Abstract
Green peach aphid Myzus persicae (Sulzer) is a serious pest of potato and causes severe yield loss to potato production worldwide. The volatiles emitted from potato plants influence host selection by M. persicae. A laboratory experiment was conducted to study how these volatile emissions vary among four potato varieties (IdaRose, Desiree, Chipeta and Russet Burbank) having different genetic backgrounds, and to compare the aphid responses to the volatiles emitted by these varieties. Results indicated that headspace volatiles detected, and aphid responses, differed significantly among the varieties. Myzus persicae arrestment was greater in response to Desiree and Chipeta than on Russet Burbank and arrestment on IdaRose was intermediate and did not differ significantly from any of the other varieties, but the arrestment was not related to the total concentration of volatiles released by these varieties. This indicates that the blend of volatiles was important for eliciting arrestment. Eigenvector weightings of principal component 1 were predominantly sesquiterpenes (especially caryophyllene, sesquiphellandrene, α-farnesene and longifolene), whereas those for principal component 2 were predominantly aldehydes and green leaf volatiles (n-hexana1, (Z)-3-hexenol, (E)-2-hexenal and nonanal. The pedigrees of the varieties are not associated with the volatile blends or aphid responses.
Resumen
El áfido verde del durazno Myzus persicae (Sulzer) es una plaga seria de la papa y causa severas pérdidas de rendimiento en la producción de papa en todo el mundo. Los compuestos volátiles emitidos de la planta de papa influencian la selección de hospedante por M. persicae. Se condujo un experimento de laboratorio para estudiar cómo éstas emisiones de volátiles varían entre cuatro variedades de papa (IdaRose, Desiree, Chipeta, y Russet Burbank) con contenidos de diferentes antecedentes genéticos, y para comparar las respuestas del áfido a los volátiles emitidos por estas variedades. Los resultados indicaron que los volátiles detectados en espacios libres y la respuesta de los áfidos, diferían significativamente entre las variedades. La permanencia de M. persicae era mayor en respuesta a Desiree y Chipeta que en Russet Burbank y la retención en IdaRose fue intermedia, y no se diferenció significativamente de cualquiera de las otras variedades, pero la retención no estuvo relacionada a la concentración total de volátiles liberados por estas variedades. Esto indica que la mezcla de volátiles era importante para inducir la permanencia. La ponderación del vector propio del componente principal 1 fue predominantemente sesquiterpenos (especialmente cariofileno, sesquifelandreno, α-farneseno y longifoleno), mientras que aquellos para el componente principal 2 fueron predominantemente aldehidos y volátiles de hoja verde (n-hexana1, (Z)-3-hexenol, (E)-2-hexenal y nonanal). No se asocia al pedigrí de las variedades con las mezclas de volátiles o con las respuestas de los áfidos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green peach aphid (GPA), Myzus persicae (Sulzer) (Hemiptera: Aphididae), is an important pest of potato (Solanum tuberosum L.) due to the direct damage to the plant caused by its feeding (Petitt and Smilowitz 1982) and the indirect damage caused by viruses, especially Potato leafroll virus, for which GPA is a principal vector (Broadbent 1953; Kennedy et al. 1962). Growers manage this pest with insecticides, but the risks of environmental contamination and the economic cost of insecticide treatments necessitate new strategies for this and other insect pests. Therefore, it will be useful to learn more about how GPA responds to its host plants as a basis for potential novel approaches to pest management.
Interspecific variation in the suitability of host plants for colonization and survival of insect herbivores is well documented in natural systems and as part of efforts to develop resistant crop varieties (Painter 1951; Smith 1989; Fritz and Simms 1992), including potato (Flanders and Radcliffe 1992). Traits of importance include volatile organic compounds (VOC) (Bruce et al. 2005; Pichersky et al. 2006; Baldwin et al. 2006; Kleine and Müller 2011), which are employed during host selection and host acceptance by phytophagous insects (Miller and Strickler 1984; Bernays and Chapman 1994; Dudareva et al. 2006; Webster et al. 2012). Aphids in general do not actively forage for host plants, but they respond to host plant VOC (Pickett et al. 1992; Visser et al. 1996; Martin and Jander 2010; Webster 2012) and can discriminate based on individual VOC (Nottingham et al. 1991) or blends of VOC characteristic of their host plants (Webster et al. 2010) during host selection. Although it is a broad generalist (Emden et al. 1969; Blackman and Eastop 1984), GPA responds to host VOC (Pickett et al. 1992) and can distinguish relatively subtle differences in host VOC blends including those elicited by virus infection (Eigenbrode et al. 2002; Ngumbi et al. 2007; Werner et al. 2009). Despite this evidence of relatively fine discrimination among VOC signals by GPA, no studies are available comparing its responses to VOC from different varieties or genotypes of its host plants, including potato.
Documenting variation in VOC of potato varieties and GPA responses to those VOC would help to understand host selection by the aphid and establish a baseline for assessing volatile profiles of different cultivar under biotic and abiotic stress. Although cultivated varieties of potato do not exhibit aphid resistance, some aphid resistant lines and accessions exist (e.g., Flanders and Radcliffe 1992). Furthermore, differences in behavior that do not constitute resistance, per se, can affect infestation patterns and ecologically important behavior such as vectoring plant viruses. With this view, a study was conducted to compare the volatile profile of four potato varieties (Chipeta, Desiree, IdaRose and Russet Burbank) and to evaluate behavioral response of GPA to these varieties.
Materials and Methods
Aphids
Myzus persicae used in this study were from the clone OUR, which has been in continuous culture at the University of Idaho (UI) Parma Research and Extension Center for 30 years. This clone is routinely maintained on Indian mustard, Brassica juncea L. cultivar Florida Broadleaf. In spring 2004, a non viruliferous colony derived from OUR was established at the UI campus in Moscow ID on B. juncea plants kept in an environmental chamber at 22 ± 2 °C, 40–60 % RH, and L 16: D 8. Late-instar aphids from this colony were used in the bioassays in this study.
Treatment Plants
Certified virus-free potato (cultivars Russet Burbank, IdaRose and Chipeta) seedlings were obtained as agar grown explants from the UI Potato Nuclear Seed Program. Virus-free tubers of another potato cultivar, Desiree, was obtained commercially and tested by ELISA to ensure the material was free of virus. Microtubers were used to propagate plants for tests and bioassays. These four varieties were chosen because they represent distinct pedigrees within cultivated Solanum tuberosum. Seedlings were transplanted into 10-cm2 pots filled with Sunshine Mix No. 1 (Sun Gro Horticulture Canada, Vancouver, Canada). The plants were then grown in the greenhouse at 20 ± 2 °C with supplementary lighting to achieve an 18 h day length. For the bioassay and head space volatile collection, plants were used 60 days after planting.
Analysis of Headspace Volatiles from Plants
Volatiles were trapped for analysis from pairs of plants from each of the varieties that were used in bioassays with aphids, and 10–14 plants were used for each variety. To trap the volatiles, the plants were enclosed in a glass chamber for 2 h and carbon filtered air was drawn through the chamber with a pull pump at 300 cm3/min. After extraction, the plant tissue was removed, dried and weighed. The exhaust from the chamber passed through a trap of Super-Q adsorbent resin. Traps were eluted with methylene chloride and the elutant standardized to 200 μl. A 1-μl sample was injected onto a Hewlett-Packard 6890 gas chromatograph with a Hewlett-Packard 5973 Mass Selective Detector (Agilent Technologies, Palo Alto, CA). The column (30 m × 0.2 mm ID; HP-1) was held at 40 °C for 2 min, then heated to 250 °C at 10 °C/min and held for 10 min using the method of Eigenbrode et al. (2002). Peaks were identified based on the National Institute of Standards and Technology (NIST) library mass spectra and interpretation was based on fragmentation and spectra of authentic standards when available. Quantities were calculated as ng/g dry weight (dw)/h. Total volatile concentration in the headspace from the treatments was compared using ANOVA followed by a least significant difference (LSD) means separation procedure (PROC GLM in SAS 2010). To reduce the complexity of multivariate volatile data principal component analysis (PCA) was carried out using SAS (PROC PRINCOM). Results were visualized on a plot of PC1 vs PC2.
Emigration Bioassay
Bioassays were conducted following the method of Eigenbrode et al. (2002), at room temperature (22 ± 2 °C). The bioassay arena was made using a 150-mm diameter plastic Petri dish with a polyethylene screen (mesh size 0.5 mm) attached to the bottom as described by Eigenbrode et al. (2002). This device was placed 3 mm above the surface of a leaflet still attached to the treatment plant, allowing aphids to move freely on the screen, but preventing them from directly contacting the leaflet surface. The bottom of the Petri dish, which was covered in aluminum foil to create a darkened environment, covered the entire arena. Thus, the aphids were prevented from accessing tactile, gustatory, or visual cues from the plants. For an individual test, 30 M. persicae were placed on the screen directly over the leaflet. The number of aphids remaining directly above the leaflet was counted every 10 min for 60 min. At each 10-min interval, those aphids not directly over the leaflet were removed and counted. A filter paper (Whatman No. 2) leaf model was included as a control. This procedure allowed estimate of an emigration rate based on all the observations in each replicate. The emigration rate was estimated using maximum likelihood estimation in SAS (PROC NLMIXED, SAS Institute 2010). The data were fitted to the equation \( P=\mathrm{m}\times \left( {1-{{\mathrm{e}}^{{-\beta \mathrm{x}}}}} \right) \) where x = time of emigration (min), P = proportion of aphids emigration at time x, m = maximum proportion of emigration at the end of the experiment, β = rate of emigration, e is the base of natural logarithm. Planned contrasts after ANOVA were used to compare maximum emigration (m) and the emigration rate (β) between the four varieties and the paper model control. The bioassay was conducted in the greenhouse where the plants had been grown and completed for each plant within 1d and used 9–12 replications for each treatment.
Results
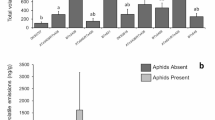
The total VOC concentrations (ng/g plant dry weight/h) detected from the four potato varieties differed significantly (P < 0.0001) (Fig. 1). Based on planned contrasts, Chipeta and Russet Burbank total VOC did not differ from Desiree (P ≥ 0.60), but VOC from Chipeta was greater than VOC from IdaRose (P ≤ 0.0001); and VOC from Russet Burbank was greater than Chipeta (P = 0.03) and IdaRose (P ≤ 0.0001). A total of 47 VOCs were detected. These belonged mostly to three groups: green leaf volatiles, monoterpenes, and sesquiterpenes. The remaining compounds were in four additional groups: benzenoids, aldehyde, n-alkanes and sesquiterpene alcohols (Table 1). Among the volatiles detected, some components including hexanal, (Z)-3-hexanal, linalool, farnesene, sesquiphellandrene, and caryophyllene were predominant in all varieties, although the amounts differed, and some others, such as pentanol acetate only occurred in some varieties. Sesquiterpenes and sesquiterpene alcohols constituted 65 % to 85 % and green leaf volatile constituted 5 % to 10 % in the total volatile profile across the four varieties (Fig. 2). Sesquiterpenes were greatest in Russet Burbank and lowest in IdaRose. Green leaf volatiles were greatest in Desiree and lowest in Chipeta and Russet Burbank and in IdaRose were intermediate. Monoterpene concentrations were greatest in Russet Burbank followed by Desiree, IdaRose and Chipeta. The amount of benzenoids and sesquiterpene alcohols were greatest in Desiree and lowest in Chipeta, while Russet Burbank and IdaRose were intermediate. Based on an inspection of Fig. 2, the profiles of Russet Burbank and Chipeta appear similar, and those of Desiree and Chipeta also appear similar, except for aldehyde and sesquiterpene alcohols. PCA analysis of the individual components in the VOC blends corroborates these patterns. A scatter plot of the first and second principal components indicates that principal component 1 was more discriminating than principal component 2. Along this axis, IdaRose and Russet Burbank tend to separate from Desiree and Chipeta (Fig. 3). The first PCA accounted for 35 % and the second PCA accounted for 20 % of the total variation in VOC. Eigenvector weightings of PC 1 were predominantly sesquiterpenes (especially caryophyllene, sesquiphellandrene, α-farnesene and longifolene), whereas those for PC 2 were predominantly aldehydes and green leaf volatiles (n-hexana1, (Z)-3-hexenol, (E)-2-hexenal and nonanal).
Mean amount of total volatile organic compound emissions from four potato varieties and based on contrast Chipeta Vs Desiree (P = 0.60); Chipeta Vs IdaRose (P = < 0.0001); Chipeta Vs Russet Burbank (P = 0.03) and Ida Rose Vs Russet Burbank (P = < 0.0001). Statistical inference based on Log transformed data. Error bars are SEM
Factor scores representing the association among the four potato varieties as revealed by PCA on relative amounts of 47 volatile organic compounds emitted. The first axis accounted for 35 % and the second axis accounted for 20 % of the total variation. Symbols are as follows: Chipeta = open squares; Desiree = open triangles; IdaRose = open circles; Russet Burbank = open diamond. Quadrants of the plot are labeled with the variety with the majority of observations within that quadrant. Approximate centroids have been added, containing all observations for each variety
In the bioassay, aphid emigration data (Fig. 4) fit the model \( P=\mathrm{m}\times \left( {1-{{\mathrm{e}}^{{-\beta \mathrm{x}}}}} \right) \) for all four varieties and for the paper model (P < 0.0001). The maximum proportion of emigration (m) and emigration rate (β) for the varieties were: Chipeta (m = 58, β = 0.06), Desiree (m = 65, β = 0.04), IdaRose (m = 68, β = 0.05), Russet Burbank (m = 72, β = 0.05), and for the paper model (m = 84, β = 0.10). Based on the planned contrasts, estimates of β did not differ among the varieties. Estimated maximum emigration (m) was significantly greater on Desiree and Chipeta than on Russet Burbank. Emigration (m) for IdaRose was intermediate and did not differ significantly from any of the other varieties (Fig. 4). Maximum emigration (m) and the rate of emigration (β) from the paper model was greater than for any of the four varieties (contrast paper vs all other was significant, P < 0.0001, for both m and β).
Cumulative M. persicae apterae emigrating from released area over a leaflet of four potato varieties and a paper model. Error bars are SEM. Data were used to fit a model to estimate the rate of emigration: \( P=\mathrm{m}\times \left( {1-{{\mathrm{e}}^{{-\beta \mathrm{x}}}}} \right) \), where x = time of emigration (min), P = proportion of aphids emigration at time x, m = maximum proportion of emigration at the end of the experiment, β = rate of emigration, e is the base of natural logarithm. Cumulative number of aphids emigrating was compared by a generalized linear model. Mean separation by letters are based on P values for pairwise contrasts among the treatments for the maximum proportion of aphid emigration (m); values with the same letter were not significantly different (P ≤ 0.05). The contrasts comparing emigration rates (β) were not significant, except that β for paper was significantly greater than for any variety
Aphid behavior was not clearly related to VOC profiles in headspace. For example, Russet Burbank and IdaRose, have significantly different total VOC concentrations and profiles based on PCA (Table 1, Figs. 1 and 3), but elicited the same rate of emigration by GPA in bioassay (Fig. 4). Similarly, Chipeta and Desiree have different volatile profiles based on PCA, but these volatile blends elicited similar rates of emigration by GPA in bioassay
Discussion
This study documents differences in VOC from four varieties of potato, which in turn elicits different behavior from GPA, a principal pest of potato and an important vector of the PLRV. This suggests that the aphid can discriminate among these varieties. Although the total concentration of headspace VOC differed significantly among the varieties tested, the aphid responses were unrelated to total VOC concentration. Furthermore, the VOC blends as characterized by PCA do not help to explain aphid responses. Previous work has shown GPA responds to VOC blends Ngumbi et al. (2007) but what characteristics of these blends are essential for eliciting arrestment is not known. This is consistent with emerging evidence for the importance of VOC blends as kairomones for herbivorous insects (Tasin et al. 2007; Webster et al. 2010; Najar-Rodriguez et al. 2010). In our study, the most arrestant blends in bioassay were those from IdaRose and Russet Burbank, which differed from one another in concentration of VOC and in overall VOC profile. It is not evident what aspect of these two blends are responsible for their stronger arrestment of the GPA. Headspace of Russet Burbank and IdaRose have greater amounts of pinene and linalool and lower amounts of 2 pentanol acetate, 2 butoxy ethanol, α-farnesene as compared to the less arrestant VOC blends of Chipeta and Desiree, so these are candidate arrestants. It remains possible, however, that aphid behavior depends not on specific compounds but particular combinations and that several different combinations could be equally arrestant for GPA. Determining how the VOC components contribute and potentially interact to influence GPA behavior is beyond the scope of this study, but Ngumbi et al. (2007), and Ngumbi and Eigenbrode (unpublished) showed that removal of any one VOC class or component can alter GPA responsiveness.
VOC profiles are heritable (Baldwin et al. 2006; Dudareva et al. 2006) and intraspecific variation in volatile profiles has been reported for many cultivated species including peach Prunus persica (Staudt et al. 2010) apple, Malus sp. (Takabayashi et al. 1991), maize, Zea mays (Hoballah et al. 2002; Gouinguene et al. 2001; Degan et al. 2004); cotton, Gossypium hirsutum (Loughrin et al. 1995); rice, Oryza sativa (Lou et al. 2006); gerbera, Gerbera jamesonii (Krips et al. 2001) and Willow, Salix viminalis (Fernandez et al. 2007). We expected the more closely related potato varieties in our study would have more similar VOC profiles but this was not clearly borne out. IdaRose and Russet Burbank had similar profiles and are related since the female parent of IdaRose (Sangre) is descended from Russet Burbank (two instances in the pedigree) (Twomey et al. 1982; Love et al. 2002; Wageningen potato pedigree database 2012). Desiree and Chipeta had similar volatile profiles and each has Katahdin in its pedigree; neither of these varieties includes Russet Burbank in its parentage and neither shares any ancestry with IdaRose (Wageningen potato pedigree database 2012). These patterns are suggestive, but may be coincidental, considering the complex pedigrees of the varieties and that most potato cultivars are autotetraploid (2n = 4x = 48) and highly heterozygous.
The VOC profiles detected in this study are generally similar to those reported previously for potato (Bolter et al. 1997; Agelopoulos and Picket 1998; Eigenbrode et al. 2002; Werner et al. 2009; Karlsson et al. 2009) but the amounts and proportions of the compounds differ. Four varieties have been studied: Desiree, Surprise, Princess and Russet Burbank. All have predominant sesquiterpenes and green leaf volatiles with monoterpenes, aldehydes, and sometimes benzenoids present in their headspace blends. In every study, caryophyllene is reported as the predominant sesquiterpene and the most abundant VOC. The other compounds reported consistently are (E)-β-farnesene, α-farnesene, (Z)-3-hexenol, (E)-2-hexenal, α-pinene, β-pinene, β-linalool, nonanal, α-elemene, germacrene D, copaene, cubebene, β-elemene, limonene, and β-myrcene. Some components differ widely in concentration or are only reported in some studies (heptanal, nonane, cadinene, toluene, indole, carene, methyl salicylate, sabinene, cadinol, undecane, tetradecane). The focus of most of these studies has been response to biological stress and profiles of untreated controls are sometimes incomplete (e.g. Agelopoulos and Picket 1998). Since the studies were conducted using different plant cultivation conditions, plant ages, headspace sampling protocols, it is not possible to compare the results other than in these general terms.
This is the first report that widely grown potato varieties differ in the volatile profiles in their headspace and that these differences elicit different behavior from one of the important pests of the crop. Although none of these varieties exhibits measurable differences in aphid resistance, different rates of colonization that could arise from the behavioral responses we document potentially affect aphid populations in mixed cropping systems, the distribution of aphids within the crop and resulting spatial distribution or spread of viruses vectored by GPA. Field studies of the rate of initial colonization among these varieties might be merited. Longer term, understanding the effects of genetics or abiotic conditions on aphid behavior could inform development of novel management approaches for GPA in potatoes.
References
Agelopoulos, N.G., and J.A. Picket. 1998. Headspace analysis in chemical ecology: Effects of different sampling methods on ratios of volatile compounds present in headspace samples. Journal of Chemical Ecology 24: 1161–1172.
Baldwin, I.T., R. Halitschke, A. Paschold, C.C.V. Dahl, and C.A. Preston. 2006. Volatile signaling in plant-plant interactions: ‘talking trees’ in the genomics era. Science 311: 812–815.
Bernays, E.A., and R.F. Chapman. 1994. Host plant selection by phytophagous Insects. New York: Chapman and Hall.
Blackman, R.L., and V.F. Eastop. 1984. Aphids on the World’s crops: An identification and information guide. London: John Wiley and Sons.
Bolter, C.J., M. Dicke, J.J.A. Vanloon, J.H. Visser, and M.A. Posthumus. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. Journal of Chemical Ecology 23: 1003–1023.
Broadbent, L. 1953. Aphids and potato virus diseases. Biological Review 28: 350–380.
Bruce, T.J.A., L.J. Wadhams, and C.M. Woodcock. 2005. Insect host location: A volatile situation. Trends in Plant Science 10: 269–274.
Degan, T., C. Dillmann, F. Marian-Poll, and T.C.J. Turlings. 2004. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiology 135: 1928–1938.
Dudareva, N., F. Negre, D.A. Nagegowda, and I. Orlova. 2006. Plant volatiles: Recent advances and future perspectives. Critical Reviews in Plant Sciences 25: 417–440.
Eigenbrode, S.D., H. Ding, P. Shiel, and P.H. Berger. 2002. Volatiles from potato plants infected with Potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proceedings of the Royal Society of B: Biological Sciences 269: 455–460.
Emden, V.H.F., V.F. Eastop, R.D. Hughes, and M.J. Way. 1969. The ecology of Myzus persicae. Annual Review of Entomology 14: 197–270.
Fernandez, P.C., T. Meiners, C. Bjorkman, and M. Hilker. 2007. Electrophysiological responses of the blue willow leaf beetle, Phratora vulgatissima, to volatiles of different Salix viminalis genotypes. Entomologia Experimentalis et Applicata 125: 157–164.
Flanders, K.L., and E.B. Radcliffe. 1992. Host plant resistance in Solanum germplasm. Minnesota Agricultural Experiment Station, University of Minnesota, Station Bulletin, 599–1992.
Fritz, R.S., and E.L. Simms (eds.). 1992. Plant resistance to herbivores and pathogens. Chicago: University of Chicago Press.
Gouinguene, S., T. Degen, and T.C.J. Turlings. 2001. Variability in herbivore induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11: 9–16.
Hoballah, M.E.F., C. Tamo, and T.C.J. Turlings. 2002. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? Journal of Chemical Ecology 28: 951–968.
Karlsson, M.F., G. Bigersson, A.M.C. Prado, F. Bosa, M. Bengtsson, and P. Witzgall. 2009. Plant odor analysis of potato: response of Guatemalan moth to above- and belowground potato volatiles. Journal of Agricultural and Food Chemistry 57: 5903–5909.
Kennedy, J.S., M.F. Day, and V.F. Eastop. 1962. A conspectus of aphids as vectors of plant viruses. London: Commonwealth Institute of Entomology.
Kleine, S., and C. Müller. 2011. Intraspecific plant chemical diversity and its relation to herbivory. Oecologia 166: 175–186.
Krips, O.E., P.E.L. Willems, R. Gols, M.A. Posthumus, G. Gort, and M. Dicke. 2001. Comparison of cultivars of ornamental crop Gerbera jamesonii on production of spider mite-induced volatiles, and their attractiveness to the predator Phytoseiulus persimilis. Journal of Chemical Ecology 27: 1355–1372.
Lou, Y., X. Hua, T.C.J. Turlings, J.A. Cheng, X. Chen, and G. Ye. 2006. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. Journal of Chemical Ecology 32: 2375–2387.
Loughrin, J.H., A. Manukian, R.R. Heath, and J.H. Tumlinson. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. Journal of Chemical Ecology 21: 1217–1227.
Love, S.L., D.L. Corsini, R. Novy, J.J. Pavek, A.R. Mosley, R.E. Thornton, S.R. James, D.C. Hane, and K.A. Rykbost. 2002. IdaRose: A potato variety with bright red skin, excellent culinary quality, and long tuber dormancy. American Journal of Potato Research 79: 79–84.
Martin, V., and G. Jander. 2010. Volatile communications in plant aphid interactions. Current Opinion in Plant Biology 13: 366–371.
Miller, J.R., and K.L. Strickler. 1984. Finding and accepting host plants. In Chemical ecology of insects, ed. W.J. Bell and R.T. Carde, 127–157. Boston: Sinauer Associates.
Najar-Rodriguez, A.J., C.G. Galizia, J. Stierle, and S. Dorn. 2010. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. Journal of Experimental Biology 213: 3388–3397.
Ngumbi, E., S.D. Eigenbrode, N.A. Bosque-Pérez, H. Ding, and A. Rodriguez. 2007. Myzus persicae is arrested more by blends than by individual compounds elevated in headspace of PLRV-infected potato. Journal of Chemical Ecology 33: 1733–1747.
Nottingham, S.F., J. Hardie, G.W. Dawson, A.J. Hick, J.A. Pickett, L.J. Wadhams, and C.M. Woodcock. 1991. Behavioural and electrophysiological responses of aphids to host and non-host plant volatiles. Journal of Chemical Ecology 17: 1231–1242.
Painter, R.H. 1951. Insect resistance in crop plants. New York: Macmillan.
Petitt, F.L., and Z. Smilowitz. 1982. Green peach aphid feeding damage to potato in various plant growth stages. Journal of Economic Entomology 75: 431–435.
Pichersky, E., J.P. Noel, and N. Duderava. 2006. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 311: 808–811.
Pickett, J.A., L.J. Wadhams, and C.M. Woodcock. 1992. The chemical ecology of aphids. Annual Review of Entomology 37: 67–90.
SAS Institute. 2010. SAS user’s guide statistics, version 9.2. Cary, NC.
Smith, C.M. 1989. Plant resistance to insects: A fundamental approach. New York: John Wiley and Sons.
Staudt, M., B. Jackson, H. El-Aouni, B. Buatois, J. Lacroze, J. Poësse, and M. Sauge. 2010. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiology : 1–15.
Takabayashi, J., M. Dicke, and M.A. Posthumus. 1991. Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: Relative influence of plant and herbivore. Chemoecology 2: 1–6.
Tasin, M., A.C. Backman, M. Coracini, D. Casado, C. Ioriatti, and P. Witzgall. 2007. Synergism and redundancy in a plant volatile blend attracting grapevine moth females. Phytochemistry 68: 203–209.
Twomey, J.A., J.J. Pavek, D.G. Holm, M. Workman, and D.L. Corsini. 1982. Sangre: an oval, high quality red potato. American Potato Journal 59: 435–437.
Visser, J.H., P.G.M. Piron, and J. Hardie. 1996. The aphid’s peripheral perception of plant volatiles. Entomologia Experimentalis et Applicata 80: 35–38.
Wageningen potato pedigree database. 2012. http://www.plantbreeding.wur.nl/potatopedigree, accessed July 1.
Webster, B. 2012. The role of olfaction in host selection. Physiological Entomology 37: 10–18.
Webster, B., T. Bruce, J. Pickett, and J. Hardie. 2010. Volatiles functioning as host cues in a blend become non host cues when presented alone to the black bean aphid. Animal Behaviour 79: 451–457.
Webster, B., E. Qvarfordt, U. Olsson, and R. Glinwood. 2012. Different roles for innate and learnt behavioral responses to odors in insect host location. Behavioral Ecology 172. doi:10.1093/beheco/ars172.
Werner, B.J., T.M. Mowry, N.A. Bosque-Pérez, H. Ding, and S.D. Eigenbrode. 2009. Changes in green peach aphid responses to Potato leafroll virus-induced volatiles emitted during disease progression. Environmental Entomology 38: 1429–1438.
Acknowledgments
The research was supported in part by award number 2009-65104-05730 from the National Institute for Food and Agriculture. We thank William Price for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajabaskar, D., Ding, H., Wu, Y. et al. Behavioral Responses of Green Peach Aphid, Myzus persicae (Sulzer), to the Volatile Organic Compound Emissions from Four Potato Varieties. Am. J. Potato Res. 90, 171–178 (2013). https://doi.org/10.1007/s12230-012-9282-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-012-9282-z