Abstract
Bacterial soft rot is a serious disease in potato (Solanum tuberosum L.), causing rapid tuber tissue maceration and, consequently, marketable yield loss. Soft rot bacteria, including Pectobacterium carotovorum subsp. carotovorum (Pbc), are favored by moist conditions, which are prevalent in large potato storage facilities. However, although most potatoes in North America are stored before use, there are no published surveys of soft rot resistance in cultivars exposed to long-term storage conditions. Thus, we tested 65 cultivars and 13 breeding lines for soft rot resistance after 6 months of storage. There was a significant effect of cultivar and production environment on soft rot resistance score. During 6 months of storage, tuber soft rot resistance in resistant clones did not change, while it changed in susceptible clones. The three most resistant cultivars to soft rot were Freedom Russet, Anett, and Alaska Red Eye.
Resumen
La pudrición blanda bacteriana es una enfermedad seria en papa (Solanum tuberosum L.), causando una maceración rápida del tejido del tubérculo, y consecuentemente, pérdida en rendimiento comercial. Las bacterias de la pudrición blanda, incluyendo Pectobacterium carotovorum subsp. Carotovorum (Pbc), son favorecidas por condiciones húmedas, que son prevalecientes en grandes instalaciones de almacenamiento de papa. No obstante, aun cuando la mayor parte de las papas en Norteamérica se almacenan antes de su uso, no hay estudios publicados de resistencia a la pudrición blanda en variedades expuestas a condiciones de almacenamiento por largo tiempo. De aquí que probamos 65 variedades y 13 líneas de mejoramiento para resistencia a la pudrición blanda después de seis meses de almacenamiento. Hubo un efecto significativo de variedad y de ambiente de producción en la calificación de resistencia a la pudrición blanda. Durante seis meses de almacenamiento, la resistencia del tubérculo a la pudrición blanda en clones resistentes no cambió, mientras que sí cambió en clones susceptibles. Las tres variedades más resistentes a la pudrición blanda fueron Freedom Russet, Anett, y Alaska Red Eye.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial soft rot disease during storage of potato tubers (Solanum tuberosum L.) is mostly caused by Pectobacterium spp. (Pérombelon and Kelman 1980). The disease causes tissue maceration in tubers, resulting in marketable yield loss at harvest and after storage. Soft rot disease causes approximately one billion dollars of damage annually in potato worldwide (Pérombelon and Kelman 1980). Soft rot inducing bacteria include P. carotovorum subsp. atrosepticum (Pca), P. carotovorum subsp. carotovorum (Pcc), and Dickeya spp. (Pch) (Pérombelon 2002). The three soft rot bacteria differ in their geographic distributions. Pca, and Pcc are found in cool climate zones and in warmer temperate and tropical zones, respectively. Soft rot bacteria infect tubers through natural openings such as lenticels and wounds (Lyon 1989; Stewart et al. 1994).
During the growing season, soft rot bacteria, especially Pcc, are favored by wet field conditions (Pérombelon and Kelman 1980). Thus, infection levels fluctuate across years, as precipitation patterns are variable. Once a field is heavily contaminated with Pcc in a favorable season for soft rot, even soil clinging to healthy harvested tubers may carry the pathogen, and can serve as a source of inoculum for neighboring tubers during storage.
Most potatoes in temperate regions are harvested in the fall, but utilized year-round. Potato tubers are stored at cool temperatures to minimize shrinkage and disease loss (Robert et al. 2002). The optimum long-term storage temperature for processing potatoes is approximately 7 °C. For fresh market potatoes, a cooler temperature (4 °C) is common. Reducing sugars accumulate at this colder temperature, but they do not interfere with quality as they do with processing potatoes. Seed potatoes may be stored at slightly lower temperatures (3 °C to 4 °C) to prevent premature sprouting.
Factors affecting soft rot resistance in potato tubers, such as calcium, dry matter and sugar content of tubers, and oxygen levels in storage, have been studied (McGuire and Kelman 1984, 1986; Otazu and Secor 1981; Pérombelon and Lowe 1975; Tzeng et al. 1990; Workman and Holm 1984). Calcium binds to cell membranes and pectic components in the middle lamella, increasing cell wall integrity (Demarty et al. 1984; Hirschi 2004; Palta 1996; Seling et al. 2000). However, calcium alone is not responsible for resistance to soft rot. High starch content is more consistently associated with resistance (McGuire and Kelman 1986; Zimnoch-Guzowska and Lojkowska 1993). High starch levels and, consequently, low water content may be unfavorable for bacterial growth. Reducing sugar levels influence tuber soft rot resistance, but not consistently across cultivars (Cother and Cullis 1987). Factors that affect resistance to soft rot interact in very complex ways depending on the genetic background of each cultivar. Consequently, it is not possible to predict cultivar resistance to soft rot without carrying out resistance tests.
When conducting resistance evaluations, it is important to know if the resistance response changes as a result of tuber storage. Storage could affect resistance levels due to physiological changes in tubers, such as response to cold stress, respiration under low oxygen conditions, and loss of dormancy. Physiological aging of tubers during long-term storage causes changes in membrane integrity, respiration, enzyme and substrate levels, calcium movement, and plant growth regulators (Coleman 2000). These changes are directly or indirectly related to cell wall integrity in the tuber. Loss of integrity results in leakage of both organic and inorganic substrates, which would foster intercellular bacterial proliferation (Workman et al. 1976). However, there is a lack of published literature on the effect of long-term storage time on resistance to soft rot. In one study, no significant change in soft rot resistance score of inoculated tubers was found after 90 days of storage at 4, 8, and 12 °C (Kushalappa and Zulfiqar 2001).
It is valuable to know potato cultivar responses to Pbc in order to minimize production and storage losses. However, most reports for soft rot resistance in potato cultivars are limited to only major cultivars and focus on factors that affect soft rot resistance, such as calcium content (Bartz et al. 1992; Bain and Pérombelon 1988; Haynes et al. 1997; Koppel 1993; Lapwood et al. 1984; Lapwood and Read 1985, 1986; Lojkowska and Kelman 1994; McGuire and Kelman 1984; Van Ittersum et al. 1990; Wolters and Collins 1995).
We hypothesized that resistance to soft rot changes during storage and that each cultivar has a different pattern for that change. Thus, we investigated the effect of storage time on soft rot resistance in potato cultivars. The objective of this study was to provide comprehensive information on soft rot resistance in 78 potato cultivars and breeding clones representing a broad array of germplasm utilized in the United States. This information is informative to pathologists, breeders, managers of storage facilities, and farmers.
Materials and Methods
Production of Tubers
On May 4, 2010, 6-hill plots of 65 cultivated potato clones were planted using an augmented experimental design at the Lelah Starks Potato Research Farm, Rhinelander, WI. These cultivars represent both current and historical cultivars from all three major market classes grown in the United States and 13 breeding lines. An identical trial was planted on May 6, 2010 at the Hancock, Wisconsin, Agricultural Research Station. At both locations, spacing between plants was 76 cm, between plots 91 cm, and between rows 91 cm. Both trials were maintained using standard cultural practices, including overhead irrigation. Vines were killed on August 16 and August 20, and tubers were lifted on September 1 and September 3, 2010, at Rhinelander and Hancock, respectively. Tubers were picked up by hand and transported to Madison immediately after harvest, where they remained at room temperature for 1 week to allow tubers to heal before they were placed in a walk-in cooler at 4 °C.
Disease Resistance Screening
All clones (cultivars and breeding clones) were screened four times, once immediately after harvest and three more times at 2 month intervals. At each sample time, three randomly selected, medium-sized tubers of each clone were rinsed with distilled water and dried overnight before inoculation. Pectobacterium carotovorum isolate WPP14 (provided by Dr. Amy Charkowski, UW-Madison) was cultured on agar plates and 10 μl of prepared bacterial suspension (1.0 × 108 CFU/ml, OD value at 0.20, which was measured with the wavelength at 600 nm) was used as the inoculum source (Yap et al. 2004). A sterilized pipette tip was used to make a 7 mm deep hole in the middle of each tuber, avoiding lenticels. Then, the inoculum was injected into the hole using a new pipette tip. A new tip was used for each inoculation. In the susceptible cultivar Atlantic, each tuber was inoculated once with the bacterium and once with water as a negative control. After a 72-h incubation period in the dark at >80 % relative humidity and room temperature (23 °C), each inoculated tuber was cut in half along the inoculated hole. Lesion diameter on the cut tuber was measured.
Statistical Analyses
Data were analyzed using the statistical program, R (version 2.10.1). After graphical visual analysis of data for trends over locations, statistical tests for normality and homogeneity of variance were performed on the soft rot resistance score over locations. One way ANOVA techniques were used to test for clone effects on soft rot resistance score. Clone was considered a fixed effect, while year and location were considered random effects in the model. An F-test was then performed to evaluate the significance of fixed effects. Markov chain Monte Carlo methods were conducted to calculate 95 % prediction intervals for random effects. If a fixed effect was significant, then Fisher’s least significant differences were calculated to evaluate soft rot resistance score differences among clones. Pearson correlation was used to correlate the soft rot resistance score from two different locations using PROC CORR (Version 9.0, SAS Institute, Cary, NC).
Results and Discussion
Soft Rot Resistance in Freshly Harvested Tubers
Significant variation for lesion diameter was found among the 78 clones (Table 1). When data from the two trials were combined, the coefficient of variance (CV) was 17.97. This relatively low value indicates that the experiments were consistent and repeatable. The mean lesion diameter of the three most resistant clones (Freedom Russet, Anett, and Alaska Red Eye) was 6.17, 6.33, and 6.67 mm, respectively, while that of the three most susceptible clones (LaChipper, Penobscot, and Taebok Valley) was 12.60, 12.84, and 13.17 mm, respectively (Table 2).
Effect of Storage on Soft Rot Resistance
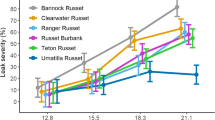
Significant variation for soft rot resistance was found among 78 clones over four storage times, based on regression analysis of the two trials (Table 3). We modeled an intercept and then modeled additional intercepts for clone and time effects. The time adjustment to the intercept was not significant while the clone adjustment was significant. Notably, average tuber soft rot resistance levels combined over both locations did not change during 6 months of storage. However, there was a significant interaction between clones and time. This indicates that clones differ in their soft rot resistance levels over storage time and must be analyzed separately. Thus, standardized residuals to fitted values were examined (data not shown). This analysis evaluated variation (as represented by errors) over soft rot resistance score. The fan-shape residual revealed a trend for susceptible clones to be more variable than more resistant clones.
To determine whether the variation in susceptible clones is due to an interaction between soft rot resistance and storage time, all clones were grouped by quartiles based on their mean of soft rot resistance scores. A significant effect of clone was found only in the lowest quartile (the most susceptible clones) (Table 4). Within each of the other groups, there was no significant effect of storage time or the interaction between clones and storage time. The P value of the lowest quartile was 0.10, while P values for other groups were very high (results not shown). Since the small number of clones in the lowest quartile was 20, a P value of 0.10 could indicate a Type II error. It appears, then, that the response to P. carotovorum in susceptible clones may change during storage, while non-susceptible clones are stable during storage for 6 months.
At each time point, correlations between the soft rot resistance score at the two locations were highly significant, ranging from 0.37 to 0.49 (Table 5). This result is consistent with the report by Tzeng et al. (1990) that resistance rankings of tubers from different cultivars to bacterial soft rot was similar for two locations with different soil types. We do not know why the level of resistance to soft rot in susceptible clones tends to fluctuate over time. It might be that 6 months is not enough time to change tuber physiology, such as electrolytic leakage, cell turgidity, membrane permeability, water loss, and calcium movement to cause soft rot in the more resistant clones, but it is enough for susceptible clones. Possibly resistance mechanisms in the most resistant clones might be stable over time and variability in susceptible clones is not related to those resistance mechanisms. However, resistance levels did change in a few resistant clones over time and the opposite was true in a few susceptible clones.
Each clone has a different genetic combination contributing to factors affecting soft rot resistance, including electrolyte composition, membrane permeability, reducing sugar levels, dry matter content, and calcium levels, as described in the introduction. The presence of only one or a few factors would likely not guarantee resistance to soft rot. This might explain the inconsistency in soft rot resistance after storage in other published studies, which used a limited number of clones.
Soft rot resistance mechanisms are complex in cultivated potatoes. It is important to carefully choose test clones when comparing cultivars with the goal of examining factors that affect soft rot resistance. Most importantly, this study provides valuable information to researchers, farmers and industry about soft rot resistance during storage. Resistant clones likely tend to be more consistent following storage than susceptible clones.
References
Bain, R.A., and M.C.M. Pérombelon. 1988. Methods of testing potato cultivars for resistance to soft rot of tubers caused by Erwinia carotovora subsp. atroseptica. Plant Pathology 37: 431–437.
Bartz, J.A., S.J. Locascio, and D.P. Weingartner. 1992. Calcium and potassium fertilization of potatoes grown in north Florida. II. Effects on the bacterial soft rot potential in the tubers. American Potato Journal 69: 39–50.
Coleman, W.K. 2000. Physiological ageing of potato tubers: A review. Annals of Applied Biology 137: 189–199.
Cother, E.J., and B.R. Cullis. 1987. Seed tuber susceptibility to Erwinia chrysanthemi: Evaluation of altered tuber physiology as a means of reducing incidence and severity of soft rot. Potato Research 30: 229–240.
Demarty, M., C. Morvan, and M. Thellier. 1984. Calcium and the cell wall. Plant Cell and Environment 7: 441–448.
Haynes, K.G., W.J.E. Potts, and R.W. Goth. 1997. Evaluation of the reliability of determining soft rot resistance in potatoes by the tuber slice method. American Potato Journal 74: 265–275.
Hirschi, K.D. 2004. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiology 136: 2438–2442.
Koppel, M. 1993. Methods of assessing potato tubers for resistance to bacterial soft rot. Potato Research 36: 183–188.
Kushalappa, A.C., and M. Zulfiqar. 2001. Effect of wet incubation time and temperature on infection, and of storage time and temperature on soft rot lesion expansion in potatoes inoculated with Erwinia carotovora ssp. carotovora. Potato Research 44: 233–242.
Lapwood, D.H., and P.J. Read. 1985. A simplified slice method for assessing tuber susceptibility of potato cultivars to Erwinia carotovora subsp. atroseptica. Plant Pathology 34: 284–286.
Lapwood, D.H., and P.J. Read. 1986. A comparison of methods of seed tuber inoculation for assessing the susceptibility of potato cultivars to blackleg (Erwinia carotovora subsp. atroseptica) in the field. Annals of Applied Biology 109: 287–297.
Lapwood, D.H., P.J. Read, and J. Spokes. 1984. Methods for assessing the susceptibility of potato tubers of different cultivars to rotting by Erwinia carotovora subspecies atroseptica and carotovora. Plant Pathology 33: 13–20.
Lojkowska, E., and A. Kelman. 1994. Comparison of the effectiveness of different methods of screening for bacterial soft rot resistance of potato tubers. American Potato Journal 71: 99–113.
Lyon, G.D. 1989. The biochemical basis of resistance of potatoes to soft rot Erwinia spp.—a review. Plant Pathology 38: 313–339.
McGuire, R.G., and A. Kelman. 1984. Reduced severity of Erwinia soft rot in potato tubers with increased calcium content. Phytopathology 74: 1250–1256.
McGuire, R.G., and A. Kelman. 1986. Calcium in potato tuber cell walls in relation to tissue maceration by Erwinia carotovora pv. atroseptica. Phytopathology 76: 401–406.
Otazu, V., and G.A. Secor. 1981. Soft rot susceptibility of potatoes with high reducing sugar content. Phytopathology 71: 290–295.
Palta, J.P. 1996. Role of calcium in plant responses to stresses: Linking basic research to the solution of practical problems. HortScience 31: 51–57.
Pérombelon, M.C.M. 2002. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathology 51: 1–12.
Pérombelon, M.C.M., and A. Kelman. 1980. Ecology of soft rot Erwinias. Annual Review of Phytopathology 18: 361–387.
Pérombelon, M.C.M., and R. Lowe. 1975. Studies on the infection of bacterial soft rot in potato tubers. Potato Research 18: 64–82.
Robert, W.B., J.C. Leslie, Y.Y. Rickey, and G.M. Alejandro. 2002. Changes in compositional parameters of tubers of potato (Solanum tuberosum) during low-temperature storage and their relationship to chip processing quality. Journal of Agricultural Food Chemestry 50: 4545–4553.
Seling, S., A.H. Wissemeier, P. Cambier, and P.P. Van Cutsem. 2000. Calcium deficiency in potato (Solanum tuberosum ssp. tuberosum) leaves and its effect on the pectic composition of the apoplastic fluid. Physiologia Plantarum 109: 44–50.
Stewart, H.E., J.E. Bradshaw, and R.L. Wastie. 1994. Correlation between resistance to late blight in foliage and tubers in potato clones from parents of contrasting resistance. Potato Research 37: 429–434.
Tzeng, K.-C., R.G. McGuire, and A. Kelman. 1990. Resistance of tubers from different potato cultivars to soft rot caused by Erwinia carotovora subsp. atroseptica. American Potato Journal 67: 287–305.
Van Ittersum, M.K., K. Scholte, and L.J.P. Kupers. 1990. A method to assess cultivar differences in rate of physiological ageing of seed tubers. American Potato Journal 67: 603–613.
Wolters, P.J.C.C., and W.W. Collins. 1995. Estimation of genetic parameters for resistance to Erwinia soft rot, specific gravity, and calcium concentration in diploid potatoes. Crop Science 35: 1346–1352.
Workman, M., and D.G. Holm. 1984. Potato clone variation in blackspot and soft rot susceptibility, redox potential, ascorbic acid, dry matter, and potassium. American Journal of Potato Research 61: 723–733.
Workman, M., E. Kerschner, and M. Harrison. 1976. The effect of storage factors on decay by Erwinia carotovora var. atroseptica and Fusarium roseum var. sambucicum. American Journal of Potato Research 53: 191–204.
Yap, M.-N., J.D. Barak, and A.O. Charkowski. 2004. Genomic diversity of Erwinia carotovora subsp. carotovora and its correlation with virulence. Applied and Environmental Microbiology 70: 3013–3023.
Zimnoch-Guzowska, E., and E. Lojkowska. 1993. Resistance to Erwinia spp. in diploid potato with a high starch content. Potato Research 36: 177–182.
Acknowledgements
We thank Emily Heenan for assisting with disease screening and Andy Hamernik for providing the tubers for this study. Partial funding was provided by USDA-NRI Grant 2009-55605-05219.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, Y.S., Goeser, N.J., Cai, X. et al. The Effect of Long Term Storage on Bacterial Soft Rot Resistance in Potato. Am. J. Potato Res. 90, 351–356 (2013). https://doi.org/10.1007/s12230-013-9311-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-013-9311-6