Abstract
The potato psyllid, Bactericera cockerelli, is a key pest of potato and important vector of the pathogen that causes zebra chip disease. Control of zebra chip relies entirely on the use of insecticides to reduce populations of this vector. The development of potato varieties resistant to B. cockerelli would contribute to cost-effective control of this insect. Wild potato germplasm are key sources for desirable traits including pest resistance to develop new potato cultivars. Our objective was to screen Solanum bulbocastanum germplasm for resistance to B. cockerelli. The combined use of choice and no-choice assays demonstrated considerable variability among S. bulbocastanum populations in their susceptibility to psyllids. At least six S. bulbocastanum populations exhibited resistance to B. cockerelli: PI 243512, PI 243513, PI 255518, PI 275194, PI 275197, and PI 283096. The documentation of the variability among S. bulbocastanum germplasm populations in their susceptibility to B. cockerelli can aid the development of potato cultivars that are naturally resistant to the potato psyllid.
Resumen
El psílido de la papa, Bactericera cockerelli, es una plaga clave de la papa y un vector importante del patógeno que causa la enfermedad del tubérculo rayado o “zebra chip”. El control de esta enfermedad se confía completamente en el uso de insecticidas para reducir las poblaciones de este vector. El desarrollo de variedades de papa resistentes a Bactericera cockerelli contribuirá al control costeable de este insecto. El germoplasma silvestre de papa es una fuente clave para características deseables incluyendo resistencia a plagas para el desarrollo de nuevas variedades. Nuestro objetivo fue evaluar germoplasma de Solanum bulbocastanum para resistencia a B. cockerelli. El uso combinado de ensayos de selección y no selección demostró variabilidad considerable entre poblaciones de S. bulbocastanum en su susceptibilidad a los psílidos. Por lo menos seis poblaciones de S. bulbocastanum exhibieron resistencia a B. cockerelli: PI 243512, PI 243513, PI 255518, PI 275194, PI 275197, y PI 283096. La documentación de la variabilidad entre las poblaciones del germoplasma de S. bulbocastanum respecto a su susceptibilidad a B. cockerelli puede ayudar en el desarrollo de variedades de papa que son resistentes de forma natural al psílido de la papa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae) is a key pest of Solanaceous crops (Solanales: Solanaceae) (Wallis 1955; Teulon et al. 2009; Munyaneza 2012). Feeding by this insect causes plant decline, decreased yields, and foliar symptoms known as ‘psyllid yellows’ (Richards 1933; Munyaneza 2012). Although B. cockerelli has long been considered a pest of Solanaceous crops, the pest-status of this insect recently increased after it was identified as the vector of a newly recognized bacterial plant pathogen, “Candidatus Liberibacter solanacearum” (aka “Ca. Liberibacter psyllaurous”) (Munyaneza et al. 2007; Liefting et al. 2008, 2009; Secor et al. 2009; Teulon et al. 2009; Munyaneza 2012). Crop damage by “Ca. Liberibacter solanacearum” was first reported in the southern United States and northern Mexico in the mid-1990’s, but the pathogen has since spread to other potato growing regions of the western United States, Mexico, Central American, and New Zealand (Munyaneza 2012). This pathogen causes plant mortality and decreased yields in all Solanaceous crops, and is the causal agent of zebra chip disease of potato (Munyaneza 2012). Zebra chip disease is characterized by the development of striped patterns in tubers that render the potatoes unmarketable leading to millions of dollars in losses to potato growers in the United States (Munyaneza 2012).
Management programs for “Ca. Liberibacter solanacearum” rely solely on the use of insecticides to target the insect vector, potato psyllid (Butler and Trumble 2012). Management of this pathogen and vector is difficult even with the use of insecticides, and current research efforts seek to provide new management strategies (Munyaneza 2012). The development of potato varieties which exhibit resistance to the pathogen, vector, or both would provide cost-effective tools to reduce the risk of zebra chip disease. Efforts are reportedly underway to identify potato varieties with resistance or tolerance to “Ca. Liberibacter solanacearum,” (Munyaneza 2012), and two studies have identified variation in host suitability to B. cockerelli among breeding clones (Butler et al. 2011; Diaz-Montano et al. 2013).
Wild potato germplasm provide sources of resistance to diseases and insects in the development of new crop cultivars (Plaisted and Hoopes 1989; Spooner and Bamberg 1994; Jansky 2000). Previous reports indicated that certain populations of wild potato germplasm exhibit resistance to aphids (Hemiptera: Aphididae), which have phloem-feeding strategies and behaviors similar to those of psyllids (Graham et al. 1959; Flanders et al. 1992, 1997; Park et al. 2005; van der Vossen et al. 2005; Le Roux et al. 2007; Pelletier et al. 2010; Davis et al. 2012). However, wild potatoes have not yet been screened for resistance to potato psyllids. The objective of our study was to compare the susceptibility to B. cockerelli among germplasm populations of the wild potato species, S. bulbocastanum Dun. We chose to screen S. bulbocastanum because the native range this potato species overlaps with that of B. cockerelli, increasing the probability that psyllid resistance has evolved in populations of S. bulbocastanum (Flanders et al. 1997; Walters 2011).
Materials and Methods
Plants and Insects
S. bulbocastanum germplasm (all 53 populations available in 2012) were obtained from the US Potato Genebank in Sturgeon Bay, WI. Full details on these populations are available at http://www.ars-grin.gov/npgs/acc/acc_queries.html under the specific Genebank sample code for each accession (Table 1). Experimental plants were grown from seed in either 450-cm3 plastic pots for the choice screening assays, or in 900-cm3 plastic pots for the no-choice performance assays. For both sets of experiments, plants were grown in soil consisting of a 1:2:2 ratio of pumice, sand, and peat mixed with Osmocoat slow-release fertilizer (Scotts Miracle-Gro Company, Marysville, OH). The plants were maintained in a greenhouse with supplemental lighting to provide a 16:8 (L:D) h photoperiod, and were fertilized once every 2 weeks with Miracle-Gro (Scotts Miracle-Gro Company). S. bulbocastanum plants of similar size were selected for assays about 3.5 months after sowing seeds. Four-week old ‘Russet Burbank’ potatoes grown from virus-free tubers were included in the performance assays as a general comparison between psyllid performance on S. bulbocastanum and S. tuberosum.
Potato psyllids were obtained from a laboratory colony maintained on ‘Ranger Russett’ potato and ‘Moneymaker’ tomato at 25 °C with a 16:8 (L:D) h photoperiod and ≈ 50 % relative humidity. The colony was originally established from psyllids (western haplotype) collected from fields of potato near Prosser, WA in the spring of 2012. Subsets of colony insects were routinely screened for the presence of “Ca. Liberibacter solanacearum” using PCR to ensure that the colony was maintained pathogen-free.
Choice Pre-Screening Assays
Table 1 provides a complete list of germplasm included in our pre-screening assays. Three populations were not included in our assays due to low numbers of usable plants at the start of the experiments. Fifty plants, one from each germplasm population, were placed into each of two dome cages. Each cage was infested with 100 adult potato psyllids, which were evenly distributed throughout the cages. Twenty-one days after releasing adults, the numbers of eggs, early instars (first through third instars), and late instars (fourth and fifth instars) present on each plant were counted. The 21-day duration was chosen because the eggs oviposited on the day of adult releases should reach the fifth instar by this time, but not yet molt to adult (Tran et al. 2012). The experiment was repeated three times with different cohorts of plants and insects for a total of 6 cages. The large number of plant populations included in the assays prevented meaningful statistical comparisons in susceptibility to psyllids among germplasm populations. Instead, plant susceptibility to B. cockerelli was ranked based on the mean numbers of eggs, early instars, and late instars observed on the plants at the end of the experiment. The lower and upper quartiles associated with the mean numbers of each insect age class, regardless of germplasm population, were calculated using SAS 9.3 (SAS Institute 2012). The least susceptible populations were identified as those with mean numbers of eggs, early instars, and late instars each below the lower quartile of their respective insect age class. The most susceptible populations were identified as those with mean numbers of eggs, early instars, and late instars above the upper quartile of their respective age class. Germplasm that supported numbers of at least one insect age class between the lower and upper quartiles were ranked as having intermediate susceptibility to B. cockerelli.
No-Choice Performance Assays
Oviposition rates and nymph survival and development were compared using no-choice performance assays among a subset of S. bulbocastanum populations selected based on results of the choice pre-screening assays. Three reproductively mature (>7 d old) female psyllids were confined to each of 5 seedlings per germplasm using a cage made of fine mesh. A rubber band enclosed the mesh cage around the rim of the pot, and a bamboo rod supported the weight of the cage. The adults were removed from the cages after 4 days, and the number of eggs on each plant was counted with the aid of an illuminated bench-top magnifier (2.25×). Twenty-one days after insect releases, each plant was examined for numbers of early instars (first through third), fourth instars, fifth instars, and adults, and offspring development was estimated using the weighted mean life-stage present on each plant (Pfeiffer and Burts 1983).
All statistical analyses were performed using SAS 9.3 (PROC GLIMMIX, SAS Institute 2012). In separate ANOVAs, the numbers of eggs, surviving offspring, and mean life-stage of offspring were compared among germplasm populations. The proportion of surviving offspring was compared among germplasm using logistic regression with offspring/eggs as the dependent variable. Germplasm population was included as the fixed effect in each analysis. Because of the difficulty imposed by compound leaves, curled leaflets, and dense leaf pubescence in accurately counting eggs on ‘Russet Burbank’, these plants were not included in analyses of oviposition rates or proportion of surviving offspring. Data were examined for heterogeneity of variance and non-normality of errors by inspecting residual and normal quantile-quantile plots, respectively. Based on these plots, count data for analyses of numbers of eggs and surviving offspring were modeled assuming a Poison distribution. Where differences among populations were indicated, means were compared using the simulation-based multiple comparison (Edwards and Berry 1987).
Results and Discussion
Numbers of eggs, early instars (first through third) and late instars (fourth and fifth) varied among the 50 S. bulbocastanum germplasm populations screened in our choice assays (Table 1). The mean numbers of insects observed among germplasm populations ranged from 3 to 141 eggs per plant, 7 to 247 early instars per plant, and 0 to 50 late instars per plant. Six S. bulbocastanum populations supported mean numbers of eggs, early instars, and late instars that were each below the lower quartile of their respective insect age classes observed on all S. bulbocastanum (Table 1, lower quartile). The low numbers of eggs and nymphs observed on plants from these germplasm suggests that these plants may exhibit traits that reduce B. cockerelli oviposition preference or the rate of nymph development. Several germplasm populations, including PI 243513, PI 275192, PI 275198, and PI 545751, had mean numbers of eggs or early instars that were above the lower quartile for their respective age class, but supported few late instars (Table 1). Although females may readily oviposit on these plants, nymph survival or development rates may be lower relative to those on most other germplasm populations. Four populations supported mean numbers of eggs, early instars, and late instars that were each above the upper quartiles observed for their respective age classes (Table 1, upper quartile), and may represent the most psyllid-susceptible S. bulbocastanum populations included in our study.
Although sources of psyllid resistance may not be limited to germplasm populations that appear resistant based on results of the choice assays, these assays were designed to identify the most psyllid-resistant populations. However, these choice assays do not provide reliable information about whether plant traits confer antixenosis or antibiosis to psyllids. Antixenosis alters the behavior of an insect and is typically expressed as an insect preferring to oviposit or feed upon a susceptible plant compared with a resistant plant. Antibiosis affects the biology of an insect and is often expressed as increased mortality of insects on resistant plants compared with susceptible plants. The efficacy of host-plant resistance used for crop protection on ecological scales may differ depending upon whether the resistance traits confer antixenosis, antibiosis, or both. It is therefore necessary to perform no-choice performance assays to identify the mechanisms of psyllid resistance in order to assess the agronomic value of the resistance traits.
Ten germplasm populations were selected for inclusion in no-choice performance assays based on results of the choice assays. These included the six germplasm that appeared resistant to B. cockerelli (Table 1, lower quartile), three of the most susceptible germplasm (PI 255516, PI 347757, and PI 604073), and one additional germplasm population that did not support high populations of nymphs in the choice assays (PI 243513). However, the germplasm population PI 243510 was not included in the assays due to a low number of vigorous plants at the start of the experiment. ‘Russet Burbank’ potato was also included in the no-choice assays as a general comparison of psyllid performance on S. tuberosum and S. bulbocastanum. Limiting the number of germplasm populations included in the performance assays allowed statistically relevant comparisons in psyllid performance among the germplasm that could not be performed if all 50 populations were included. In addition, the use of no-choice performance assays provided valuable knowledge on the basic mechanisms of potential psyllid resistance in S. bulbocastanum.
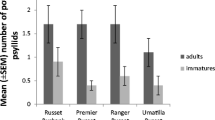
There were significant differences among plants for each measure of psyllid performance: number of eggs oviposited after 4 days, number of surviving offspring after 21 days, proportion of surviving offspring after 21 days, and mean life-stage of offspring after 21 days (Table 2). In general, results of the no-choice performance assays were consistent with results of the choice screening assays. The germplasm PI 604073 was more suitable to B. cockerelli performance compared to other S. bulbocastanum populations in each parameter measured. Where meaningful comparisons could be made, psyllid performance on this germplasm did not differ from that on ‘Russet Burbank.’ However, these comparisons should be interpreted cautiously due to the differences between S. bulbocastanum and ‘Russet Burbank’ in plant age and propagation methods. The germplasm PI 255516 was also more susceptible to B. cockerelli compared to most other S. bulbocastanum populations with regards to numbers of eggs and surviving offspring. These germplasm should be useful for use as susceptible controls in future studies to characterize psyllid resistance in S. bulbocastanum.
Two S. bulbocastanum populations, PI 283096 and PI 275197, exhibited strong resistance to B. cockerelli relative to other S. bulbocastanum populations. Both populations supported few eggs and nymphs, and a lower proportion of surviving offspring compared with other germplasm (Table 2). The reduced oviposition and survival by psyllids on these plants relative to that on other germplasm suggest that these plants exhibit both antixenosis to adults and antibiosis to nymphs. Another population, PI 255518, supported few eggs and nymphs, but the proportion of surviving offspring and the mean life-stage of offspring on these plants did not differ significantly from that on the susceptible population, PI 604073. This population may exhibit antixenosis to adults, yet lack antibiosis that reduces nymph survival and development rates. The remaining four germplasm populations (PI 243512, PI 243513, PI 275194, and PI 347757) exhibited an intermediate susceptibility relative to other populations. The high survival but young life stage of psyllids on PI 243513 and PI 275194 suggest that psyllids may develop slower on these plants compared with other germplasm. These results indicate that these six germplasm populations may provide valuable sources of resistance to B. cockerelli.
Plantings of potato cultivars with B. cockerelli resistance could provide a cost-effective control strategy for this pest and for “Ca. Liberibacter solanacearum.” If sources of resistance to “Ca. Liberibacter solanacearum” are also discovered (Munyaneza 2012), then the combined use of resistance to Liberibacter and B. cockerelli could strengthen the long-term durability of pathogen resistance. The documentation of variation in susceptibility to B. cockerelli among S. bulbocastanum germplasm and the identification of populations exhibiting psyllid resistance should facilitate the development of new potato cultivars that are resistant to the potato psyllid. Further studies are needed to determine whether resistance traits from different populations can be combined for enhanced psyllid resistance, and to determine how much of the resistance from S. bulbocastanum can be transferred to the background of a marketable potato cultivar. Several R-genes that confer resistance to late blight (Phytophthora infestans), including Rpi-blb2 from S. bulbocastanum, are expressed in interspecific hybrids involving S. bulbocastanum and S. tuberosum (Hermsen and Ramanna 1973), and in somatic hybrids of S. bulbocastanum PI 243510 (Table 1, lower quartile) and S. tuberosum (Helgeson et al. 1998; Song et al. 2003; van der Vossen et al. 2005; Park et al. 2005). These same somatic transfusions are also resistant to potato aphid and green-peach aphid (Davis et al. 2012). Therefore, future studies should also screen these somatic hybrids for psyllid resistance. It is also important to screen resistant plants against all B. cockerelli haplotypes identified in the United States (Swisher et al. 2012, 2013, 2014) to predict the potential durability resistance traits.
References
Butler, C.D., and J.T. Trumble. 2012. The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae): life history, relationship to plant disease, and management strategies. Terrestrial Arthropod Reviews 5: 87–111.
Butler, C.D., B. Gonzalez, K.L. Manjunath, R.F. Lee, R.G. Novy, J. Creighton Miller, and J.T. Tumble. 2011. Behavioral responses of adult potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae), to potato germplasm and transmission of Candidatus Liberibacter psyllaurous. Crop Protection 30: 1233–1238.
Davis, J.A., E.B. Radcliffe, C.A. Thill, and D.W. Ragsdale. 2012. Resistance to aphids, late blight and viruses in somatic fusions and crosses of Solanum tuberosum L. and Solanum bulbocastanum Dun. American Journal of Potato Research 89: 489–500.
Diaz-Montano, J., B.G. Vindiola, N. Drew, R.G. Novy, J. Creighton Miller, and J.T. Tumble. 2013. Resistance of selected genotypes to the potato psyllid (Hemiptera: Triozidae). American Journal of Potato Research. doi:10.1007/s12230-013-9356-6.
Edwards, D., and J.J. Berry. 1987. The efficiency of simulation-based multiple comparisons. Biometrics 43: 913–928.
Flanders, K.L., J.G. Hawkes, E.B. Radcliffe, and F.I. Lauer. 1992. Insect resistance in potatoes: sources, evolutionary relationships, morphological and chemical defenses, and ecogeographical associations. Euphytica 61: 83–111.
Flanders, K.S., E.B. Radcliffe, and J.G. Hawkes. 1997. Geographical distribution of insect resistance in potatoes. Euphytica 93: 201–221.
Graham, K.M., J.S. Niederhauser, and L. Servin. 1959. Studies on fertility and late blight resistance in Solanum bulbocastanum Dun. in Mexico. Canadian Journal of Botany 37: 41–49.
Helgeson, J.P., J.D. Pohlman, S. Austin, G.T. Haberlach, S.M. Wielgus, D. Ronis, L. Zambolim, P. Tooley, J.M. McGrath, R.V. James, and W.R. Stevenson. 1998. Somatic hybrids between Solanum bulbocastanum and potato: a new source of resistance to late blight. Theoretical and Applied Genetics 96: 738–742.
Hermsen, J.G., and M.S. Ramanna. 1973. Double-bridge hybrids of Solanum bulbocastanum and cultivars of S. tuberosum. Euphytica 22: 457–466.
Jansky, S.H. 2000. Breeding for disease resistance in potato. Plant Breeding Review 19: 69–155.
Le Roux, V., E.D.M. Campan, F. Dubois, C. Vincent, and P. Giordanengo. 2007. Screening for resistance against Myzus persicae and Macrosiphum euphorbiae among wild Solanum. Annals of Applied Biology 151: 83–88.
Liefting, L.W., Z.C. Perez-Egusquiza, G.R.G. Clover, and J.A.D. Anderson. 2008. A new ‘Candidatus Liberibacter’ species in Solanum tuberosum in New Zealand. Plant Disease 92: 1474.
Liefting, L.W., P.W. Sutherland, L.I. Ward, K.L. Paice, B.S. Weir, and G.R.G. Clover. 2009. A new ‘Candidatus Liberibacter’ species associated with diseases of solanaceous crops. Plant Disease 93: 208–214.
Munyaneza, J.E. 2012. Zebra chip disease of potato: biology, epidemiology, and management. American Journal of Potato Research 89: 329–350.
Munyaneza, J.E., J.M. Crosslin, and J.E. Upton. 2007. Association of Bactericera cockerelli (Homoptera: Psyllidae) with “zebra chip”, a new potato disease in southwestern United States and Mexico. Journal of Economic Entomology 100: 656–663.
Park, T.H., J. Gros, A. Sikkema, V.G. Vleeshouwers, M. Muskens, S. Allefs, E. Jacobsen, R. Visser, and E.A. van der Vossen. 2005. The late blight resistance locus Rpi-blb3 from Solanum bulbocastanum belongs to a major late blight R gene cluster on chromosome 4 of potato. Molecular Plant-Microbe Interactions 18: 722–729.
Pelletier, Y., J. Pompon, P. Dexter, and D. Quiring. 2010. Biological performance of Myzus persicae and Macrosiphum euphorbiae (Homoptera: Aphididae) on seven wild Solanum species. Annals of Applied Biology 156: 329–336.
Pfeiffer, D.G., and E.C. Burts. 1983. Effect of tree fertilization on numbers and development of pear psylla (Homoptera: Psyllidae) and on fruit damage. Environmental Entomology 12: 898–901.
Plaisted, R.L., and R.W. Hoopes. 1989. The past record and future prospects for the use of exotic potato germplasm. American Potato Journal 66: 603–627.
Richards, B.L. 1933. Psyllid yellows of the potato. Journal of Agricultural Research 46: 189–216.
SAS Institute. 2012. SAS release 9.3 ed. SAS Institute, Cary, NC.
Secor, G.A., V. Rivera-Varas, J.A. Abad, I.M. Lee, G.R.G. Clover, L.W. Liefting, W. Li, and S.H. De Boer. 2009. Association of ‘Candidatus Liberibacter solanacearum’ with zebra chip disease of potato established by graft and psyllid transmission, electron microscopy, and PCR. Plant Disease 93: 574–583.
Song, J., J.M. Bradeen, S.K. Naess, J.A. Raasch, S.M. Wielgus, G.T. Haberlach, J. Liu, H. Kuang, S. Austin-Phillips, and C.R. Buell. 2003. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proceedings of the National Academy of Sciences of the United States of America 100: 9128–9133.
Spooner, D.M., and J.B. Bamberg. 1994. Potato genetic resources: sources of resistance and systematics. American Potato Journal 71: 325–337.
Swisher, K.D., J.E. Munyaneza, and J.M. Crosslin. 2012. High resolution melting analysis of the cytochrome I gene identifies three haplotypes of the potato psyllid in the United States. Environmental Entomology 41: 1019–1028.
Swisher, K.D., J.E. Munyaneza, and J.M. Crosslin. 2013. Temporal and spatial analysis of potato psyllid haplotypes in the United States. Environmental Entomology 42: 381–393.
Swisher, K.D., D.C. Henne, and J.M. Crosslin. 2014. Identification of a fourth haplotypes of the potato psyllid, Bactericera cockerelli, in the United States. Journal of Insect Science in press.
Teulon, D.A.J., P.J. Workman, K.L. Thomas, and M.C. Nielsen. 2009. Bactericera cockerelli: incursion, dispersal, and current distribution on vegetable crops in New Zealand. New Zealand Plant Protection 62: 136–144.
Tran, L.T., S.P. Worner, R.J. Hale, and D.A.J. Teulon. 2012. Estimating development rate and thermal requirements of Bactericera cockerelli (Hemiptera: Triozidae) reared on potato and tomato by using linear and nonlinear models. Environmental Entomology 41: 1190–1198.
van der Vossen, E.A.G., J. Gros, A. Sikkema, M. Muskens, D. Wouters, P. Wolters, A. Pereira, and S. Allefs. 2005. The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog conferring broad-spectrum late blight resistance in potato. The Plant Journal 44: 208–222.
Wallis, R.R. 1955. Ecological studies on the potato psyllid as a pest of potatoes. USDA Technical Bulletin 1107: 24pp.
Walters, D.R. 2011. Plant Defense: Warding off attack by pathogens, herbivores, and parasitic plants. Ames: Wiley-Blackwell Publishing.
Acknowledgments
Pauline Anderson and Heather Headrick provided technical assistance. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooper, W.R., Bamberg, J.B. Variation in Bactericera cockerelli (Hemiptera: Triozidae) Oviposition, Survival, and Development on Solanum bulbocastanum Germplasm. Am. J. Potato Res. 91, 532–537 (2014). https://doi.org/10.1007/s12230-014-9384-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-014-9384-x