag2S from the traps was filtered, and 0.3 g from both distillation steps was packed with 1 mg of vanadium oxide and analyzed for δ34S at Iso-analytical, UK. Samples to determine the δ34S of sulfate in seawater were prepared by centrifuging seawater (10 min, 1,500×g) followed by boiling of the supernatant under acidic conditions (3 M HCl) and precipitating sulfate with BaCl2 as BaSO4. Isotopic analyses of BaSO4 were made at Iso-analytical, UK.
δ34S in Seagrasses
Seagrasses take up sulfur from three different sources: seawater sulfate through the leaves, porewater sulfate, and/or sulfide through the roots (Rennenberg et al. 1984). Due to the bacterial fractionation by sulfate-reducing bacteria, the sulfide produced in the sediments is isotopically lighter (lower δ34S) than seawater and porewater sulfate (Kaplan et al. 1963). These differences allow quantification of sulfide invasion into the different parts of the seagrasses (e.g., Frederiksen et al. 2006). In order to estimate how much of the total sulfur in the plants was derived from sediment sulfide, the parameter F sulfide was estimated using the following mixing equation:
where δ34Stissue is the value measured in the leaf, rhizome or root, δ34Ssulfate is the value measured in the seawater, and δ34Ssulfide is the value measured in the AVS pool which includes porewater sulfide potentially invading the seagrasses. The CRS pool was used for C. taxifolia, as it was not possible to obtain sufficient material for analysis from the AVS pool. This may have underestimated the F sulfide with up to 3% based on lower δ34SCRS (−21.94‰) compared to δ34SAVS (average of C. prolifera and C. racemosa sites = −13.93‰).
Sedimentation Rate
In the laboratory, the contents of one to three cylinders from the sediment traps were combined and collected on a combusted, pre-weighed Whatman GF/F filter (final replication 2 to 5). Dry weight of total sediment deposition was obtained after drying the filters at 60°C to constant weight. Sedimentation rates were estimated according to Blomqvist and Håkanson (1981) and Hargrave and Burns (1979) as described in detail in Gacia et al. (1999). The trap material was analyzed for nutrient contents (POC and PON) as described above for the sediments.
Statistical Analysis
A two-way analysis of variance (ANOVA) model (Systat 7.0) was used to test differences in sediment characteristics, sedimentation, and plant characteristics between sections and locations. Prior to analysis, data were tested for homogeneity, and normality assumption was verified by a Shapiro–Wilks test. The data were log-transformed if necessary. The ANOVA tests included tests for interactions and were followed by a Tukey post hoc test with a significance level of p < 0.05.
Results
In the PO sections, no significant difference existed in P. oceanica shoot density among the three Caulerpa sp. locations (771 to 1,154 shoots per square meter; Table 1, Fig. 2). However, P. oceanica shoot density was significantly lower (45–58%) in the Mix sections that had both Caulerpa sp. and P. oceanica compared to the PO sections (Fig. 2). Similarly, the aboveground biomass of P. oceanica meadows in the PO section ranged between 874 and 1,974 g DW m−2 and was threefold higher than that of Mix sections (data not shown). Caulerpa frond biomass differed at the three locations, as C. prolifera attained the highest biomass (93 g DW m−2) compared to C. taxifolia (58 g DW m−2) and C. racemosa (8 g DW m−2; Fig. 2). At the C. prolifera location, the highest biomass was found in the Mix section, whereas the highest macroalgae biomass was observed in the Cau sections at the two other locations.
Average aboveground macroalgae biomass and seagrass shoot density at the three study locations (n = 3, ±SE). Bare unvegetated site, Cau Caulerpa section, Mix Caulerpa sp. and P. oceanica together, PO P. oceanica alone. Significant differences are given for P. oceanica. Small letters, significant (p < 0.05) difference between locations (C. prolifera vs. C. racemosa vs. C. taxifolia). Capital letters, significant difference between Mix and PO
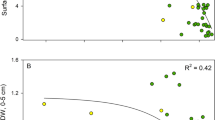
Sedimentation rates were not enhanced in the Cau section compared to the PO sections, but the sedimentation rates in Cau section for C. racemosa and C. taxifolia were higher compared to Bare (Tables 1 and 2, Figs. 3 and 4). The sedimentation varied significantly between locations, with highest rates at the C. prolifera location. Here, the Bare section had significantly higher sedimentation rate, and a high C/N ratio (18.8) compared to other sections indicating a relatively higher fraction of refractory material, which may be due to resuspension of sediments (Table 2). Despite the lack of difference in rates of sedimentation between vegetation type, the sediment conditions varied considerably, with much higher POC (up to 16 times higher) and PON contents (up to five times higher) in the Caulerpa-dominated sections compared to the P. oceanica sections at all three locations (Figs. 3 and 4). The average sediment C/N ratios were generally high in Cau sections compared to Bare and PO sections, except at the C. taxifolia location where the C/N ratio was lower compared to the PO section (16.3 compared to 30.3, Table 2).
Rates of sedimentation (upper panel) and sediment content of organic carbon (lower panel) at Bare, Cau, and PO sections (n = 3, ±SE). Significant differences are given for the sedimentation rate and not for the sediment POC due to lack of normality (Shapiro–Wilk p < 0.05). Small letters, significant (p < 0.05) difference between locations (C. prolifera vs. C. racemosa vs. C. taxifolia). Capital letters, significant (p < 0.05) difference between Bare, Cau, or PO sections
Rates of sedimentation (upper panel) and sediment content of organic nitrogen (lower panel) at Bare, Cau, and PO sections (n = 3, ±SE). Significant differences are given for the sedimentation rate (log-transformed) and not for the sediment PON due to lack of normality (Shapiro–Wilk p < 0.05). Small letters, significant (p < 0.05) difference between locations (C. prolifera vs. C. racemosa vs. C. taxifolia). Capital letters, significant (p < 0.05) difference between Bare, Cau, or PO sections
SRR were enhanced at the vegetated sections compared to Bare (Table 1, Fig. 5), and in particular presence of Caulerpa prolifera-enhanced SRR, which was about four times higher compared to the Bare section. The Bare sections generally reflected the sedimentation rates with highest rates at the C. prolifera location and lowest at C. taxifolia. Similarly, the pools of sulfides (TRS = AVS + CRS, where AVS <1% of TRS) were higher in the vegetated sections compared to Bare and followed the SRR. The accumulation of TRS at the Bare sections reflected the sedimentation rates and sulfate reduction rates with highest accumulation at the C. prolifera location. On the other hand, the sulfide front was consistently closest to the sediment surface in the Cau and Mix sections at all locations where sulfide was found already at 0.4- to 0.9-cm depth. At the C. prolifera location, no precipitate was found on the silver stick in the examined depth (=10 cm) in the PO section despite elevated TRS pools compared to the Bare and Cau sections.
Depth integrated sulfate reduction rates (SRR, 0–10 cm, n = 3, ±SE, upper), total pools of sulfide (TRS, mid), and the depth of the sulfide-free zone in the sediments (lower) at the three study locations. The sulfide front was >10 cm for P. oceanica section at the C. prolifera location. Significant differences are given for the sulfate reduction rates (log-transformed), sulfide pools and for the sulfide front. Small letters, significant (p < 0.05) difference between locations (C. prolifera vs. C. racemosa vs. C. taxifolia). Capital letters, significant (p < 0.05) difference between Bare, Cau, Mix, or PO sections (n = 1 for sulfide free zone at PO for C. prolifera and Bare at C. taxifolia)
The sulfate reduction rates were enhanced up to seven times in the Mix sections compared to the PO sections at two of the locations (C. prolifera and C. racemosa), whereas no significant differences between vegetation type were found at the location with C. taxifolia (Table 1). SRR were particularly high at the C. prolifera location where the highest Caulerpa biomass was found, and a positive correlation between Caulerpa biomass and SRR (R 2 = 0.64, p < 0.001) indicates that the abundance of macroalgae affects the sulfate reduction rates. Consistent with the high SRR, the TRS were high at the Mix sections at the C. prolifera and C. racemosa locations. Variability in sediment pools of sulfides was, however, not coupled to changes in Caulerpa biomass (regression analysis, p > 0.05).
The δ34S of the seawater sulfate ranged between 19.6‰ and 20.5‰ (Table 3). The AVS pools were most depleted at the C. prolifera location (−16.5‰), whereas the δ34SCRS pools ranged between −19.1‰ and −21.9‰. The δ34S of P. oceanica varied between the plant tissues, with the isotopic values in the roots and rhizomes more negative than those in the leaves (Table 4). The δ34S of P. oceanica rhizomes at the C. prolifera location were significantly lower compared to the two other locations (Table 1), and there were significant differences between δ34S at the Mix and the PO sections with higher values at the Mix section (p = 0.03). F sulfide followed the pattern observed for δ34S, although sedimentary sulfides were only detected in the leaves at PO section at the C. prolifera location (Table 4). The highest fraction of sulfides was found in the rhizomes and roots at the C. prolifera location where up to 40% of the total sulfur in the roots was derived from sedimentary sulfides. The contribution was up to 14% at the two other locations.
Discussion
The presence of Caulerpa sp. in P. oceanica meadows was coupled with a lower seagrass shoot density, suggesting either a negative effect of the macroalgae presence on the seagrass population dynamics or that the seagrasses were in decline, allowing for colonization by the macroalgae. There was no significant difference between the shoot densities in the PO sections at the three locations, whereas the shoot density was 45–58% lower in sections with Caulerpa sp. present. Seagrass population dynamics, e.g., assessed through counts in permanent plots or changes in cover, have not been measured at the three locations, but observations between 2003 and 2005 suggest that the P. oceanica population in Cala d’Or with C. taxifolia present was in steady state or slightly expanding, whereas in Cala Llonga with C. profilera, it was declining (decreased from 30% to 20% cover in 2 years; Terrados et al. 2009) along with an increase in C. prolifera cover (from 78% to 95%; Terrados et al. 2009). There are no observations for the C. racemosa location. The success of C. prolifera in Cala Llonga may thus be amplified by the modifications taking place in the seagrass meadow, similar to observations by Chisholm et al. (1997) for C. taxifolia in degrading P. oceanica meadows.
The presence of C. prolifera, and to some extent also C. racemosa, in P. oceanica meadows coincided with significant differences in sediment biogeochemical conditions, with enhanced sulfate reduction rates, shallower sulfide front depth, and increased pools of sulfides in the sediments compared to seagrass sediments. Sulfate reduction rates in the mixed macroalgae–seagrass sections and in the C. prolifera stands were similar to observations from similar habitats underneath fish farm net cages in the Mediterranean (Holmer and Frederiksen 2007). Fish farm sediments receive extremely high organic loading compared to natural sediments, resulting in significantly enhanced sulfate reduction rates (Holmer and Frederiksen 2007). Similar high sulfate reduction rates have been found in C. prolifera stands in a nearby bay (Holmer et al. 2004), suggesting that high abundance of C. prolifera stimulates the sulfate reduction rates in the sediments. The sulfate reduction rates were not as high at the C. racemosa location, probably due to a lower biomass, but a similar pattern with rates almost twice as high as in Mix compared to PO sections suggest a similar mechanism for this species. Our results suggest that the effect of C. taxifolia on sediment biogeochemistry is less, as the sulfate reduction rates were similar in Mix and PO sections despite relatively high macroalgae biomass. Chisholm et al. (1997); however, found major changes in sediment biogeochemistry in degrading P. oceanica meadows colonized by C. taxifolia, suggesting that the health of the P. oceanica meadow may play a role and that invaders are more successful in degraded environments as proposed by Didham et al. (2005).
Environmental differences at the three locations may also play a role for the observed differences. The sedimentation of organic matter was significant higher at C. racemosa and C. prolifera sites, which may negatively affect P. oceanica, as the rates (∼1 g DW OM per square meter per day, estimated from POC sedimentation considering POC = 50% of OM) were close to the threshold value (1.5 g DW OM per square meter per day) for net population decline found by Diaz-Almela et al. (2008). In particular, sedimentation of allochthonous material may contribute to the enhanced microbial activity (Holmer et al. 2004). Sedimentation of nutrients was up to two times higher at the vegetated compared to the bare sections, but there were no significant differences between the Caulerpa-dominated and the PO sections. Regrettably, sedimentation was not measured at the Mix sections where it may differ from the PO sections due to the smaller seagrass shoots (Marbà et al., unpublished) with openings in the less dense meadow. The higher rates of sedimentation in the vegetated sections were reflected in higher sediment organic contents, although the organic enrichment did not strictly follow the sedimentation rates. Caulerpa-dominated sections showed much higher organic enrichment than PO sections, suggesting that Caulerpa detritus contributed directly to the sediment organic pools and indirectly through enhanced trapping of organic matter (Piazzi et al. 2007). The enrichment was quite substantial at the C. prolifera and C. racemosa locations where the sediment organic carbon content was up to 11 times higher than found in the PO section. An important effect of Caulerpa presence may thus be an organic enrichment of the sediments, leading to the observed enhanced microbial activity in the sediments (Chisholm et al. 1997).
The significant and major decline in δ34S values in roots (δ34S decline = 8–13‰) and rhizomes (δ34S decline = 6–7‰) of P. oceanica at the C. prolifera location suggests a high intrusion of sedimentary sulfides into the belowground parts (up to 40%). Depleted δ34S values in P. oceanica belowground tissues have been found in sediments enriched by organic matter from phytoplanktonic inputs (decline 6–8‰; Marbà et al. 2007) and from fish farm facilities (decline 6–13‰; Frederiksen et al. 2007), where the sulfide exposure of the plants is enhanced due to high rates of sulfate reduction fuelled by high sedimentation of organic matter. There was, however, higher sulfide invasion in the PO sections compared to the Mix section, which is puzzling but may be due to a number of reasons: (1) The δ34S values show large heterogeneity, indicating that the intrusion of sulfide is highly variable probably controlled by individual shoot characteristics, which could be addressed by analyzing a larger number of replicates. (2) P. oceanica shoots are long lived (years) and sulfide intrusion could have occurred earlier. (3) P. oceanica shoots are connected through rhizomes (Marbà et al. 2002) and sulfides may be translocated between shoots growing in the Mix and PO sections, as the distance between them was small (<1–2 m). Data on shoot size showed smaller shoots (17–55%) at the Mix sections probably as a result of decreased meristematic activity compared to the PO sections (Garcias-Bonet et al. 2008). Reduced growth upon sulfide exposure has been found for a number of different seagrasses, e.g., Halophila ovalis (Kilminster et al. 2008), Zostera marina (Goodman et al. 1995; Holmer and Bondgaard 2001), Thalassia testudinum (Koch et al. 2007), including P. oceanica (Marbà et al. 2007; Pérez et al. 2007). The observations of the seagrass population dynamics at the C. prolifera (declining) and C. taxifolia (steady state/expanding) locations are consistent with the generally negative effects on P. oceanica with C. prolifera presence and limited effects with C. taxifolia.
In conclusion, the presence of Caulerpa prolifera and C. racemosa in P. oceanica meadows was coincident with deteriorated sediment quality. Sediment organic matter pools were enhanced in Caulerpa sp. beds, whereby sediments became reduced almost to the sediment surface, and sulfate reduction rates and sulfide production were stimulated similar to findings in organic-enriched sediments. Sulfate reduction rates and sulfide pools were further enhanced in the P. oceanic meadows with co-occurrence of Caulerpa sp. δ34S values in the seagrasses were depleted and a high fraction of sedimentary sulfides were found accumulating in the seagrass rhizomes and roots, suggesting a high invasion of sulfides into the plants. Low shoot density and small shoots in the Mix sections suggest that altered sediment biogeochemistry could eventually lead to seagrass decline being enhanced in modified environments. Caulerpa sp. seems to benefit from the deterioration of the sediments with high biomass in organic-enriched sediments. These results suggest that stimulation of high abundance of Caulerpa sp., in particular of C. prolifera and C. racemosa, could be displacing seagrass meadows through the modifications of sediment conditions to turn these adverse to support seagrass growth. This is a powerful mechanism for interactions between rapid colonizers and native seagrass species, in particular in habitats where the native species already are stressed by other factors.
References
Blomqvist, S., and L. Håkanson. 1981. A review on sediment traps in aquatic environments. Archieves of Hydrobiologia 91: 101–132.
Calleja, M.L.l., N. Marbà, and C.M. Duarte. 2007. The relationship between seagrass (Posidonia oceanica) decline and sulfide porewater concentration in carbonate sediments. Estuarine, Coastal and Shelf Science 73: 583–588. doi:10.1016/j.ecss.2007.02.016.
Ceccherelli, G., and D. Campo. 2002. Different effects of Caulerpa racemosa on two co-occurring seagrasses in the Mediterranean. Botanica Marina 45: 71–76. doi:10.1515/BOT.2002.009.
Ceccherelli, G., and N. Secchi. 2002. Nutrient availability in the sediment and the reciprocal effects between the native seagrass Cymodocea nodosa and the introduced rhizophytic alga Caulerpa taxifolia. Hydrobiologia 474: 57–66. doi:10.1023/A:1016514621586.
Ceccherelli, G., L. Piazzi, and F. Cinelli. 2000. Response of the non-indigenous Caulerpa racemosa (Forsskål) J. Agardh to the native seagrass Posidonia oceanica (L.) Delile: Effect of density of shoots and orientation of edges of meadows. Journal of Experimental Marine Biology and Ecology 243: 227–240. doi:10.1016/S0022-0981(99)00122-7.
Chisholm, J.R.M., and P. Moulin. 2003. Stimulation of nitrogen fixation in refractory organic sediments by Caulerpa taxifolia (Chlorophyta). Limnology and Oceanography 48: 787–794.
Chisholm, J.R.M., F.E. Fernex, D. Mathieu, and J.M. Jaubert. 1997. Wastewater discharge, seagrass decline and algal proliferation on the Côte d’Azur. Marine Pollution Bulletin 34: 78–84. doi:10.1016/S0025-326X(96)00072-0.
Cline, J.D. 1969. Spectrophotometric determination of hydrogen sulphide in natural waters. Limnology and Oceanography 14: 454–458.
Diaz-Almela, E., E. Alvarez, R. Santiago, N. Marbà, M. Holmer, T. Grau, R. Danovaro, M. Argyrou, Y. Karakassis, and C.M. Duarte. 2008. Benthic inputs as predictors of seagrass (Posidonia oceanica) fish farm-induced decline. Marine Pollution Bulletin 56:1332–1342.
Didham, R.K., J.M. Tylianakis, M.A. Hutchinson, R.M. Ewers, and N.J. Gemmell. 2005. Are invasive species the drivers of ecological change? Trends in Ecology and Evolution 20: 470–474. doi:10.1016/j.tree.2005.07.006.
Fossing, H., and B.B. Jørgensen. 1989. Measurement of bacterial sulphate reduction in sediments: Evaluation of a single-step chromium reduction method. Biogeochemistry 8: 205–222. doi:10.1007/BF00002889.
Frederiksen, M.S., M. Holmer, J. Borum, and H. Kennedy. 2006. Temporal and spatial variation of sulfide invasion in eelgrass (Zostera marina) as reflected by its sulfur isotopic composition. Limnology and Oceanography 51: 2308–2318.
Frederiksen, M.S., M. Holmer, E. Diaz-Almela, N. Marbà, and C.M. Duarte. 2007. Sulfide invasion in the seagrass Posidonia oceanica along gradients of organic loading at Mediterranean fish farms: Assessment by stable sulfur isotopes. Marine Ecology Progress Series 345: 93–104. doi:10.3354/meps06990.
Gacia, E., C.M. Duarte, and T. Granata. 1999. An approach to the measurement of particle flux and sediment retention within seagrass (Posidonia oceanica) meadows. Aquatic Botany 65: 255–268. doi:10.1016/S0304-3770(99)00044-3.
Garcias-Bonet, N., N. Marbà, M. Holmer, and C.M. Duarte. 2008. Effects of sediment sulfides on seagrass (Posidonia oceanica) meristematic activity. Marine Ecology Progress Series 372:1–6.
Goodman, J.L., K.A. Moore, and W.C. Dennison. 1995. Photosynthetic responses of eelgrass (Zostera marina) to light and sediment sulfide in a shallow barrier island lagoon. Aquatic Botany 50: 37–47. doi:10.1016/0304-3770(94)00444-Q.
Hargrave, B.T., and N.M. Burns. 1979. Assessment of sediment trap collection efficiency. Limnology and Oceanography 24: 1124–1136.
Holmer, M., and E.J. Bondgaard. 2001. Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquatic Botany 70: 29–38. doi:10.1016/S0304-3770(00)00142-X.
Holmer, M., and M.S. Frederiksen. 2007. Stimulation of sulfate reduction rates in Mediterranean fish farm sediments. Biogeochemistry 85: 169–185. doi:10.1007/s10533-007-9127-x.
Holmer, M., C.M. Duarte, H.T.S. Boschker, and C. Barrón. 2004. Carbon cycling and bacterial carbon sources in pristine and impacted Mediterranean seagrass sediments. Aquatic Microbial Ecology 36: 227–237. doi:10.3354/ame036227.
Jørgensen, B.B. 1978. Comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments 2. Calculation from mathematical-models. Geomicrobiology Journal 1: 29–47.
Jousson, O., J. Pawlowski, L. Zaninetti, F.W. Zechman, F. Dini, G. Di Guiseppe, R. Woodfield, A. Millar, and A. Meinesz. 2000. Invasive alga reaches California—The alga has been identified that threatens to smother Californian coastal ecosystems. Nature 408: 157–158. doi:10.1038/35041623.
Kaplan, I.R., K.O. Emery, and S.C. Rittenberg. 1963. The distribution and isotopic abundance of sulphur in recent marine sediments off Southern California. Geochimica et Cosmochimica Acta 27: 297–331. doi:10.1016/0016-7037(63)90074-7.
Kilminster, K.L., D.I. Walker, P.A. Thompson, and J.A. Raven. 2008. Changes in growth, internode distance and nutrient concentrations of the seagrass Halophila ovalis with exposure to sediment sulphide. Marine Ecology Progress Series 361: 83–91. doi:10.3354/meps07479.
Koch, M.S., S. Schopmeyer, C. Kyhn-Hansen, and C.J. Madden. 2007. Synergistic effects of high temperature and sulfide on tropical seagrass. Journal of Experimental Marine Biology and Ecology 341: 91–101. doi:10.1016/j.jembe.2006.10.004.
Kristensen, E., and F.Ø. Andersen. 1987. Determination of organic carbon in marine sediments: a comparison of two CHN-analyzer methods. Journal of Experimental Marine Biology and Ecology 109: 15–23. doi:10.1016/0022-0981(87)90182-1.
Marbà, N., M.A. Hemminga, M.A. Mateo, C.M. Duarte, Y.E.M. Mass, J. Terrados, and E. Gacia. 2002. Carbon and nitrogen translocation between seagrass ramets. Marine Ecology Progress Series 226: 287–300. doi:10.3354/meps226287.
Marbà, N., C.M. Duarte, E. Díaz-Almela, J. Terrados, E. Álvarez, R. Martinez, R. Santiago, E. Gacia, and A.M. Grau. 2005. Direct evidence of imbalanced seagrass (Posidonia oceanica) shoot population dynamics in the Spanish Mediterranean. Estuaries 28: 53–62. doi:10.1007/BF02732753.
Marbà, N., M. Calleja, C.M. Duarte, E. Álvarez, E. Díaz-Almela, and Holmer. 2007. Iron additions reduce sulfide intrusion and reverse seagrass (Posidonia oceanica) decline in carbonate sediments. Ecosystems 10: 745–756. doi:10.1007/s10021-007-9053-8.
Pedersen, M.F., P.A. Staehr, T. Wernberg, and M.S. Thomsen. 2005. Biomass dynamics of exotic Sargassum muticum and native halidrys siliquosa in Limfjorden, Denmark—Implications of species replacements on turnover rates. Aquatic Botany 83: 31–47. doi:10.1016/j.aquabot.2005.05.004.
Pérez, M., O. Invers, J.M. Ruiz, M.S. Frederiksen, and M. Holmer. 2007. Physiological responses of the seagrass Posidonia oceanica to elevated organic matter content in sediments: an experimental assessment. Journal of Experimental Marine Biology and Ecology 344:149–160.
Piazzi, L., and G. Ceccherelli. 2006. Persistence of biological invasion effects: Recovery of macroalgal assemblages after removal of Caulerpa racemosa var. cylindracea. Estuarine, Coastal and Shelf Science 68: 455–461. doi:10.1016/j.ecss.2006.02.011.
Piazzi, L., D. Balata, G. Ceccherelli, and F. Cinelli. 2005. Interactive effect of sedimentation and Caulerpa racemosa var. cylindracea invasion on macroalgal assemblages in the Mediterranean Sea. Estuarine, Coastal and Shelf Science 64: 467–474. doi:10.1016/j.ecss.2005.03.010.
Piazzi, L., D. Balata, L. Foresi, C. Cristaudo, and F. Cinelli. 2007. Sediment as a constituent of Mediterranean benthic communities dominated by Caulerpa racemosa var. cylindracea. Scientia marina 71: 129–135. doi:10.3989/scimar.2007.71n1129.
Rennenberg, H. 1984. The fate of excess sulfur in higher-plants. Annual Reviews in Plant Physiology and Plant Molecular Biology 35: 121–153. doi:10.1146/annurev.arplant.35.1.121.
Terrados, J., N. Marbà, S. Deudero, and A. Box. 2009. Biomass development of Caulerpa prolifera, Caulerpa taxifolia and Caulerpa racemosa var. cylindracea (Chlorophyta) under a common seasonal forcing. Journal of Phycology (in press).
Tyler, A.C., and K.J. McGlathery. 2006. Uptake and release of nitrogen by the macroalgae Gracilaria vermiculophylla (Rhodophyta). Journal of Phycology 42: 515–525. doi:10.1111/j.1529-8817.2006.00224.x.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Verlaque, M., C.F. Boudouresque, A. Meinesz, and V. Gravez. 2000. The Caulerpa racemosa complex (Caulerpales, Ulvophyceae) in the Mediterranean Sea. Botanica Marina 43: 49–68. doi:10.1515/BOT.2000.005.
Verlaque, M., J. Afonso-Carrillo, M.C. Gil-Rodriguez, C. Durand, C.F. Boudouresque, and Y. Le Parco. 2004. Blitzkrieg in a marine invasion: Caulerpa racemosa var. cylindracea (Bryopsidales, Chlorophyta) reaches the Canary Islands (north-east Atlantic). Biological Invasions 6: 269–281. doi:10.1023/B:BINV.0000034589.18347.d3.
Acknowledgment
This research is a contribution to the MarBEF Network of Excellence, the project “Invasoras” funded by the Spanish Ministry of Education, and the project “Praderas” funded by the BBVA Foundation. MH was supported by Danish Research Council (grant no. 212-05-0408) and Thresholds (EU contract no. 003933).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holmer, M., Marbà, N., Lamote, M. et al. Deterioration of Sediment Quality in Seagrass Meadows (Posidonia oceanica) Invaded by Macroalgae (Caulerpa sp.). Estuaries and Coasts 32, 456–466 (2009). https://doi.org/10.1007/s12237-009-9133-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-009-9133-4