Abstract
Living botanic garden plant collections are a fundamental and underutilized worldwide resource for plant conservation. A common goal in managing a botanical living collection is to maintain the greatest biodiversity at the greatest economic and logistic efficiency. However to date there is no unified strategy for managing living plants within and among botanic gardens. We propose a strategy that combines three indicators of the management priority of a collection: information on species imperilment, genetic representation, and the operational costs associated to maintaining genetic representation. In combination or alone, these indicators can be used to assay effectiveness and efficiency of living collections, and to assign a numeric conservation value to an accession. We illustrate this approach using endangered palms that have been studied to varying degrees. Management decisions can be readily extended to other species based on our indicators. Thus, the conservation value of a species can be shared through existing databases with other botanic gardens and provide a list of recommendations toward a combined management strategy for living collections. Our approach is easily implemented and well suited for decision-making by gardens and organizations interested in plant conservation.
Resumen
Las colecciones vivas en jardines botánicos son una parte fundamental y poco utilizada en la conservación de plantas a nivel mundial. Un objetivo común en el manejo de las colecciones botánicas vivas es mantener la mayor biodiversidad al menor costo económico y logístico. Sin embargo hasta ahora no existe una estrategia unificada para el manejo de plantas vivas dentro y entre jardines botánicos. Aquí proponemos una estrategia que combina tres indicadores para establecer prioridades de manejo de una colección: información acerca del riesgo en estado silvestre de la especie, representación genética, y los costos operativos asociados a mantener la representación genética. En combinación o solos, estos indicadores pueden ser utilizados para evaluar la efectividad y eficiencia de las colecciones vivas, y para asignar un valor numérico de conservación a un espécimen. Demostramos esta estrategia con palmas que tienen diversos tipos de estudios e información disponible. Decisiones de manejo basadas en nuestros indicadores se pueden extender y aplicar fácilmente a otras especies de manera similar a como lo demostramos aquí. Además, el valor de conservación de un especie puede ser compartido con otros jardines botánicos utilizando bases de datos pre-existentes y así proveer una serie de recomendaciones hacia el manejo integrado de las colecciones vivas. Nuestra estrategia se puede implementar fácilmente y es apropiada para la toma de decisiones en jardines y organizaciones interesadas en la conservación de recursos en plantas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite progress in plant species conservation efforts, the increasing loss of plant species worldwide demands rethinking and invigorating unexploited conservation strategies that are both effective and resource-efficient for plant conservation. One such strategy is to characterize and make use of the vast number of plant species maintained at botanic gardens. Botanic gardens are central to an integrated in situ and ex situ conservation strategy (Maunder et al., 2001; Kramer et al., 2011). The Global Strategy for Plant Conservation highlights that need by requiring a minimum of 75 % of threatened plant species within ex situ collections, with at least 20 % available for recovery and restoration (Wyse Jackson & Kennedy, 2009).
Living collections are already an important element of robust conservation programs (Havens et al., 2004c; Griffith & Husby, 2010; Namoff et al., 2010; Oldfield, 2010). Botanic gardens are increasing in number and intensifying their focus on living conservation collections (Dosmann, 2006; Crane et al., 2009; Oldfield, 2009). There are over 3,000 botanic gardens and arboreta internationally (BGCI, 2011). Five hundred of them are registered in the US as members of the American Public Gardens Association, an increase from 360 within the past 20 years (Watson et al., 1993). Increased focus on living plant collections can be seen in the growth of the North American Plant Collections Consortium, which currently lists 51 nationally recognized collections, and rising at a rate of around six new collections per year. Gardens have various strategies to handle collections, which include long-term storage methods like seed-banking (Martyn et al., 2009), tissue culture (Yam et al., 2010), and cryopreservation (Volk, 2010). The fundamental conservation contribution of botanic gardens remains in cultivating living plant collections of imperiled species (Griffith et al., 2011).
Despite this progress, most living collections in botanic gardens are an underutilized source of plant genetic and species diversity in conservation and management. There is no unified management strategy for conserving threatened plants among living collections (Wyse Jackson & Sutherland, 2000; CBD, 2002; Havens et al., 2004a, b, 2006; Farnsworth et al., 2006; Seaton et al., 2010). Furthermore, it can be difficult to assess the conservation value of an ex situ collection (Schaal & Leverich, 2004; Namoff et al., 2010) and as a result evaluation of conservation value is not often performed. Perhaps a strictly unified strategy will not best serve the diversity of plant life histories but a unified concept can be broadly adaptable by many gardens.
Here, we suggest that management and use of living collections can be better aligned with threatened species conservation goals. We propose using species threat rank, and genetic tools and their association to the operational costs of maintaining a living collection. We exemplify this approach with current practices at the Arnold Arboretum at Harvard University and the Montgomery Botanical Center (MBC). We propose a strategy for an efficient and sustainable use of living collections towards threatened species conservation via an integrated Conservation Value assigned to a species within a collection. This approach can be implemented in many gardens worldwide, shared through existing databases, and used in decision-making by other interested organizations.
Indicator 1: Species Rarity and Imperilment Assessment - Arnold Arboretum
Since its founding in 1872, the Arnold Arboretum of Harvard University has been a leader in living collections management and the study and preservation of woody plant species. Recent efforts have focused on collections management to support threatened species conservation within the Arboretum’s living collection (Hird, 2010). With the goal to establish and maintain an ex situ collection of living plants that has direct, on-the-ground conservation value for natural populations (Havens et al., 2004b), we began by a better understanding of the Arboretum’s current living collections in light of plant conservation, and then strategically utilize and build upon that foundation during a two-year process.
The first task was to determine which species in the Arboretum’s living collection were threatened in the wild. Plant records database were a tool for dynamic collections information and analysis. Typically, existing functional conservation status fields contained minimal contemporary data that could be used to recognize or categorize threatened species. A survey of American Public Garden Association (APGA) member gardens revealed that most gardens maintain plant records databases, and also maintain threatened species (Hird & Dosmann, unpublished data). A majority of those gardens did not track threat ranks and could not provide basic statistics such as the total number of threatened species maintained in their collections. Similar to the Arnold Arboretum’s plant records at that time, we found a nationwide deficiency in threatened species information actually integrated into living plant collections.
An obstacle was discovered when we sought out basic threatened species information such as threat rank (Yatskievych & Spellenberg, 1993). We found numerous and varied threatened species lists and ranking schemes at various geographic levels all relevant to the Arboretum’s living collections, such as the International Union for Conservation of Nature (IUCN) Red List of globally threatened species, the NatureServe global (G), national (N), and subnational (S) ranks for North American species, and the Massachusetts Endangered Species Act (MESA). The wide variety of threat lists and ranks confounded efforts to assign meaningful and consistent conservation information to each taxon and specimen in the living collection. To compare threat level among threatened species lists, as well as evaluate taxa with multiple, sometimes conflicting threat ranks, an Arnold Arboretum Conservation Value was developed using a threatened species “Rosetta Stone” (Table 1, Indicator 1). Threat ranks and descriptions were compared and harmonized into the “Rosetta Stone”, which served to unify threatened species information and help us, for the first time, assess and prioritize the Arnold Arboretum living collections according to a single, meaningful Conservation Value scale (C-value).
Once relevant threatened species lists were identified and threat ranks harmonized, an additional obstacle arose in incorporating threatened species information into the plant records database for the nearly 4,000 total taxa in the Arnold Arboretum living collections. To avoid a manual, taxon-by-taxon comparison with each threatened species list, the BGCI Plant Upload was utilized to assign threat ranks to the entire living collection and connect our collections to a global botanical community (BGCI, 2011). The Plant Upload cross-referenced IUCN Red List (IUCN, 2001), CITES, and NatureServe G-rank (NatureServe, 2009) data with the Arboretum’s taxa list, and threat ranks were entered into the plant records database. We found this to be a fairly efficient approach for assigning relevant threatened species data at the global and North American levels. The “Rosetta Stone” was then used to assign an Arnold Arboretum Conservation Value to each threatened species in the living collections, and entered in the database.
Once threatened species information was incorporated into the plant records database in a meaningful way, further analyses could be done on threatened taxa in the living collections. The Arnold Arboretum Conservation Value allowed us to filter taxa according to greatest threat, and then include other collections parameters such as provenance and current health condition to identify greatest conservation, curatorial, educational, horticultural, and research needs and uses. Gaps and strengths found in the living collections provided focal points for increasing genetic diversity via future acquisitions; distribution of backup germplasm to other institutions; appropriate management and documentation of living specimens; and interpretation and research opportunities. This was the first time we were able to assess the value of the Arnold Arboretum living collections in light of threatened species conservation, and make informed collections management decisions for the future.
Indicator 2. Genetic Tools to Evaluate the Conservation Value of Living Collections – Montgomery Botanical Center
Living plant collections strive to represent natural variation and serve as a reservoir for potential reintroductions. It is thus critical to include knowledge of the genetic representation of the collections. In particular, a structured collecting method is essential to maintain genetically diverse living collections in ex situ collection management decisions. A broader sample maintained can enable studies on minimum viable populations and breeding strategies, such as those that currently take place in zoos and aquaria (DeSalle & Amato, 2004).
At many botanic gardens, progress has been made towards maximizing the genetic diversity in plant collections through cultivation of multiple individuals from several populations (Valois, 1994; Vaxevanidou et al., 2006).
A main concern in this effort is to avoid genetic drift in botanic garden collections, which could lead to the fixation of detrimental variation common in small populations. Very substantial drift was found for Cochlearia polonica E. Fröhl (Rucińska & Puchalski, 2011), a biennial mustard in protective cultivation for over 30 years. For Cynoglossum L. (Boraginaceae), genetic drift in garden collections appeared to increase with the duration of cultivation and in particular in plants with short generation times such as annuals, biennials, or short-lived monocarpic perennials, compared to long-lived pleonanthic perennials, such as most tree species (Enßlin et al., 2011). Other species depend entirely on living collections for their survival and reintroduction to the wild, such as the Talipot Rendah, Corypha taliera Roxb. (Arecaceae). This taxon is known from perhaps fewer than 20 living individuals and is extinct in the wild (Dhar, 1996). Its century-long, monocarpic life history adds further complexity to its management. Likewise, the Wollemi Pine, Wollemia nobilis W. G. Jones, K. D. Hill & J. M. Allen (Araucariaceae), has no resolvable genetic variation in the wild (Peakall et al., 2003), so any single-specimen garden collection could be considered a complete genetic collection for this Critically Endangered species.
At MBC we have sought to integrate and apply population genetic information into a generalized decision-making framework that can be adapted at other botanic gardens to establish conservation value of species within their collections. This framework consists of three measures of genetic variation that can be applied to answer questions related to collection management and future field planning: (1) Allelic Capture, (2) Baseline Genetic Variation, and (3) Reintroduction Potential (Fig. 1).
Framework to evaluate the conservation value of living collections from a genetic standpoint. Three measures of genetic variation can be applied to answer questions related to collection management and future field planning: Allelic Capture, Baseline Genetic Variation and Reintroduction Potential. Allelic Capture is a measure of optimal sampling for ex situ conservation based on mean genetic diversity (H E ), genetic distance, and percentage of polymorphic loci. Baseline Genetic Variation is based on measures of allelic richness and heterozygosity, and Reintroduction Potential is based on the likelihood of a sample being assigned to its population of origin
We previously proposed Allelic Capture as an indicator of the degree of genetic variation found through a population-based collection protocol in botanic gardens, and as a measure of optimal sampling for ex situ conservation (Namoff et al., 2010). We used the living collection of Leucothrinax morrisii (H. Wendl.) C. Lewis & Zona (Arecaceae) derived from a single collecting event (Namoff et al., 2010) to test this as a proof-of-principle. We estimated mean genetic diversity (H E ), genetic distance, and percentage of polymorphic loci of Intersimple Sequence Repeat (ISSRs) markers using the freely available software GenAlEx v6.4 (Peakall & Smouse, 2006). Genetic capture was studied by comparing 58 specimens from an ex situ collection with 100 individuals from the parent population. Same sample sizes with different numbers of accessions –a common scenario in living collections- were compared regarding change in allele capture, as measured by the percent of private alleles. A 3-parameter logistic model (Meyer et al., 1999) was fitted by least squares using Loglet Lab 3.0 (Rockefeller University) to evaluate the value of increasing accessions. Allelic capture was greater than 94 % in the living collection of L. morrisii, while resampled collections of different sizes captured from 48 % to 94 % of alleles. This study supported that: (1) larger collections conserve greater genetic diversity; (2) as collection size increases, genetic capture increases at a diminishing rate, and (3) there is a point at which increased investment in a collection (as reflected by more plants maintained) does not appreciably increase the conservation value of the collection (Namoff et al., 2010).
Here we detail two new genetic estimates of a species’ conservation value in botanical collections: Baseline Genetic Variation and Reintroduction Potential. Both estimates are indicators of how representative a living collection is compared to the population from which it was collected, or compared to a population of known genetic background. Both estimates can be easily obtained via free software (Genalex v6.4; Peakall & Smouse, 2006).
Baseline Genetic Variation is based on measures of allelic richness and heterozygosity. Allelic richness is critical to long-term evolutionary processes, while heterozygosity is an indicator of the extent of inbreeding in a population (Petit et al., 1998). Both are important evolutionary factors in small populations. Allelic richness is measured as the number of alleles per locus and population (N a ), and the number of private alleles (N p ). Heterozygosity as the unbiased expected heterozygosity (H E ), and observed heterozygosity (H O ) corrected for sample size (Nei, 1978).
To measure Reintroduction Potential we used the likelihood of a sample being assigned to its population of origin. If one assumes that the best-case scenario for reintroductions is to maintain the original patterns of genetic variation, then one can infer an accession’s reintroduction potential based on how well the accession represents the population of interest. Accessions that are most likely assigned to the original population or population of interest are presumed to be the best sources for reintroduction of seeds or whole plants (i.e. minimize outbreeding depression) unless the goal of managing collections is not to maximize variation. We used a frequency-based assignment test which calculated a log likelihood value for each sample to estimate if that sample is more likely assigned to itself (Population 1) or to another population (Population 2) (Paetkau et al., 2004).
As a proof-of-principle of this approach we estimated the Baseline Genetic Variation and Reintroduction Potential of 23 individuals of the tropical understory palm Chamaedorea ernesti-augustii H. Wendl. from the MBC living collections. These samples were originally collected from Mexico (n = 8) and Belize (n = 15). Chamaedorea ernesti-augustii is a small, dioecious, perennial and long-lived palm (Hodel, 1992). Chamaedorea Willd. species are prominent ecological components of the understory in Neotropical evergreen forests, constituting up to 50 % of the number of understory species in these habitats (Oyama et al., 1992). They are among the most traded ornamental palms worldwide with more than 17 million seeds and cut leaves sold annually, 99 % extracted from Mexico’s natural populations (Sosa-Martinez, 1996; Ramírez, 2001; CEC, 2002; Hodel, 2002; Eccardi, 2003). This genus provides a good example of the conservation value of living collections as several Chamaedorea species have gone locally extinct due to overharvesting and habitat loss (Escalera-Más, 1993; Radachowsky et al., 2004), including C. ernesti-augustii in some parts of Guatemala (Reyes-Rodas et al., 2006) and Mexico (Ramírez, 2001; De los Santos et al., 2003). Chamaedorea is also biologically interesting as wild populations are characterized by varying gradients of population abundance over short geographic distances (Zarco, 1999; Garwood et al., 2006) that often represent unique genetic groups at a local scale (Cibrián-Jaramillo, 2007). These patterns of human use and biological distribution have direct implications on ex situ collection management of representative samples of wild populations.
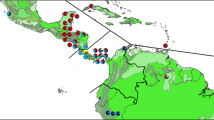
We compared estimates for the 23 ex situ individuals to assays of wild populations from Mexico and Belize. In Mexico we sampled 158 individuals from five sites within the tropical evergreen broadleaf forests of Los Tuxtlas, Veracruz; while in Belize we used information from 138 individuals from eight sites (Fig. 2) (Cibrián-Jaramillo et al., 2009; Cibrián-Jaramillo, In press).
The 23 MBC accessions from Mexico and Belize fall within the Baseline Genetic Variation found in wild populations, with an average number of alleles N a = 6.3 and 6.2; number of private alleles N p = 1.3 and 1.5; and expected heterozygosity H E = 10.0 and 8.3; and observed heterozygosity H O = 8.0 and 5.2, respectively (Fig. 3). Wild populations of this species have generally high allelic richness and high inbreeding compared to other palms (Cibrián-Jaramillo, 2007). These results suggest that MBC sampling and management have been adequate to maintain the genetic variation found in some natural populations in this species.
Baseline Genetic Variation found in wild populations and living collections (arrow) of Chamaedorea ernesti-augustii in Mexico and Belize. There is an average number of alleles N a = 6.3 and 6.2; number of private alleles N p = 1.3 and 1.5; and expected heterozygosity H E = 10.0 and 8.3; and observed heterozygosity H O = 8.0 and 5.2, respectively
Figure 4 shows the proportion of assigned MBC accessions to wild populations of C. ernesti-augustii based on the calculated Reintroduction Potential. About half of the MBC accessions from Belize were assigned to wild populations of Belize, while most of Mexico’s MBC collections were not assigned to the majority of Mexico’s wild populations, possibly due to the smaller sample size (n = 8) of the living collections. Samples from the Cerro Borrego wild populations were similarly distant from the general variation in Mexico. Other population genetic parameters of interest are discussed elsewhere (Cibrián-Jaramillo et al., 2009; Cibrián-Jaramillo, In press).
Proportion of assigned MBC accessions to wild populations of Chamaedorea ernesti-augustii. Population 1 refers to the population of origin, and Population 2 to a population other than the one of origin. A portion of MBC accessions from Belize were assigned to wild populations of Belize, while most of Mexico’s MBC collections were not assigned to the Mexican wild populations. Samples from the wild populations of Cerro Borrego were similarly distant from the general variation in Mexico
These two measures show that MBC’s collections from Mexico and Belize have similar genetic variation compared to wild populations. However, only the Belize MBC accessions would be recommended as a source of seeds for reintroduction in Belize wild populations. If maintaining a high level of genetic variation in the living collection is the primary goal, further targeted collecting of C. ernesti-augustii material from specific sites from both countries will be needed (Table 2). Assay for percent allelic capture via the garden collection (Namoff et al., 2010) shows that capture of allelic variation for each population ranges from 15 % to 43 % when the MBC plants are treated as a single group (Table 2). Compared to the previous Leucothrinax C. Lewis & Zona study (Namoff et al., 2010), this is a low percent allelic capture. This low percentage can likely be explained by this assay’s design as a post hoc assessment, without strictly congruent geographic sampling between the ex situ collection and the populations of interest.
Indicator 3: Integrating Genetic Tools and Management Operations
A desired goal in managing an ex situ living plant collection is to conserve greatest diversity at the greatest economic efficiency. Inclusion of new molecular tools with robust accounting of expenses is an innovative way to assay effectiveness and efficiency of ex situ conservation collections. To accomplish this we compared direct measure of genetic capture by living collections to operations costs. Specifically, data was collected on three parameters. First, using molecular tools, genetic diversity in wild populations is measured as described in the previous section. Then population diversity is compared to living collections genetic diversity via the same assay. Concurrently, the total monetary cost of building and maintaining the living collection is closely evaluated. These metrics can be synthesized to produce an efficiency model.
A preliminary model has been proposed for exploring the relationships among living collections conservation, cost, and efficient application of resources (Griffith & Husby, 2010). As described in the previous section, we explored the population genetics of an extensive living collection of Leucothrinax morrisii derived from a single collecting event (Namoff et al., 2010). From an economic standpoint, this study supported that: (1) larger collections conserve greater genetic diversity; (2) as collection size increases, genetic capture increases at a diminishing rate, and (3) there is a point at which increased investment in a collection (as reflected by more plants maintained) does not appreciably increase the in situ conservation value of the collection (Namoff et al., 2010). Exploring the relationship between monetary investment over the life of the collection (obtaining, documenting, germinating, and caring for the plants) and the efficacy of conservation was performed by integrating these two metrics. The interrelationship of these results for the case study can be represented as an easily interpreted model (Fig. 5). The interaction of these three variables – number of plants in collection, genetic capture, and investment – has implications for living conservation collections management. Evaluating the MBC sampling and management protocol through this case study, it was found that the existing ex situ conservation target of 15 individuals per population (Walters & Decker-Walters, 1991) can provide for a healthy level of genetic capture, consistent with institutional goals. Highest efficiency in conservation value versus resource investment occurs at around five individuals, although planned redundancy may call for a larger number (cf. Li & Pritchard, 2009, for seed bank work).
Relationship between conservation collections investment and outcomes (Adapted from Griffith & Husby, 2010). “Unit cost of conservation” is % genetic capture divided by the cost of that collection. The rate of increase in genetic capture diminishes while the cost of maintaining the collection increases steadily, as the collection size increases. Position of the Y-intercept (arrow 1) is equal to the fixed cost of bringing plants to the garden, and is never equal to zero. There are an optimum number of plants to reach an efficient “unit cost of conservation,” at the lowest Y-value (arrow 2). Greater numbers of plants will result in a steadily increased unit cost of conservation (arrow 3)
In generalized terms, this approach recommends maximizing genetic capture represented in living collections (which motivates larger collections), balanced with careful allocation of resources (which motivates smaller collections). Finding the most efficient point (Fig. 5) helps balance these two parameters.
Finally, how feasible is it to characterize collections genetically? Genetic characterization of plant germplasm collections offers valuable information (Meerow, 2005), but efficacy and economics -- time and resources -- of the genetics work must also be considered. Table 3 compares three commonly used assay methods: Random Amplified Polymorphic DNA (RAPDs; Williams et al., 1990), Amplified Fragment Ligase Polymorphism (AFLP; Vos et al., 1995), and Simple Sequence Repeat (SSR; Powell et al., 1996), based on experiences at the USDA-ARS Subtropical Horticulture Research Station (i.e. Chapman Field). Development and assay using RAPDs is easiest and least expensive, and requires only modest lab equipment. Issues with reproducibility (McGregor et al., 2000) can limit application of RAPD data for comparative purposes. AFLP analysis offers a more reliable approach (Meerow et al., 2004), but the costs are greater. SSR data offers a more reliable and finer-scale assay approach (Meerow et al., 2002, 2007), but development, assay, and equipment costs for this work are the most expensive of the three. Microsatellite discovery assays using increasingly cheaper next-generation sequencing technology (Castoe et al., 2009; Santana et al., 2009; Csencsics et al., 2010) offers a cost effective alternative approach to laborious isolation procedures (e.g., Edwards et al., 1996), and can yield significantly more informative loci.
For many botanic gardens (e.g., MBC), DNA lab infrastructure and equipment may not be a priority. Thus, a collaborative, project-based approach involving botanic garden plants and university or research collaborators can provide the expertise and lab facilities needed for collections characterization (Griffith et al., 2011). Reciprocally, botanic gardens can benefit by cultivating collections used in genetics research, such as the genotyped Cocos nucifera L. ‘Niu Leka’ (‘Fiji Dwarf’) collections currently kept at MBC (Meerow et al., 2002).
Information Sharing Across Gardens
An important aspect of successful ex situ plant conservation is to connect gardens and researchers to plants and information available in living plant collections. Facilitating the use of living collections to support research, and literally connecting individual collections to the rest of the world, is crucial as the conservation community wrestles with large-scale issues such as climate change, species and habitat loss, invasive species, and pollution. Collaboration with the broader public garden and plant conservation communities can increase the value and use of an individual collection, and support broader conservation efforts. A single garden may hold extremely rare specimens, or valuable local germplasm, and the synergy that can exist among collections has been identified as a very powerful tool for plant conservation and collection-building efforts (Kramer et al., 2011).
Sharing plant material can serve as an insurance policy against loss of that germplasm at a single institution. Institutions can also share their unique expertise and facilities to support efforts such as specialized propagation and horticultural protocols (e.g. Kay et al., 2011, for Microcycas). Furthermore, each time exchange takes place, an auditing process occurs to verify the identity and accuracy of the material and associated data, which can lead to data corrections or improvements and even new botanical discoveries.
Online facilities such as BGCI’s PlantSearch database (BGCI, 2011) are emerging as effective tools for connecting researchers directly to plants in living collections. PlantSearch is the only global database of gardens and the plants they cultivate. Gardens can upload a taxon list for free via the BGCI Plant Upload (BGCI, 2011). The benefits of the Upload are three-fold. First, the upload process completes a basic names audit via the International Plant Names Index, through which misspellings and similar database errors can be identified and later corrected. Additionally, the Plant Upload cross-checks all valid plant names with the global threatened plant lists: the IUCN Red List of Threatened Species (IUCN, 1994, 2001; Walter & Gillette, 1998), Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and NatureServe G-ranks (NatureServe, 2009). Finally, all valid names uploaded to BGCI are included in PlantSearch queries; and blind email requests for plant material and information connect online users (i.e., researchers, other garden staff, etc.) to collection managers at gardens maintaining species of interest.
For the individual garden, BGCI’s Plant Upload supplies relevant information to a living collection that can aid in strategically building ex situ collections, as demonstrated with the Arnold Arboretum Conservation Value (Hird & Dosmann, unpublished data). As living collections become more accessible to a broader community via BGCI’s PlantSearch database and others, exciting possibilities arise. One possibility is to include the quantitative C-value estimated from our indicators (Table 1) into global databases. The North American Collections Assessment demonstrates how PlantSearch data can be used to guide broader plant conservation efforts (Kramer et al., 2011). This assessment utilized the framework of PlantSearch, combined with a broad call for data sharing, to assess gaps between imperiled plant species and botanic garden living collections. This approach outlines a clear path forward for continued collections development.
Integrating Indicators into a Conservation Value - Conclusions and Recommendations
The current world situation includes limited resources and imperiled plants, and this is expected to continue going forward. Allocating botanic garden resources for maximum conservation value within their living collections is therefore important for future efforts in plant conservation. Additionally, strategic efforts in the short term will allow for more effective long term planning.
We proposed that botanic gardens are an important and currently underutilized resource for threatened species management and conservation. The first step to make better use of these collections is to determine their value. We have discussed here three main areas to consider when assessing the value of living collections for plant conservation: their conservation status in situ via a species rarity and imperilment assessment; how well they represent wild populations genetically; and the cost of management operations in maintaining a specimen.
We suggest that these three measures of a living botanical collection can be used to decide the species’ Conservation Value (Fig. 6). We have summarized this idea in an expanded version of the Conservation Value “Rosetta Stone” discussed previously (Table 1). A botanic garden manager can characterize a species based on these three metrics using the approach and tools highlighted in this paper. We add here a list of specific recommendations to facilitate the integration of our approach into the utilization of plant resources in botanic gardens to support ex situ plant conservation:
-
(1)
Single individuals and multiple plants help capture genetic diversity. A single individual can help capture significant genetic diversity -- one individual of an imperiled plant is better than none at all. However, multiple individuals tend to capture greater genetic diversity and have direct conservation applications. Larger collection sizes ensure adequate genetic representation. Coordination of management methods and plant records among multiple collections can increase the effective ex situ collection size and genetic capture for a species.
-
(2)
Increasing sample size will eventually yield diminishing returns on genetic capture. Balancing allocation of limited resources against these diminishing returns requires careful consideration of specific circumstances. As an example, growing excessive acres of a single imperiled species may tie up resources that may be better utilized maintaining multiple species.
-
(3)
Genetic data on conservation collections offers direct insight. Investing resources to assay diversity of cultivated plants can help advance understanding of the behavior of conservation genetics within botanic garden living collections. This is a strategic investment of resources that will benefit ex situ conservation in the long term. We encourage conservation genetic experts to run samples of living collections parallel to the other samples in their investigations. In most cases, this additional sampling should not increase the resources or workload for modern population genetic studies by a significant amount, given the relative sizes of cultivated collections and wild populations. This will, however provide useful information for collection holders and the collective efforts to preserve a given species.
-
(4)
Including garden plant collection genetic assay costs in grants can improve management. Researchers can factor in the cost of processing a subset of their wild collections for the genetic profile we propose here into their grants. Even a few individuals processed for genetic data would be a valuable contribution to botanic gardens where corresponding living collections are kept.
-
(5)
Conservation journals can encourage ex situ investigation. Much like requirements for deposit of type specimens (Mottram & Gorelick, 2008), enabling open-access of findings and data (Lawrence, 2001), and ensuring lack of conflict of interest, conservation journal editors can encourage parallel study of garden living collections for inclusion in basic research.
-
(6)
Conservation journals can encourage expert recommendations for botanic gardens. Conservation genetic study or review can include clear interpretation of results for botanic garden curators and managers. Such interpretation may be as simple as a bullet point list or a priority of management units for developing ex situ collections.
-
(7)
Gardens can incorporate threatened species data into living collections. Gardens can build their living collections around plant conservation objectives more effectively by incorporating threatened species ranks into their plant records databases. This will allow for strategic collections management and prioritization that more effectively supports threatened species conservation.
-
(8)
Gardens must connect their collections. Gardens should share their collections data with broader databasing efforts, such as BGCI’s PlantSearch database. By completing the BGCI Plant Upload, gardens gain valuable threatened species data about their collection, and connect and integrate their collections with the botanical research and conservation communities.
Strategy towards evaluating the conservation value of living collections in botanical gardens. At least one of three main factors should be taken into account to evaluate a collection: the status of a species in the wild (species risk assessments), the genetic representation of the collection in the context of wild variation or among the collection itself, and the operational cost of maintaining collections. A conservation value (C-value) of a species can be shared via online databases (e.g. BGCI’s PlantSearch database), leading towards a unified effort of ex situ conservation in Botanic Gardens
The need for effective ex situ plant conservation, and the role living collections can play, is only going to grow in the future. The obstacles and solutions presented above are examples of how to take the first steps toward effective ex situ collections management of living plant collections. Simply put, ex situ plant conservation cannot be an effective component of living collections management if threatened species are not easily recognized, documented, assayed and monitored within a collection. Further, effective collections management will ultimately allow ex situ collections of threatened species to be accessible and useful for conservation, education, horticulture and research applications.
Literature Cited
BGCI. 2011. GardenSearch Database. Available on the internet at: www.bgci.org/garden_search.php.
Castoe, T. A., A. W. Poole, W. Gu, A. P. J. De Koning, J. M. Daza, E. N. Smith & D. D. Pollock. 2009. Rapid identification of thousands of copperhead snake (Agkistrodon contortrix) microsatellite loci from modest amounts of 454 shotgun genome sequence. Molecular Ecology Resources 10: 341–347.
CBD. 2002. Global strategy for plant conservation. Secretariat of the Convention on Biological Diversity, Quebec.
CEC. 2002. In search of a sustainable palm market in North America, Commission for Environmental Cooperation, North American Agreement for Environmental Cooperation.
Cibrián-Jaramillo, A. 2007. Genetic connectivity in wild populations of understory Chamaedorea palms. PhD dissertation. Columbia University, New York.
——— In press. Genética de la conservación de la palma de sotobosque Chamaedorea ernesti-augustii. In: V. Reynoso Rosales (ed.), Avances y perspectivas en la investigación de bosques tropicales y sus alrededores: Los Tuxtlas. Editorial UNAM, Mexico.
———, C. D. Bacon, N. C. Garwood, R. M. Bateman, M. M. Thomas, S. Russell, C. D. Bailey, W. J. Hahn, S. G. M. Bridgewater & R. DeSalle. 2009. Population genetics of the understory fishtail palm Chamaedorea ernesti-augusti in Belize: high genetic connectivity with local differentiation. BMC Genetics 10: 65.
Crane, P., S. D. Hopper, P. H. Raven & D. W. Stevenson. 2009. Plant science research in botanic gardens. Trends in Plant Science 14: 575–577.
Csencsics, D., S. Brodbeck & R. Holderegger. 2010. Cost-effective, species-specific microsatellite development for the endangered dwarf bulrush (Typha minima) using next generation sequencing technology. Journal of Heredity 101: 789–793.
De los Santos, E. J., P. J. López, R. A. González & M. M. Bolaños. 2003. Proyecto de comercialización de productos forestales no maderables: factores de éxito y fracaso. Palma camedora (Chamaedorea spp.). Comunidad Monte Tinta, Ayotzintepec, Tuxtepec, Oaxaca, México. Grupo Mesófilo A. C., Oaxaca, Mexico.
DeSalle, R. & G. Amato. 2004. The expansion of conservation genetics. Nature Reviews Genetics 5: 702–712.
Dhar, S. 1996. Corypha taliera: endangered palm extinct in the wild. The Palm Journal 130: 10–11.
Dosmann, M. S. 2006. Research in the garden: averting the collections crisis. Botanical Review 72: 207–234.
Eccardi, F. 2003. La palma camedora. Biodiversitas 50: 1–7.
Edwards, K. J., J. H. A. Baker, A. Daly, C. Jones & A. Karp. 1996. Microsatellite libraries enriched for several microsatellite sequences in plants. BioTechniques 20: 758–760.
Enßlin, A., T. M. Sandner & D. Matthies. 2011. Consequences of ex situ cultivation of plants: genetic diversity, fitness and adaptation of the monocarpic Cynoglossum officinale L. in botanic gardens. Biological Conservation 144: 272–278.
Escalera-Más, C. E. 1993. Caracterización de los factores ecológicos relevantes en las comunidades donde el shate (Chamaedorea spp.) es componente, en San Miguel la Palotada, Petén. Facultad de Agronomía, Universidad de San Carlos de Guatemala, Guatemala.
Farnsworth, E. J., S. Klionsky, W. E. Brumback & K. Havens. 2006. A set of simple decision matrices for prioritizing collection of rare plant species for ex situ conservation. Biological Conservation 128: 1–12.
Garwood, N. C., S. G. M. Bridgewater & R. M. Bateman. 2006. Conservation and sustainable management of Chamaedorea palms in Belize. Abstracts Botany 2006 meeting, Chico, California. Available on the internet at: http://2006.botanyconference.org/engine/search/index.php?func=detail&aid=719.
Griffith, P. & C. Husby. 2010. The price of conservation: measuring the mission and its cost. BGJournal 7: 12–14.
———, ———, C. E. Lewis & J. Francisco-Ortega. 2011. Palm conservation at a botanic garden: a case study of the Keys Thatch Palm. Palms 55: 93–101.
Havens, K., E. O. Guerrant & M. Maunder. 2004a. Conservation research and public gardens. Public Garden 19: 40–43.
———, ———, ——— & P. Vitt. 2004b. Guidelines for ex situ conservation collection management: minimizing risks. Pp 454–473. In: E. O. Guerrant, K. Havens, & M. Maunder (eds). Ex situ plant conservation. Island Press with the Society for Ecological Restoration, Washington, DC.
———, ——— & ——— (eds). 2004c. Ex situ plant conservation. Island Press with the Society for Ecological Restoration, Washington, DC.
———, P. Vitt, M. Maunder, E. O. Guerrant & K. Dixon. 2006. Ex situ plant conservation and beyond. BioScience 56: 525–531.
Hird, A. 2010. Center for Plant Conservation Collections Assessment. The Arnold Arboretum of Harvard University, Boston.
——— & M. Dosmann. In Preparation. A conservation assessment model for living plant collections. The Arnold Arboretum of Harvard University. Boston, MA.
Hodel, D. R. 1992. Chamaedorea palms: the species and their cultivation. Allen Press, Lawrence, KS.
——— 2002. In search of a sustainable palm market in North America. T. C. f. E. C. i. N. America. Comission for Environmental Cooperation in North America, Montreal, Quebec.
IUCN. 1994. IUCN red list categories. Prepared by the IUCN Species Survival Commission. IUCN, Gland, Switzerland.
——— 2001. IUCN red list categories. Prepared by the IUCN Species Survival Commission. IUCN, Gland, Switzerland.
Kay, J., A. Strader, V. Murphy, L. Nghiem-Phu, M. Calonje & M. P. Griffith. 2011. Palma Corcho: A Case Study in Botanic Garden Conservation Horticulture and Economics. HortTechnology 21(4): 474–481.
Kramer, A., A. Hird, K. Shaw, M. Dosmann & R. Mims. 2011. Conserving North America’s threatened plants: progress towards target 8 of the Global Strategy for Plant Conservation. Botanic Gardens Conservation International U.S.
Lawrence, S. 2001. Free online availability substantially increases a paper’s impact. Nature 411: 521.
Li, D.-Z. & H. W. Pritchard. 2009. The science and economics of ex situ plant conservation. Trends in Plant Science 14: 614–621.
Martyn, A., D. Merritt & S. Turner. 2009. Seed banking. Pp 63–85. In: C. A. Offord & P. F. Meagher (eds). Plant germplasm conservation in Australia: strategies and guidelines for developing, managing and utilizing ex situ collections, ed. 2nd. Australian Network for Plant Conservation Inc., Canberra.
Maunder, M., B. Lyte, J. Dransfield & W. Baker. 2001. The conservation value of botanic garden palm collections. Biological Conservation 98: 259–271.
McGregor, C. E., C. A. Lambert, M. M. Greyling, J. H. Louw & L. Warnich. 2000. A comparative assessment of DNA fingerprinting techniques (RAPD, ISSR, AFLP and SSR) in tetraploid potato (Solanum tuberosum L.) germplasm. Euphytica 113: 135–144.
Meerow, A. W. 2005. Molecular genetic characterization of new floricultural germplasm. Acta Horticuturae 683: 43–62.
———, R. J. Wisser, J. S. Brown, D. N. Kuhn, R. J. Schnell II & T. K. Broschat. 2002. Analysis of genetic diversity and population structure within Florida coconut (Cocos nucifera L.) germplasm using microsatellite DNA, with special emphasis on the Fiji Dwarf cultivar. Journal of Theoretical and Applied Genetics 106: 715–726.
———, R. J. Schoellhorn & M. Kartuz. 2004. Four cultivars of Iochroma. HortTechnology 39: 194–197.
———, D. Stevenson, J. Moynihan & J. Francisco-Ortega. 2007. Unlocking the coontie conundrum: the potential of microsatellite DNA studies in the Caribbean Zamia pumila complex (Zamiaceae). Memoirs of New York Botanical Garden 98: 484–518.
Meyer, P. S., J. W. Yung & J. H. Ausubel. 1999. A primer on logistic growth and substitution: The mathematics of the loglet lab software. Technological Forecasting and Social Change 61: 247–271.
Mottram, R. & R. Gorelick. 2008. Proposal to require prior deposition of types. Taxon 57: 314.
Namoff, S., C. E. Husby, J. Francisco-Ortega, L. R. Noblick, C. E. Lewis & M. P. Griffith. 2010. How well does a botanical garden collection of a rare palm capture the genetic variation in a wild population? Biological Conservation 143: 1110–1117.
NatureServe. 2009. NatureServe Explorer: An online encyclopedia of life Version 7.1. Arlington, Virginia. Available on the internet at: www.natureserve.org/explorer.
Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583–590.
Oldfield, S. F. 2009. Botanic gardens and the conservation of tree species. Trends in Plant Science 14: 581–583.
——— 2010. Botanic gardens: modern-day arks. MIT Press, Cambridge.
Oyama, K., R. Dirzo & G. Ibarra-Manríquez. 1992. Population structure of the dominant palm species in the understory of a Mexican lowland rain forest. Tropics 2: 23–28.
Paetkau, D., R. Slade, M. Burden & A. Estoup. 2004. Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Molecular Ecology 13: 55–65.
Peakall, R. & P. E. Smouse. 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6: 288–295.
———, D. Ebert, L. J. Scott, P. F. Meagher & C. A. Offord. 2003. Comparative genetic study confirms exceptionally low genetic variation in the ancient and endangered relictual conifer, Wollemia nobilis (Araucariaceae). Molecular Ecology 12: 2331–2343.
Petit, R. J., A. El Mousadik & O. Pons. 1998. Identifying populations for conservation on the basis of genetic markers. Conservation Biology 12: 844–855.
Powell, W., G. C. Machray & J. A. Provan. 1996. Polymorphism revealed by simple sequence repeats. Trends in Plant Science 1: 215–222.
Radachowsky, J., V. H. Ramos, R. García, J. López & A. Fajardo. 2004. Effects of managed extraction on populations of the understorey palm, xaté (Chamaedorea sp.) in northern Guatemala: Monitoring ecological integrity of the Maya Biosphere Reserve, Péten, Guatemala. Wildlife Conservation Society.
Ramírez, F. 2001. La extracción de palmas camedoras en México: un grave riesgo de perdida de diversidad biológica. Proyecto de Sierra de Santa Marta. Available on the internet at: http://www.raises.org/documentacion/documentos/manejocampesino/ArtPalmas3.pdf.
Reyes-Rodas, R., R. Landivar & R. P. Wilshusen. 2006. El rol de los productos naturales en el desarrollo rural, el alivio a la pobreza y gobernabilidad en el manejo del recurso. El caso de la palma de Xate (Chamaedorea spp) en la region del Peten, Guatemala. USAID.
Rucińska, A. & J. Puchalski. 2011. Comparative molecular studies on the genetic diversity of an ex situ garden collection and its source population of the critically endangered Polish endemic plant Cochlearia polonica E. Fröhlich. Biodiversity and Conservation 20: 401–413.
Santana, Q. C., P. A. M. Coetzee, E. T. Steenkamp, O. X. Mlonyeni, G. N. A. Hammond, M. J. Wingfield & B. D. Wingfield. 2009. Microsatellite discovery by deep sequencing of enriched genomic libraries. BioTechniques 46: 217–223.
Schaal, B. & W. J. Leverich. 2004. Population genetic issues in ex situ plant conservation. Pp 267–285. In: E. O. Guerrant, K. Havens, & M. Maunder (eds). Ex situ plant conservation: supporting species survival in the wild. Island Press with the Society for Ecological Restoration, Washington, DC.
Seaton, P. T., H. Hu, H. Perner & H. W. Pritchard. 2010. Ex situ conservation of orchids in a warming world. Botanical Review 76: 193–203.
Sosa-Martinez, A. 1996. Factibilidad agroecologica para la produccion de palma camedor (Chamaedorea elegans Max) y nuez de macadamia (Maxcadamia integrifolia Maiden &Betche) en los cafetales de la region de Atoyac, Veracruz. M.Sc. Thesis.Instituto de Recursos Naturales, Colegio de Posgraduados, Campus de Veracruz, Veracruz.
Valois, A. C. C. 1994. Genetic resources of palms. Acta Horticulturae 360: 113–120.
Vaxevanidou, Z., S. C. González-Martínez, J. Climent & L. Gil. 2006. Tree populations bordering on extinction: a case study in the endemic Canary Island pine. Biological Conservation 129: 451–460.
Volk, G. M. 2010. Application of functional genomics and proteomics to plant cryopreservation. Current Genomics 11: 24–29.
Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper & M. Zabeau. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research 23: 4407–4414.
Walter, K. & H. Gillette (eds). 1998. 1997 IUCN red list of threatened plants. WCMC IUCN, Gland Switzerland and Cambridge, U.K.
Walters, T. W. & D. S. Decker-Walters. 1991. Patterns of allozyme diversity in the West Indies cycad Zamia pumila (Zamiaceae). American Journal of Botany 78: 436–445.
Watson, G. W., V. Heywood & W. Crowley. 1993. North American botanic gardens. Horticultural Reviews 15: 1–62.
Williams, J. G. K., A. Kubelik, K. J. Livak, J. A. Raflski & S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18: 6531–6535.
Wyse Jackson, P. S. & K. Kennedy. 2009. The global strategy for plant conservation: a challenge and opportunity for the international community. Trends in Plant Science 14: 578–580.
——— & L. A. Sutherland. 2000. International agenda for botanic gardens in conservation. Botanic Gardens Conservation International, U.K.
Yam, W. T., J. Chua, F. Tay & P. Ang. 2010. Conservation of the native orchids through seedling culture and reintroduction—A Singapore experience. Botanical Review 76: 263–274.
Yatskievych, G. & R. W. Spellenberg. 1993+. In Flora of North America Editorial Committee, eds.. Chapter 10: Plant Conservation in the Flora of North America Region. Volume 1 (http://fna.huh.harvard.edu/Volume/V01/Chapter10). New York and Oxford.
Zarco, E. V. 1999. Patrones biogeográficos y filogeográficos del género Chamaedorea. B.Sc. Thesis. Facultad de Ciencias. Universidad Nacional Autonoma de Mexico, Mexico City.
Acknowledgments
The authors thank Laurie Danielson, Michael Dosmann, Chad Husby, Andrea Kramer, Carl Lewis, Sandra Namoff, Larry Noblick, Sandra Rigotti and Arantza Strader, for their assistance and input; AH acknowledges the Arnold Arboretum Putnam Fellowship, ACJ acknowledges funding from the MBC, the Sackler Institute of Comparative Genomics of the American Museum of Natural History, and the Dorothy and Lewis Cullman Postdoctoral Fellowship; MPG acknowledges the International Palm Society and the Kelly Foundation for generous funding. This is contribution number 221 from the Tropical Biology Program of Florida International University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cibrian-Jaramillo, A., Hird, A., Oleas, N. et al. What is the Conservation Value of a Plant in a Botanic Garden? Using Indicators to Improve Management of Ex Situ Collections. Bot. Rev. 79, 559–577 (2013). https://doi.org/10.1007/s12229-013-9120-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-013-9120-0