Abstract
We assessed the effects of hypoxia on macrobenthic communities in the York and Rappahannock Rivers, Chesapeake Bay, in box-core samples before and after hypoxic episodes in 2003 and 2004. Hypoxia occurred in both years and was associated with a decrease in biomass and a shift in community structure toward opportunistic species in both rivers. Long-term data indicate that the frequency of hypoxia in the York has increased over the last 22 years. In previous work from ∼20 years ago, the macrobenthic community structure did not change in response to hypoxia in the York; however, in the present study hypoxia was associated with a reduction in community biomass and a change in community structure. We conclude that currently hypoxia is a more important environmental problem in the York than in previous years. Hypoxia likely negatively affects the estuarine food web, as lower macrobenthic biomass could decrease food availability to epibenthic predators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypoxia, a dissolved oxygen (DO) concentration below 2.0 mg l−1(Vaquer-Sunyer and Duarte 2008), is a serious ecological problem in aquatic systems ranging from freshwater lakes to continental shelves around the world (Diaz and Rosenberg 1995; Diaz 2001; and Gray et al. 2002) that has been reported in 400 systems (Diaz and Rosenberg 2008). Exacerbated by land-based nutrient runoff, seasonal hypoxia primarily affects the benthic macrofauna by keeping longer-lived ‘climax’ species from dominating. Many macrobenthic species are adapted to mild hypoxic conditions of short duration (less than 2 weeks) and low intensity (DO of 1.0–2.0 mg l–1), but increasing severity of hypoxia can defaunate large areas of benthic habitat (Diaz and Rosenberg 1995; Kemp et al. 2005).
The Chesapeake Bay is one of the most productive estuarine systems in the United States that suffers from hypoxia (Officer et al. 1984; Seliger et al. 1984). The extent and duration of hypoxia in this system has increased in recent decades (Diaz 2001; Hagy et al. 2004), but the effects have not been fully quantified. In Chesapeake Bay, areas that experience mild hypoxia, such as the York River, historically have shown no change in the structure of the macrobenthos as compared with similar normoxic areas, which usually include a mix of long- and short-lived species with the biomass dominated by bivalves (Boesch and Rosenberg 1981; Dauer et al. 1992; Diaz et al. 1992). In contrast, areas experiencing severe hypoxia, such as the Rappahannock River and the Chesapeake Bay mainstem, show large differences in the macrobenthic communities compared to normoxic areas (Dauer et al. 1992; Llansó 1992; Kemp et al. 2004). In such regions, species diversity and total biomass tend to be lower than those in normoxic areas and the community is dominated by short-lived opportunistic species, such as euryhaline annelids (Dauer et al. 1992), instead of longer-living species, such as large bivalves.
Two tributaries of the Chesapeake Bay, the York and the Rappahannock Rivers (Fig. 1), offer an opportunity to look at the effects of differing degrees of hypoxia on macrobenthos. Both rivers have similar macrobenthic communities, depths, temperature, and salinity regimens, and both experience periodic hypoxia, but hypoxia in the Rappahannock tends to be more severe and longer-lasting (Kuo and Neilson 1987). The benthic community in these rivers represents an important natural resource, as it is vital in the food web and serves as the food base for the largest fishery in the Chesapeake Bay, the blue crab (Hines et al. 1990). We designed this study as a large-scale survey of the benthos to establish the extent and effects of hypoxia in two subestuaries that differ in the severity of hypoxia. Given the large-scale trend of increased hypoxic stress in the Chesapeake, we hypothesized that hypoxia and its effects had worsened over time, especially in the York River.
Maps showing Chesapeake Bay (top) with insets (a) Rappahannock River and (b) York River and extent of hypoxia in early summer 2004. DO contours calculated using inverse distance weighing interpolation (ESRI ArcMap v9.1 software). Open triangles sampling sites, filled diamonds Chesapeake Bay Program water quality sites
Materials and Methods
Our study sites were in the middle and lower reaches of the York and Rappahannock Rivers (Fig. 1). Both rivers are partially mixed mesohaline subestuaries of Chesapeake Bay and both experience summer hypoxia, with the Rappahannock having longer and more intense hypoxia than the York (Kuo and Neilson 1987). To look at trends in dissolved oxygen in the York River over the last two decades, we examined water quality data from the Chesapeake Bay Program’s water quality website (Chesapeake Bay Program 2006). We used bottom dissolved oxygen data from 1984–2005 at all the sites sampled by the Chesapeake Bay Program that are near or within our study area: LE4-1, LE4-2, and LE4-3 (Fig. 1). The depths of these sites are 11, 16, and 21 m, respectively, and represent the deep channel areas of the river. We restricted our analysis to May–September, when hypoxia occurs. During this period the observations are made once every 2 to 4 weeks. We calculated the percent of hypoxic measurements (DO < 2.0 mg l–1) recorded during each summer for each of the sites to estimate the frequency of hypoxia. We then smoothed the data by applying a five-point running average and regressed the frequencies against year at each site.
We sampled the infaunal community in 2003 and 2004 using a box corer. We used a random, stratified design to sample the rivers, dividing the rivers into three depth strata (<3 m, 3–6 m, and >6 m) and randomly selecting sites in each stratum. In 2003, we sampled ten sites per stratum in the York and five in the Rappahannock (due to logistical constraints), and in 2004, we sampled ten sites per stratum in both rivers. Stratification by depth was used because depth is a good proxy for hypoxia (Powers et al. 2005); deep sites are more likely to experience hypoxia than shallow, and we wanted to ensure that we sampled a sufficient number of hypoxic sites. Benthic box-core samples were taken in the York before hypoxia on June 19, 2003 and May 25, 2004 and after hypoxia on October 14, 2003 and November 11, 2004. The Rappahannock was sampled with a box-corer before hypoxia on June 24, 2003 and June 7, 2004 and after hypoxia on February 10, 2004 and November 19, 2004. October is generally about 1 month after the end of hypoxia in these systems and represents the community before substantial post-hypoxia recruitment. November captures the beginning of the post-hypoxic recruitment pulse that occurs in the fall. The bottom temperature, salinity, and dissolved oxygen were measured with a DO probe (YSI Model 85, Yellow Springs Instruments, Dayton, OH, USA) at each site when we sampled and when we revisited the sites during hypoxia in July of 2003 and in June, July, August, and September of 2004.
In 2003, two rounds of sampling were planned, one in June before hypoxia and one in October after hypoxia. Many samples from the June (before hypoxia) sampling were lost in Hurricane Isabel (September 18, 2003). These samples had been sorted to remove all macrofauna but only the bivalves had been identified. The hurricane led us to postpone the second round of sampling in the Rappahannock River until February 2004, to quantify system recovery after fall recruitment.
In 2003, each site was sampled with a 25 × 25-cm (0.0625 m2) Gray O’Hara box-corer. Sub-cores were taken from each sample; one 10-cm diameter core (0.0081 m2) down to 15 cm was taken and sieved on a 0.5-mm mesh screen to quantify smaller infauna and a 2.5-cm diameter core was taken from the top 5 cm for grain-size analysis. The remaining sediment was sieved through a 2-mm mesh. All samples were frozen and transported back to the lab for processing. Bottom temperature, salinity, and dissolved oxygen were measured, as well as water depth and core penetration depth. Samples with <5 cm box-corer penetration were discarded, samples with >5 cm penetration and <15 cm were sub-cored and the community and biomass data from the 0.5-mm sieve were analyzed but the remainder was discarded and samples with >15 cm penetration were fully processed. Community and biomass data from both the 0.5-mm and the 2-mm sieve were analyzed to ensure that we adequately sampled the biota in each size class (Hines and Comtois 1985). In 2004, the same procedure was used, except that three box-core samples were taken at each site. Only the first core had sub-cores taken from it; the last two cores were sieved in entirety through a 2-mm mesh.
The 0.5-mm sieved samples were stained with Rose Bengal dye before all invertebrates were removed and identified to the lowest taxonomic level possible. The 2-mm sieved samples were sorted and identified to the lowest taxonomic level. For the after-hypoxia samples, new bivalve recruits (shell length <3 mm) were considered separately in all analyses of community structure to differentiate between individuals that had survived hypoxia and those that had recruited after hypoxia. Wet, dry, and ash-free dry mass (AFDM) were determined for each of the major taxonomic groups (bivalves, crustaceans, and annelids) for each sample. Grain-size analysis was conducted using standard wet sieving and pipetting and the sediment was classified as muddy (sand:mud >1) or sandy (sand:mud <1; Folk 1974). We analyzed differences in sediment grain size among treatments for all sites, including those with <15-cm core penetration, but did not analyze the effect of sediment type on community structure because ∼90% of our sites were muddy and any analysis would be grossly unbalanced.
The community-structure data from the 0.5-mm and 2-mm sieved samples were divided by the planar area sampled to calculate the density (individuals m–2) and pooled. For large invertebrates (e.g., bivalves) we used the densities from the 2-mm sieved samples; for smaller invertebrates, we used those from the 0.5-mm sieved samples. For invertebrates whose size range overlapped the sieve sizes, we calculated the density from both the 2-mm and the 0.5-mm sieve samples and used whichever was greater. We treated the three 2-mm sieved cores taken in 2004 as a single sample with planar area of 0.179 m2. The data were square-root transformed and compared among samples in Bray–Curtis similarity matrices. Many of the samples from the Rappahannock River in 2003 had core penetrations between 5 and 15 cm and thus we could not use the 2-mm sieved samples; therefore, we did a separate analysis of the 0.5-mm sieved samples. Sites were designated ‘hypoxic’ if we observed hypoxic conditions there at least once during any of the sampling periods and ‘normoxic’ if we never observed hypoxic conditions. Thus, in samples taken before hypoxia, a hypoxic site was one that had not experienced hypoxic conditions that year at the time of sampling, but would experience hypoxia later that summer. Given our limited temporal coverage of the dissolved oxygen, it is possible that some of the sites designated as ‘normoxic’ experienced hypoxia on dates other than our sampling dates. Differences in community structure between hypoxic sites and normoxic sites were analyzed using a non-metric multi-dimensional scaling (nMDS) analysis and an analysis of similarity (ANOSIM; Primer v6.1.6; Clarke and Warwick 2001). Where hypoxic and normoxic sites differed significantly, a similarity percentages (SIMPER) analysis was performed on the untransformed densities to determine which species most contributed to that difference. As we had data on the bivalve community from both before and after hypoxia in 2003, we analyzed the bivalve community subset separately (2-mm sieved samples). We excluded sites where no bivalves were present (similarity matrixes cannot be calculated if no species are present in multiple sites) to look at changes over time, using nMDS and a 2-way ANOSIM with river and hypoxia level as factors.
The biomass data were separated into 2-mm and 0.5-mm sieved samples and analyzed separately. All bivalves >2 mm were considered to be part of the 2-mm sieved samples even if they were sampled in the 0.5-mm sieved sub-core. Polychaetes and other annelids were only analyzed for the 0.5-mm sieved samples. In 2003, the samples were regressed against the minimum dissolved oxygen observed during the summer using the least squares method. In 2004, because we had more observations of DO than in 2003, we used the average of three DO measurements in the York and two in the Rappahannock taken at each site from May–August for regressions.
Results
Physical Parameters
In general, hypoxia was more extensive in the Rappahannock River than in the York (Fig. 1) and less extensive in 2003 than in 2004. In 2003, we observed hypoxia at 47% (seven of 15) of the sites in the Rappahannock and at 17% (five of 29) of sites in the York. In 2004, we observed hypoxia at 47% (14 of 30) of the Rappahannock and at 26% (eight of 31) of the York sites (Appendix 1). Also, in 2003, hypoxia in the Rappahannock was more intense than that in the York, with an average DO at hypoxic sites (sites where DO < 2 mg l–1 was observed at least once during the sampling period) of 0.5 mg l–1 in the Rappahannock versus 1.3 mg l–1 in the York (one-way ANOVA, F 1,21 = 12.17, p = 0.002). In 2004, the trend was reversed, with an average DO of 0.7 mg l–1 in the Rappahannock and 0.3 mg l–1 in the York. However, because the limited temporal coverage of our DO measurements does not capture the full temporal variability present in the system, these data must be interpreted cautiously, especially in the more episodically hypoxic York River. At Chesapeake Bay Program benthic monitoring site LE4-3 in the York, there was a fourfold increase in the frequency of hypoxia between 1984 and 2003 (Fig. 2) and some indication that most of this increase has been in the years since 1994. Hypoxia was seldom observed at sites farther upriver (LE4-2 and LE4-1); there were only four hypoxic observations in 20 years at LE4-2 (all after 1998) and two at LE4-1. Despite the limited spatial and temporal coverage, these data are a useful time-series of DO and the only long-term DO data set available from this region. Sediments in both rivers across all sampling periods were mainly muddy (90% of sites), and grain size was similar among depth strata: shallow (20.9% sand ± 4.1% SE), mid (17.7% sand ± 4.5% SE), and deep sites (18.0% sand ± 4.7% SE) (Appendix 1).
Community Structure
Bivalves recruited to all areas of both rivers in the spring of 2003 but hypoxic sites had higher mortalities of the biomass dominant, Macoma balthica, over the summer. In the spring, before the onset of hypoxia, there was no difference in the bivalve community among sites for both rivers (ANOSIM, Global R = –0.013, p = 0.213). After hypoxia, there was a significant difference between hypoxic and normoxic sites in both rivers (ANOSIM, Global R = 0.698, p = 0.031); hypoxic areas were dominated by Mulinia lateralis, whereas normoxic areas were dominated by M. balthica and Macoma mitchelli (SIMPER). There was no significant difference in bivalve community structure between the rivers either before (ANOSIM, Global R = 0.213, p = 0.081) or after (ANOSIM, Global R = 0.300, p = 0.070) hypoxia.
After hypoxia in 2003 in the York River, the macrobenthic community in hypoxic sites differed from that in normoxic sites in both the whole core (ANOSIM, Global R = 0.267, p = 0.025) and the 0.5-mm sieved sub-core (ANOSIM, Global R = 0.341, p = 0.007) (samples for the benthic community before hypoxia in 2003 were lost in Hurricane Isabel). Small polychaetes and crustaceans were associated with sites where hypoxia was observed (hypoxic sites) while large bivalves and crustaceans were common in normoxic sites (Fig. 3; Table 1). In the Rappahannock, hypoxic and normoxic sites did not differ in community structure by February, following fall recruitment. This was consistent in both the whole core (ANOSIM, Global R = 0.026, p = 0.464) in samples from eight sites and the 0.5-mm sieved sub-core (ANOSIM, Global R = –0.012, p = 0.501) in samples from 14 sites.
Density of abundant taxa (based on SIMPER results) in hypoxic and normoxic sites (mean + SE) in the York River after hypoxia in 2003, arranged in order of abundance in hypoxic sites. Patterns in 2004 for both the York and the Rappahannock were similar (see Table 1)
Following hypoxia, patterns in community structure in 2004 were similar to those in 2003. In the York hypoxic sites, macrobenthic community structure differed from that in the normoxic sites both before and after hypoxia (Fig. 4a, b). In the Rappahannock, there was no significant difference between hypoxic and normoxic sites before the onset of hypoxia in June, similar to the findings from February, but after hypoxia there was a significant difference (Fig. 4c, d).
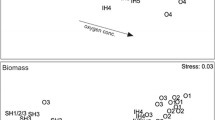
Community structure nMDS plot (2-mm and 0.5-mm sieved cores combined) for the York a before and b after hypoxia in 2004 and for the Rappahannock c before and d after hypoxia in 2004. Global R and p values are from ANOSIM analysis for differences between hypoxic (black triangles) and normoxic (gray triangles) sites
The species that were associated with normoxic sites included the bivalves M. balthica and M. mitchelli and the amphipod Leptocheirus plumulosus (Table 1, Fig. 5). Some of the larger infaunal polychaetes, as well as other crustaceans, such as Mysidacea species and Cyathura polita, were only abundant in normoxic sites. The polychaete species Neanthes (Nereis) succinea was consistently characteristic of normoxic areas. The taxa associated with hypoxic areas included small, typically opportunistic polychaetes, including Spionidae, Capitellidae, Glycinde solitaria, and Leitoscoloplos sp. (Table 1, Fig. 5). Small crustaceans in the family Caprellidae and order Cumacea were also abundant in hypoxic areas. In the York after hypoxia, some larger species of polychaetes, such as Pectinaria gouldii and Amphitrite ornata, were abundant at hypoxic sites.
Community structure and species nMDS plots for the Rappahannock after hypoxia in 2003 showing the distribution of selected species in hypoxic (black triangles) and normoxic (gray triangles) sites. Circle area proportional to the relative density of each taxon at each site (Table 1); letter H indicates hypoxic site and N indicates normoxic site. Stress value same for each plot
Community Biomass
The effect of hypoxia on the community structure was also observed in the community biomass. We examined several curves to describe the relationship between dissolved oxygen and biomass, including linear, sigmoid, and exponential; the best fit curve was generally an exponential growth curve (F tests). After hypoxia, in October 2003, sites in the York River that had a minimum DO < ∼3 mg l–1 had lower biomass than sites that had >∼3 mg l–1 in both the 2-mm sieved samples and the 0.5-mm sieved samples (Fig. 6a, b) and there was a positive correlation between biomass and DO in 0.5-mm sieved samples (Fig. 6b). Biomass in the Rappahannock samples collected in February, after the fall recruitment, showed no difference between hypoxic and normoxic areas in 2-mm or 0.5-mm sieved samples (Fig. 6c, d).
AFDM plotted against minimum observed dissolved oxygen. a York River 2-mm sieved samples. b York River 0.5-mm sieved samples (line is best fit exponential growth equation; p = 0.001; R 2 = 0.18). c Rappahannock River 2-mm sieved samples. d Rappahannock River 0.5-mm sieved samples. York samples were taken shortly after hypoxia in October, 2003, whereas Rappahannock samples were taken after the fall and spring recruitment events following hypoxia in February, 2004. Note that y-axis scales change among plots
In 2004 before hypoxia, community biomass in 2-mm sieved samples was significantly related to average summer DO in the York (Fig. 7a, b; p = 0.001, R 2 = 0.29) but not in the Rappahannock (Fig. 7c, d; p = 0.805, R 2 < 0.0005). Following hypoxia, there was a decrease in biomass at all sites, but it was proportionately greater in hypoxic areas. Biomass in 2-mm sieved samples was significantly related to average summer DO in both the York (Fig. 7; p = 0.04, R 2 = 0.11) and the Rappahannock (Fig. 7; p = 0.003, R 2 = 0.32). Bivalves comprised ∼80–90% of the benthic community biomass during all time periods and the majority of the bivalve species were either M. balthica or M. mitchelli.
AFDM plotted against average of three (York) and two (Rappahannock) observations of dissolved oxygen over the summer for the York and Rappahannock before (solid circles) and after (hollow circles) hypoxia in 2004; (a and c) 2-mm sieved samples (mean ± SE); (b and d) 0.5-mm sieved samples. Trend lines show best-fit exponential growth equations (see text for statistics). Note that y-axis scales change among plots
Discussion
In both the York and Rappahannock Rivers, hypoxia was associated with lower macrobenthic biomass and a macrobenthic community dominated by opportunistic species including small, short-lived polychaetes and crustaceans. While this was observed previously in the Rappahannock in similar studies (Llansó 1992), such effects of hypoxia on the macrobenthic community were not detected in the York River in two similar previous studies (Dauer et al. 1992; Diaz et al. 1992); however, Holland et al. (1987) documented a Bay-wide trend of replacement of larger bivalves with opportunistic species from 1971–1984, which may reflect worsening effects of hypoxia in the Chesapeake as a whole. A similar shift in community structure of the lower York occurred after Tropical Storm Agnes in 1972, but this was an extreme event that combined hypoxic conditions with a ∼12 psu drop in salinity and severe sediment deposition (Boesch et al. 1976a). Several possible explanations exist for the difference between our results and previous results for the York River. Our study had a larger sample size than either of the other two York River studies (Dauer et al. (1992) had only three stations in the York River), increasing the spatial resolution and power of our study. However, a likely reason for the differences in the two sets of results is an increase in the frequency of hypoxia in the York River during the intervening ∼20 years between our study and previously published work. This conclusion is supported by the increase in the frequency of hypoxia over the last two decades (Fig. 3) and similar increases in the Chesapeake Bay over the same period (Hagy et al. 2004; Kemp et al. 2005). Moreover, the benthic community in hypoxic areas in the York was dominated by small, short-lived opportunistic polychaetes, such as Spionidae, Capitellidae, G. solitaria, and Leitoscoloplos sp., and small crustaceans, similar to the community that developed after Agnes (Boesch et al. 1976a) in the more chronic hypoxic areas of the Rappahannock River (Dauer et al. 1992; Llansó 1992; this study) and other hypoxic or chronically hypoxic areas of Chesapeake Bay (Holland et al. 1987). A few larger polychaetes, Pectinaria gouldii and Amphitrite ornata, were also associated with hypoxic sites in the York. Both of these species spawn and recruit in the late summer and fall, which suggests that they recruited after the relaxation of hypoxia (Scott 1909; Franz and Harris 1988).
In both rivers, normoxic areas were numerically dominated by the amphipod L. plumulosus, the polichaete N. (Nereis) succinea, and the bivalves M. balthica, and M. mitchelli. Approximately 85% of the biomass was composed of the bivalves M. balthica and M. mitchelli. These species are all well-characterized members of the benthos of Chesapeake Bay associated with non-degraded environments. L. plumulosus is a highly productive (Holland et al. 1987), pollution-sensitive amphipod that is widely used as a bio-indicator of sediment toxicity (e.g., Schlekat et al. 1992). N. succinea is a medium-sized polychaete that responds negatively to environmental degradation (Lerberg et al. 2000). Although it is tolerant of organic enrichment, it is not tolerant of hypoxia (Detwiler et al. 2002). M. balthica and M. mitchelli are the biomass-dominant species in the mesohaline regions of Chesapeake Bay (Holland et al. 1977; Holland et al. 1987) and are moderately tolerant of hypoxic conditions (Borsuk et al. 2002; Seitz et al. 2003). All four of these species are important links in the Chesapeake Bay food web, as benthic predators feed heavily on them (Virnstein 1977; Holland et al. 1987; Baird and Ulanowicz 1989; Hines et al. 1990).
There seems to be good potential for recovery from hypoxic conditions in both Chesapeake Bay tributaries that we examined. Recolonization began with the fall recruitment such that, by February 2004, the hypoxic sites in the Rappahannock were no longer distinguishable from adjacent normoxic areas. Such a recovery is common in hypoxic areas of Chesapeake Bay (Holland et al. 1987). Larvae of benthic organisms recruit into areas exposed to hypoxia and can settle during a hypoxic event (Sagasti et al. 2003). The lower benthic biomass, and therefore decreased competition, may actually increase recruitment into hypoxic areas (Rhoads and Young 1970; Woodin 1974). When we sampled the Rappahannock in June 2004, a larger sample size still did not show any strong differences between the communities in hypoxic and normoxic areas.
Much of the recovery in the Rappahannock was attributable to the recruitment of bivalves; in February 2003, the number of M. balthica recruits alone exceeded 1,000 m–2 at sites that had been exposed to hypoxia the previous summer. In the York in 2003, before hypoxia, there was no difference in bivalve populations between sites that experienced hypoxia and those that remained normoxic, similarly indicating that recruitment was strong. However, after hypoxia, by the fall, bivalves were essentially absent from the areas that experienced hypoxia. Additionally, in previous samples taken in the 1970s, densities of M. balthica in the now-hypoxic areas of the river were similar to those we found in normoxic areas, showing that our current hypoxic zones previously supported clams (Boesch and Rosenberg 1981). In the spring of 2004 (after recruitment and some recovery), there was still a measurable difference between the hypoxic and normoxic areas in the York. Much of this difference was due to densities of M. balthica, L. plumulosus, and N. succinea in hypoxic areas, where they were present but at lower densities than in normoxic areas. This partial recovery in hypoxic areas is similar to what occurs in other systems, such as the Neuse River, where recovery was complete after a mildly hypoxic year, but only partial after a severely hypoxic year (Powers et al. 2005). This suggests that recruitment from adjacent normoxic areas allows partial recovery from hypoxia.
Biomass followed a pattern typical of hypoxic systems (e.g., Lim et al. 2006; Montagna and Ritter 2006), with lower biomass in sites that experienced hypoxia. Although our measurements of dissolved oxygen were infrequent, and thus not a complete picture of the history of dissolved oxygen at each site, they were a reasonably good predictor of biomass following hypoxia. In the Rappahannock, after hypoxia reduced biomass in summer 2003, biomass increased from fall of 2003 to spring of 2004 in areas that went hypoxic such that there was no difference between formerly hypoxic areas and normoxic areas when we sampled in spring of 2004, indicating strong recovery. In contrast, in the York River, the biomass in areas that typically go hypoxic remained lower than in normoxic areas in the spring of 2004. Similar amounts of biomass accumulate in the hypoxic areas of both rivers during the fall, winter, and spring, indicating similar rates of recovery; however, because biomass in normoxic areas is lower in the Rappahannock than in the York, the relative recovery in hypoxic areas as compared to normoxic areas was higher in the Rappahannock (i.e., biomass only needed to return to low levels). The Rappahannock, which had a greater extent of hypoxia than the York, had lower biomass in normoxic areas. As the extent of hypoxia increases, it may cause negative feedback through a decreased supply of larvae throughout the river, leading to cumulative effects (Pulliam and Danielson 1991; Long 2007). Because so much of the biomass in our systems is comprised of bivalves and these were nearly absent from hypoxic areas, the pattern of continued low biomass through the end of spring is not surprising. Full recovery of the system in the absence of hypoxia could likely be attained within the time it takes to establish a mature bivalve population, which for M. balthica is about 3 years (Long 2007). Although such a recovery did not occur within 2.5 years after Agnes (Boesch et al. 1967b), Agnes was a Bay-wide event that changed the benthic community throughout the Chesapeake, whereas the current area affected by hypoxia is relatively small and localized (square kilometers, compared to the entire Chesapeake Bay).
A decrease in biomass and shift in community structure due to hypoxia likely has a detrimental effect on the food web, as the benthic community serves as prey for many epibenthic predators (e.g., Virnstein 1977; Baird and Ulanowicz 1989). Direct mortality of benthic infauna due to hypoxic stress may result in biomass being transferred through microbes rather than to higher trophic levels (Baird et al. 2004; Altieri and Witman 2006). Although our study does demonstrate a decrease in biomass in hypoxic areas, it cannot differentiate between direct mortality and mortality due to predation.
The behavioral responses of benthic fauna when exposed to hypoxia (e.g., reduced burial depth, exposure on sediment surface, and extension of siphons or palps in the water column) may make them more vulnerable to predation (Jørgensen 1980; Dauer et al. 1992; Diaz et al. 1992; Seitz et al. 2003; Long et al. 2008). Whether predators are able to take advantage of this is still under debate. Predators generally migrate out of hypoxic areas (e.g., Pihl et al. 1991; Eby and Crowder 2004; Bell and Eggleston 2005). However, predators will enter mildly hypoxic areas to forage (Rahel and Nutzman 1994; Nestlerode and Diaz 1998). In the York, the diets of benthic predators shifted to include more infaunal species during and immediately after hypoxia (Pihl et al. 1992), and episodic hypoxia increased the predation rate on M. balthica threefold in a caging experiment (Long and Seitz 2008), with mortality due to predation under hypoxia seven times greater than mortality from hypoxic stress. These studies were done in mildly hypoxic areas; more intense hypoxia may exclude predators or decrease predation (Bell et al. 2003; Seitz et al. 2003; Montagna and Ritter 2006).
Hypoxia causes a decrease in the ecosystem services provided by the benthic macrofauna by decreasing their biomass and changing the species composition. The infauna in the York and Rappahannock Rivers filter the water and provide food for higher trophic levels, along with other secondary services such as geochemical processing, denitrification, and bioturbation. Many infaunal organisms remove particulates from the water through filter or suspension feeding (McCay et al. 2003), which increases the clarity of the water and has positive effects on other species, particularly submerged aquatic vegetation (Kemp et al. 2005). All of these main functions are influenced by the abundance, biomass, and species composition of the benthic infauna. Hypoxia has an effect on these important ecosystem services by decreasing the biomass and shifting the species composition away from larger species to smaller species, which are less-preferred prey items for large benthic predators (Hines et al. 1990). Our data suggest that hypoxia in the York River is worsening both in frequency and effects; thus, increased efforts to reduce hypoxia by limiting nutrient inputs are suggested.
References
Altieri, A.H., and J.D. Witman. 2006. Local extinction of a foundation species in a hypoxic estuary: integrating individuals to ecosystem. Ecology 87: 717–730. doi:10.1890/05-0226.
Baird, D., and R.E. Ulanowicz. 1989. The seasonal dynamics of the Chesapeake Bay ecosystem. Ecological Monographs 59: 329–361. doi:10.2307/1943071.
Baird, D., R.R. Christian, C.H. Peterson, and G.A. Johnson. 2004. Consequences of hypoxia on estuarine ecosystem function: energy diversion from consumers to microbes. Ecological Applications 14: 805–822. doi:10.1890/02-5094.
Bell, G.W., and D.B. Eggleston. 2005. Species-specific avoidance responses by blue crabs and fish to chronic and episodic hypoxia. Marine Biology 146: 761–770. doi:10.1007/s00227-004-1483-7.
Bell, G.W., D.B. Eggleston, and T.G. Wolcott. 2003. Behavioral response of free-ranging blue crabs to episodic hypoxia. II. Feeding. Marine Ecology Progress Series 259: 227–235. doi:10.3354/meps259227.
Boesch, D.F., and R. Rosenberg. 1981. Response to stress in marine benthic communities. In Stress effects on natural ecosystems, eds. G.W. Barrett, and R. Rosenberg, 179–200. New York: Wiley.
Boesch, D.F., R.J. Diaz, and R.W. Virnstein. 1976a. Effects of tropical storm Agnes on soft-botto macrobenthic communities of the James and York estuaries and the lower Chesapeake Bay. Chesapeake Science 17: 246–259. doi:10.2307/1350512.
Boesch, D.F., M.L. Wass, and R.W. Virnstein. 1976b. The dynamics of estuarine benthic communities. In Estuarine Processes Vol. 1, ed. M.L. Wiley, 177–196. New York: Academic.
Borsuk, M.E., S.P. Powers, and C.H. Peterson. 2002. A survival model of the effects of bottom-water hypoxia on the population density of an estuarine clam (Macoma balthica). Canadian Journal of Fisheries and Aquatic Science 59: 1266–1274. doi:10.1139/f02-093.
Chesapeake Bay Program. 2006. Chesapeake Bay Program Water Quality Database 1984–present. http://www.chesapeakebay.net/wquality.htm. Accessed 10 January 2006.
Clarke, K.R., and R.M. Warwick. 2001. Change in Marine Communities: an approach to statistical analysis and interpretation. 2nd edition. Plymouth: Plymouth Marine Laboratory.
Dauer, D.M., A.J. Rodi Jr., and J.A. Ranasinghe. 1992. Effects of low dissolved oxygen events on the macrobenthos of the lower Chesapeake Bay. Estuaries 15: 348–391. doi:10.2307/1352785.
Detwiler, P.M., M.F. Coe, and D.M. Dexter. 2002. The benthic invertebrates of the Salton Sea: distribution and seasonal dynamics. Hydrobiologia 473: 139–160. doi:10.1023/A:1016537903644.
Diaz, R.J. 2001. Overview of hypoxia around the world. Journal of Environmental Quality 30: 275–281.
Diaz, R.J., and R. Rosenberg. 1995. Marine benthic hypoxia: A review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanography and marine Biology. An Annual Review 33: 245–303.
Diaz, R.J., and R. Rosenberg. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929. doi:10.1126/science.1156401.
Diaz, R.J., R.J. Neubauer, L.C. Schaffner, L. Pihl, and S.P. Baden. 1992. Continuous monitoring of dissolved oxygen in an estuary experiencing periodic hypoxia and the effect of hypoxia on macrobenthos and fish. Science of the Total Environment (Suppl. 1992), 1055–1068.
Eby, L.A., and L.B. Crowder. 2004. Effects of hypoxic disturbances on an estuarine nekton assemblage across multiple scales. Estuaries 27: 342–351. doi:10.1007/BF02803390.
Folk, R.L. 1974. Petrology of sedimentary rocks. Austen: Hemphill.
Franz, D.R., and W.H. Harris. 1988. Seasonal and spatial variability in macrobenthos communities in Jamaica Bay, New York—an urban estuary. Estuaries 11: 15–28. doi:10.2307/1351714.
Gray, J.S., R.S. Wu, and Y.Y. Or. 2002. Effects of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series 238: 249–279. doi:10.3354/meps238249.
Hagy, J.D., W.R. Boynton, C.W. Wood, and K.V. Wood. 2004. Hypoxia in Chesapeake Bay, 1950–2001: long-term changes in relation to nutrient loading and river flow. Estuaries 27: 634–658. doi:10.1007/BF02907650.
Hines, A.H., and K.L. Comtois. 1985. Vertical distribution of infauna in sediments of a subestuary of central Chesapeake Bay. Estuaries 8: 296–304. doi:10.2307/1351490.
Hines, A.H., A.M. Haddon, and L.A. Wiechert. 1990. Guild structure and foraging impact of blue crabs and epibenthic fish in a subestuary of Chesapeake Bay. Marine Ecology Progress Series 67: 105–126. doi:10.3354/meps067105.
Holland, A.F., N.K. Mountford, and J. Mihursky. 1977. Temporal variation in upper bay and mesohaline communities: I. The 9-m mud habitat. Chesapeake Science 18: 370–378. doi:10.2307/1350592.
Holland, A.F., A.T. Shaughnessy, and M.H. Hiegel. 1987. Long-term variation in mesohaline Chesapeake Bay macrobenthos: spatial and temporal patterns. Estuaries 10: 227–245. doi:10.2307/1351851.
Jørgensen, B.B. 1980. Seasonal oxygen depletion in the bottom waters of a Danish fjord and its effects on the benthic community. Oikos 34: 68–76. doi:10.2307/3544551.
Kemp, W.M., W.R. Boynton, J.E. Adolf, D.F. Boesch, W.C. Boicourt, G. Brush, J.C. Cornwell, T.R. Fisher, P.M. Glibert, J.D. Hagy, L.W. Harding, E.D. Houde, D.G. Kimmel, W.D. Miller, R.I.E. Newell, M.R. Roman, E.M. Smith, and J.C. Stevenson. 2005. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series 303: 1–29. doi:10.3354/meps303001.
Kuo, A.Y., and B.J. Neilson. 1987. Hypoxia and salinity in Virginia estuaries. Estuaries 10: 277–283. doi:10.2307/1351884.
Lerberg, S.B., A.F. Holland, and D.M. Sanger. 2000. Responses of tidal creek marcrobentic communities to the effects of watershed development. Estuaries 23: 838–853. doi:10.2307/1353001.
Lim, H.S., R.J. Diaz, J.S. Hong, and L.C. Schaffner. 2006. Hypoxia and benthic community recovery in Korean coastal waters. Marine Pollution Bulletin 52: 1517–1526. doi:10.1016/j.marpolbul.2006.05.013.
Llansó, R.J. 1992. Effects of hypoxia on estuarine benthos: The lower Rappahannock River (Chesapeake Bay), a Case Study. Estuarine Coastal and Shelf Science 359: 491–515. doi:10.1016/S0272-7714(05)80027-7.
Long, W.C. 2007. Hypoxia and Macoma balthica: Ecological effects on a key benthic infaunal species. PhD dissertation, College of William and Mary, Virginia Institute of Marine Science, Gloucester Point, VA. www.vims.edu/library/Theses/Long07.pdf.
Long, W.C., and R.D. Seitz. 2008. Trophic interactions under stress: hypoxia enhances foraging in an estuarine food web. Marine Ecology Progress Series 362: 59–68. doi:10.3354/meps07395.
Long, W.C., B.J. Brylawski, and R.D. Seitz. 2008. Behavioral and lethal effects of hypoxia on Macoma balthica. Journal of Experimental Marine Biology and Ecology 359: 34–39. doi:10.1016/j.jembe.2008.02.013.
McCay, D.P.F., C.H. Peterson, J.T. DeAlteris, and J. Catena. 2003. Restoration that targets function as opposed to structure: replacing lost bivalve production and filtration. Marine Ecology Progress Series 264: 197–212. doi:10.3354/meps264197.
Montagna, P.A., and C. Ritter. 2006. Direct and indirect effects of hypoxia on benthos in Corpus Christi Bay, Texas, USA. Journal of Experimental Marine Biology and Ecology 330: 119–131. doi:10.1016/j.jembe.2005.12.021.
Nestlerode, J.A., and R.J. Diaz. 1998. Effects of periodic environmental hypoxia on predation of a tethered polychaete, Glycera americana: implications for trophic dynamics. Marine Ecology Progress Series 172: 185–195. doi:10.3354/meps172185.
Officer, C.B., R.B. Biggs, J.L. Taft, L.E. Cronin, M.A. Tyler, and W.R. Boynton. 1984. Chesapeake Bay anoxia: origin, development, and significance. Science 223: 22–27. doi:10.1126/science.223.4631.22.
Pihl, L., S.P. Baden, and R.J. Diaz. 1991. Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Marine Biology 108: 349–360. doi:10.1007/BF01313644.
Pihl, L., S.P. Baden, R.J. Diaz, and L.C. Schaffner. 1992. Hypoxia-induced structural changes in the diet of bottom feeding fish and crustacea. Marine Biology 112: 349–361. doi:10.1007/BF00356279.
Powers, S.P., C.H. Peterson, R.R. Christian, E. Sullivan, M.J. Powers, M.J. Bishop, and C.P. Buzzelli. 2005. Effects of eutrophication on bottom habitat and prey resources of demersal fishes. Marine Ecology Progress Series 302: 233–243. doi:10.3354/meps302233.
Pulliam, H.R., and B.J. Danielson. 1991. Sources, sinks and habitat selection: A landscape perspective on population dynamics. American Naturalist 37Suppl.: S50–S66. doi:10.1086/285139.
Rahel, F.J., and J.W. Nutzman. 1994. Foraging in a lethal environment: fish predation in hypoxic waters of a stratified lake. Ecology 75: 1246–1253. doi:10.2307/1937450.
Rhoads, D.C., and D.K. Young. 1970. The influence of deposit-feeding organisms on sediment stability and community trophic structure. Journal of Marine Research 28: 150–178.
Sagasti, A., J.E. Duffy, and L.C. Schaffner. 2003. Estuarine epifaunal recruit despite periodic hypoxia stress. Marine Biology 142: 111–122.
Schlekat, C.E., B.L. McGee, and E. Reinharz. 1992. Testing sediment toxicity in Chesapeake Bay with the amphipod Leptocheirus plumulosus: an evaluation. Environmental Toxicology and Chemistry 11: 225–236. doi:10.1897/1552-8618(1992)11[225:TSTICB]2.0.CO;2.
Scott, J.W. 1909. Some egg-laying habits of Amphitrite ornate Verrill. Biological Bulletin 17: 327–340. doi:10.2307/1536055.
Seitz, R.D., L.S. Marshall, A.H. Hines, and K.L. Clark. 2003. Effects of hypoxia on predator-prey dynamics of the blue crab (Callinectes sapidus) and the Baltic clam (Macoma balthica) in Chesapeake Bay. Marine Ecology Progress Series 257: 179–188. doi:10.3354/meps257179.
Seliger, H.H., J.A. Boggs, and W.H. Biggley. 1984. Catastrophic anoxia in the Chesapeake Bay in 1984. Science 228: 70–73. doi:10.1126/science.228.4695.70.
Vaquer-Sunyer, R., and C.M. Duarte. 2008. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences 105: 15452–15457. doi:10.1073/pnas.0803833105.
Virnstein, R.W. 1977. The importance of predation by crabs and fishes on benthic infauna in Chesapeake Bay. Ecology 58: 1199–1217. doi:10.2307/1935076.
Woodin, S.A. 1974. Polychaete abundance patterns in a marine soft-sediments environment: the importance of biological interactions. Ecological Monographs 44: 171–187. doi:10.2307/1942310.
Acknowledgements
We thank K. Delano, B. Brylawski, K. Knick, K. Erickson, R. Burke, J. Falls, D. Lambert, and M. Seebo for help with the field sampling; K. Knick and B. Brylawski for help with the laboratory work; and R. Diaz, D. Wolcott, J. Long, and six anonymous reviewers for help with the revision of this manuscript. W.C. Long was funded by an Environmental Protection Agency GRO fellowship. Funding was provided by National Oceanographic and Atmospheric Administration National Sea Grant, Essential Fish Habitat Program, by the Commonwealth of Virginia, and by a grant to the Blue Crab Advanced Research Consortium from the National Oceanographic and Atmospheric Administration Chesapeake Bay Program. This is contribution 2982 from the Virginia Institute of Marine Science.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 37 kb)
Rights and permissions
About this article
Cite this article
Long, W.C., Seitz, R.D. Hypoxia in Chesapeake Bay Tributaries: Worsening effects on Macrobenthic Community Structure in the York River. Estuaries and Coasts 32, 287–297 (2009). https://doi.org/10.1007/s12237-009-9132-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-009-9132-5