Abstract
The goal of this study was to review the impact of DNA sequence analyses on our understanding of Cariceae phylogeny, classification and evolution. To explore character evolution, 105 taxa from four different studies were included in an nrDNA ITS + ETS 1f analysis of all recognized genera (Carex, Cymophyllus, Kobresia, Schoenoxiphium, Uncinia) and Carex subgenera (Carex, Psyllophora, Vignea, Vigneastra). As in previous analyses, four major Cariceae clades were recovered: (1) a “Core Carex Clade” (subg. Carex, Vigneastra, Psyllophora p.p); (2) A “Vignea Clade” (subg. Vignea, Psyllophora p.p.); (3) a “Schoenoxiphium Clade” (Schoenoxiphium, subg. Psyllophora p.p.), and (4) a “Core Unispicate Clade” (Uncinia, Kobresia, subg. Psyllophora p.p.). All studies provide strong support (86–100% BS) for the Core Carex and Vignea Clades, but only weak to moderate support (<50%–78% BS) for the Core Unispicate and Schoenoxiphium Clades. The relationships of these groups are unresolved. Studies suggest that Carex is either paraphyletic with respect to all Cariceae genera or to all genera except Schoenoxiphium. Kobresia is a grade, but Uncinia and possibly Schoenoxiphium are monophyletic. The monotypic Cymophyllus is indistinct from Carex subg. Psyllophora species. Character analyses indicate that inflorescence proliferation and reduction have occurred in all major clades, and that the Cariceae’s unisexual flowers have evolved from perfect flowers. The ancestor to Cariceae possessed a multispicate inflorescence with cladoprophylls and female spikelets with tristigmatic gynoecia and closed utricles. This morphology is most similar to extant Carex subg. Carex species, which contradicts the nearly unanimous assumption that the highly compound inflorescences of Schoenoxiphium are primitive. Since taxonomic sampling and statistical support for phylogenies have generally been poor, we advocate the temporary maintenance of the four traditional Carex subgenera with androgynous unispicate species placed within subg. Psyllophora and dioecious and gynaecandrous unispicate species distributed amongst subgenera Carex and Vignea. A collective effort focused on developing new nuclear markers, on increasing taxonomic and geographic sampling, and on studying development within the context of phylogeny, is needed to develop a phylogenetic classification of Cariceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Cyperaceae (5,000 spp., 109 genera, 14 tribes; Goetghebeur, 1998) is a cosmopolitan clade (Jones et al., 2007) of mainly anemophilous, herbaceous plants that is the third largest monocot family after orchids and grasses. Even within such a remarkable family, tribe Cariceae Pax stands out for its extraordinary diversity (2,150 spp.; Goetghebeur, 1998), extensive aneuploidy (n = 6–56; Davies, 1956), varied habitats (prairies to rain forests) and diverse biogeographic patterns (e.g., Gondwanan, Amphiatlantic, Bipolar; Raymond, 1951; Croizat, 1952). The tribe is also notable because it is clearly monophyletic (Muasya et al., 1998, 2000; Starr et al., 2006) and easily separated from all other Cyperaceae by naked unisexual flowers where the female is surrounded by a sac-like prophyll known as a utricle or perigynium (Fig. 1). The above characteristics would seem to make Cariceae an ideal model for studying the evolution of biodiversity, but factors such as its high species diversity and reduced morphology have confounded traditional attempts to create a phylogenetic classification of the group (e.g., Kükenthal, 1909; Kreczetovicz, 1936; Nelmes, 1951, 1952; Savile & Calder, 1953).
Cariceae generic circumscription. For each genus, typical spikelet morphologies are portrayed next to a stylised representation of their inflorescences [highly compound (>1 lateral order), multispicate and/or unispicate]. Note that the female spikelets in Schoenoxiphium and Kobresia have open utricles (i.e., not fused to apex) and rachillae that typically have male flowers at their apex, whereas Uncinia and Carex have closed utricles and sterile rachillae (i.e., when present). Schoenoxiphium can be distinguished from Kobresia by flattened rachillae with scabrous or ciliate margins that possess more highly developed male apices. Uncinia is separated from Carex by hooked rachillae exsert from the utricle. Cymophyllus has a similar spikelet and inflorescence morphology to unispicate Carex. Note that rachillae may be present or absent in all Carex subgenera. Modified from Starr et al. (2008)
Despite the well-marked nature of the tribe, the circumscription of genera (Carex L., Cymophyllus Mack., Kobresia Willd., Schoenoxiphium Nees, Uncinia Pers.) and infrageneric groups has been contentious with many taxa possessing a mixture of features. Traditionally, genera have been distinguished on the basis of differences in the morphology of the utricle and rachilla (i.e., the pistillate spikelet; Fig. 1), the latter interpreted as the continuation of the axis that bears a single female flower. Carex, Cymophyllus, and Uncinia are characterized by completely closed utricles (Fig. 1). Cymophyllus (1 sp.) is distinguished by its unique leaf morphology, short rachillae and white, solitary androgynous spikes. Uncinia (ca. 60 spp.) is characterized by an elongate rachilla, which is exsert from the utricle and bent like a hook. Carex (ca. 2,000 spp.) usually has multiple, non-white spikes and reduced rachillae that lack a hook (Reznicek, 1990). In contrast to these genera, Kobresia (ca. 50 spp.) and Schoenoxiphium (ca. 17 spp.) have open utricles with well-developed rachillae that bear male flowers at their termini (Fig. 1). Inflorescences are often highly compound (>1 lateral order), the consequence of proliferation of both rachillae and inflorescence axes. Kobresia and Schoenoxiphium are primarily distinguished on the basis of rachilla morphology and geography (northern hemisphere vs. predominantly South African). Past hypotheses regarding primitive and derived characters states in Cariceae are based on the assumption that compound inflorescences, composed of androgynous peduncled spikes, are primitive. For this reason, Kobresia and Schoenoxiphium are usually considered the least derived members of the tribe (Reznicek, 1990).
Owing to its size and variability, Carex has a particularly complex infrageneric taxonomy. In the only comprehensive monograph of the genus, Kükenthal (1909) divided Carex into four subgenera (Fig. 2): (1) Vigneastra (Tuck.) Kük. [=Indocarex (Baill.) Kük. in Engl.; highly compound bisexual spikes with the peduncles of the primary axes subtended by cladoprophylls, but with secondary and tertiary floral aggregations associated with utricle-like inflorescence prophylls]; (2) Carex (mostly tristigmatic flowers, peduncled unisexual spikes with the peduncle of at least the lowest spike subtended by a scale-like or ocreaform cladoprophyll); (3) Vignea (P. Beauv. ex Lestib. f.) Perterm. (sessile bisexual spikes, usually distigmatic flowers, no prophylls, setaceous bracts); and (4) Psyllophora (Degl.) Peterm. (= Primocarex Kük. in Engl.; solitary, terminal spikes) (Reznicek, 1990). Subgenus Vigneastra, with its highly compound inflorescences, is regarded as being derived from a Kobresia- or Schoenoxiphium-like ancestor and is usually considered ancestral for the genus (but see Reznicek, 1990).
Carex subgeneric circumscription based on Kükenthal (1909) (see “Introduction” for details). Note that subg. Vigneastra is distinguished from all other subgenera by the presence of peduncled, bisexual spikes (androgynous) with cladoprophylls and inflorescence prophylls; subg. Carex by peduncled, predominately unisexual spikes (lateral females, terminal males) with cladoprophylls; subg. Vignea by typically sessile, bisexual spikes (androgynous or gynaecandrous), two stigmas, and the absence of cladoprophylls, and subg. Psyllophora by a unispicate inflorescence. Reprinted from Starr et al. (2008)
Significant variation, approaching the extremes found in the tribe as a whole, occurs within most Carex subgenera. For example, the highly compound inflorescences of subgenera Vignea p.p., Carex p.p. and Vigneastra, are similar to those found in Schoenoxiphium or Kobresia p.p., while the reduced unispicate inflorescences of subgenera Carex p.p. and Psyllophora, approach the morphology of Uncinia, Cymophyllus and Kobresia p.p. Although many authors consider Kükenthal’s (1909) classification as unnatural, his taxonomy is generally maintained because of its utility for organizing gross morphological types and because of the lack of any widely accepted alternatives (e.g., Raymond, 1959; Chater, 1980; Jermy et al., 1982; Kukkonen, 1978; Kukkonen & Toivonen, 1988; Egorova, 1999).
The goal of this study is to review the impact of recent DNA sequence analyses on our understanding of Cariceae phylogeny, classification and evolution. To explore character evolution we performed an nrDNA ITS + ETS 1f analysis of taxa selectively chosen from four different studies (Starr et al., 2004, 2008; Ford et al., 2006; Waterway & Starr, 2007) to represent both “typical” variation within major taxa and taxa that have played important roles in past phylogenetic scenarios. The trees from our analyses were then manipulated to reflect unique topologies among the major Cariceae clades seen in previous phylogenetic analyses to determine what effect they would have on our inference of character evolution. This gave us an insight into the origin of the caricoid flower and the evolution of some of the most important traits used in generic and subgeneric circumscription. Based on our topologies and those of previous studies, past morphological evidence, and the results of our character analyses, we also make recommendations for future Cariceae research and classification.
Materials and Methods
Phylogenetic Analyses
All ITS and ETS 1f sequences from the 52 taxa used in the previous study by Starr et al. (2004) were included in analyses, in addition to a further 53 taxa selected from the analyses of Starr et al. (2008), Ford et al. (2006), and Waterway and Starr (2007). ITS and ETS 1f sequences for the unusual Carex subg. Vignea species C. baldensis L. were generated for this study using the PCR primers, and DNA amplification and sequencing techniques described in Starr et al. (2003, 2004). Sequences for both these regions are available from GenBank by using accession numbers EF363120 (ITS) and EF363121 (ETS 1f). The taxa and taxonomy used in this analysis are given in Table 1. The aligned matrices for all analyses, including scored characters for indels and morphology can be obtained from TreeBASE (http://www.treebase.org/).
Sequences were initially aligned with Clustal X (Thompson et al., 1997) and then adjusted manually using parsimony as an objective criterion to choose between potential alignments (Starr et al., 2004). Characters 71–100, 137–140, 203–206, 239–242, 269–281, and 1107–1111 were excluded from all analyses due to alignment ambiguity or the presence of sequence repeats. Insertion/deletions (indels) were scored in Gapcoder (Young & Healy, 2003) using the ‘simple’ gapcoding method of Simmons and Ochoterena (2000). These indels were only used in parsimony analyses.
Tree searches were performed using parsimony and Bayesian methods. For both methods Eriophorum vaginatum and Scirpus polystachyus were placed in the outgroup based on previous molecular analyses that strongly suggest these taxa are sister to Cariceae (Muasya et al., 1998; Starr et al., 2006; Simpson et al., 2007). Parsimony analyses were conducted in PAUP* 4.0b10 (Swofford, 2003) using heuristic searches and a random addition sequence of taxa for 2,000 replicates with the MULTREES option on. Support for internal branches was assessed via the bootstrap (BS; Felsenstein, 1985) using a simple addition sequence for 10,000 replicates with the MULTREES option off (DeBry & Olmstead, 2000). Clade support was subjectively described (strong, weak, etc.) according to the scheme used by Starr et al. (2004) which is based on explicit intervals in BS values. Posterior probabilities (PP, see below) were not considered when describing clade support.
Bayesian analyses were performed in MrBayes 3.0b4 (Huelsenbeck & Ronquist, 2001). The posterior tree distribution was estimated via a Metropolis-coupled Markov Chain Monte Carlo (MC3) run of 1,000,000 generations with a tree sampled every 100th generation from one (the “cold” chain) of four simultaneously run Markov chains. A general-time-reversible (GTR) model incorporating a gamma distribution (G) and invariant sites (I) was enforced during the running of the chain. The model was chosen using MrModeltest 1.1b (J. A. A. Nylander, Uppsala University). A plot of log-likelihoods versus generation number was used to determine the point where the chain levelled off and began to fluctuate around a stable value (i.e., the stationary phase). Trees sampled prior to the stationary phase were discarded. To assess whether enough generations had been run to reach convergence and to determine whether sufficient mixing of the chain had occurred to provide reliable parameter estimates, three further independent analyses using the same initial parameters as above were conducted. Convergence and mixing were assessed by a comparison of likelihood values, the mean and variance of model parameters, and the topologies of 50% majority rule consensus trees that were generated via the “sumt contype = allcompat” command (i.e., with all compatible partitions). The final Bayesian topology used in character analyses also used the “sumt contype = allcompat” command to generate a consensus of all trees sampled from the stationary phase of the four independent Bayesian analyses conducted. Estimates of branch lengths on this consensus were determined from the mean branch lengths of all summarized trees.
Character Evolution
The history of character change was inferred in Mesquite 1.06 (Maddison & Maddison, 2005) for six of the most important characters used in Cariceae classification using the Bayesian “allcompat” topology and the criteria of parsimony and maximum likelihood (ML; all characters were treated as unordered). For parsimony reconstructions, the major Cariceae clades in the Bayesian consensus were rearranged manually in Mesquite to reflect unique parsimony, ML or Bayesian topologies that were recovered in either Yen and Olmstead (2000a, b), Roalson et al. (2001), Starr et al. (2004, 2008), Waterway and Starr (2007), or the present parsimony and Bayesian analyses. Only topologies that resolved all four major Cariceae clades were used. These rearrangements were done to determine how the conclusions drawn on character evolution in this study would be affected by the topologies of previous analyses. ML reconstructions were performed under a Markov k-state 1 parameter model (Mk1) of character evolution. For interpretive purposes, likelihood reconstructions were reported as proportional likelihoods (pL) adding up to 1. Nodal support for one character state over another was considered significant if reconstructions differed by two or more log-likelihood units (Edwards, 1972; Pagel, 1999; significant support is denoted by *pL in “Results”). Multiple states were reported for binary characters where neither state was supported at a node and for multistate characters where at least two states were significantly different from a third, but not from each other. All ingroup and outgroup taxa were scored for the following characters (see Figs. 1 and 2): (1) Gross Inflorescence Structure (unispicate, 0; occasionally unispicate, 1; multispicate, 2; highly compound, 3); (2) Degree of Utricle Fusion (closed, 0; open, 1); (3) Spikelet Sexuality (one-flowered pistillate, 0; bisexual, but monoecious, 1; perfect, 2); (4) Stigma Number (three, 0; two, 1); (5) Cladoprophylls (present, 0; absent, 1); (6) Inflorescence Prophylls (present, 0; absent, 1). Character 1 can be difficult to score because some Cariceae species (e.g., Carex exilis, C. curvula) are not strictly unispicate or multispicate. Highly compound inflorescences were defined as those possessing more than one lateral order. All Kobresia and Schoenoxiphium species were treated as possessing open utricles with bisexual spikelets, except for K. nepalensis and K. esenbeckii whose pistillate spikelets are strictly one-flowered (Noltie, 1994). It should be noted, however, that considerable variability in characters 2 and 3 may be observed not only between, but even within, the inflorescences of Kobresia and Schoenoxiphium species (Clarke, 1883; Noltie, 1994). Since utricles are unique to Cariceae, character 2 could not be scored for outgroup taxa. Following Reznicek (1990), cladoprophylls are defined as the tubular structures found at the bases of the first lateral order of a lateral inflorescence unit (i.e., spike), whereas inflorescence prophylls are utricle-like structures, often possessing a fertile, female flower that are seen at the bases of second lateral order spikes and above as seen in Carex subg. Vigneastra (Fig. 2). Although C. baldensis and C. gibba possess utricle-like prophylls that typically possess a fertile, female flower like subg. Vigneastra species, both taxa were scored as present for cladoprophylls because the prophyll is found at the base of the first lateral order on the inflorescence. In general, the presence/absence of cladoprophylls for all species in this analysis could not be directly scored due to a lack of material. Unfortunately, such gaps in the dataset cannot be filled from monographic works since the presence/absence and morphology of cladoprophylls has not been consistently described for all infrageneric taxa. Although we recognize that errors in scoring may have been made, for the purposes of this study, all unispicate taxa (i.e., Uncinia, Carex subg. Psyllophora, Kobresia p.p., and Carex sect. Phyllostachyae) were scored as cladoprophylls absent and all multispicate taxa were scored as cladoprophylls present, including Carex sect. Ammoglochin subsect. Herporrhizae (C. arenaria, C. brizoides, and C. praecox), which is the only Carex subg. Vignea taxon besides C. gibba known to possess cladoprophylls (Ford et al., 2006). Since the genus Schoenoxiphium possesses prophylls that are often utricle-like and fertile at the base of the first, second and above lateral orders (Kukkonen, 1983), all strictly multispicate species from this genus were scored as possessing both cladoprophylls and inflorescence prophylls. These characters were not scored for S. filiforme (often unispicate) because of a lack of material.
Results
Phylogenetic Analysis
The aligned matrix of ITS and ETS 1f sequences including 176 indels for 105 taxa produced 1,396 aligned characters of which 89 were excluded, and 629 were parsimony informative. Parsimony searches found 75 trees, 3,927 steps long (CI = 0.34; RI = 0.68). Because the strict consensus was largely similar to Bayesian results, only a summary of the relationships of the major Cariceae clades is presented for parsimony (Fig. 3). A full description of the composition of these groups is given below.
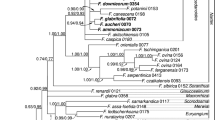
Relationships of the four major Cariceae clades in all previous analyses where one or more species from each clade was sampled and all four clades were monophyletic. The criterion used to reconstruct phylogeny is abbreviated to the left of each topology (PARS parsimony; ML maximum likelihood; Bayes Bayesian analysis). Abbreviations with darkened backgrounds represent unique topologies that were used in character reconstructions. All bootstrap values were derived from parsimony analyses and are given above branches when previously reported. Asterisks above ML or Bayesian topologies represent branches whose lengths were not significantly different from zero. Summary topologies of the present parsimony (strict consensus) and Bayesian analyses are also given for comparison. The Schoenoxiphium Clade in Roalson et al. (2001) was represented by a single Schoenoxiphium species. The Bayesian analysis of Waterway and Starr (2007) is not depicted since it has the same topology as the Bayesian tree in the present analysis. The branch lengths for the Core Carex + Vignea clade and Core Unispicate Clade in Waterway and Starr (2007) were not significantly different from zero. Squares (Core Carex Clade), open triangles (Vignea Clade), closed circles (Core Unispicate Clade) and open circles (Schoenoxiphium Clade) are used to discern differences in topologies
For Bayesian analyses, plots of log-likelihood values versus generation number indicated that the stationary phase was reached after generation 110,100 (Markov chain 1), generation 143,200 (Markov chain 2), generation 256,900 (Markov chain 3), and generation 174,500 (Markov chain 4). All trees sampled prior to these generations were excluded from majority rule consensus trees as they were not sampled from the posterior distribution. Comparisons of likelihood values, estimates of model parameters and majority rule consensus trees suggested that convergence had been reached in all four Bayesian analyses. Therefore, all 33,157 trees sampled from the stationary phase of all four independent analyses were used to generate a 50% majority rule consensus with all compatible groups in MrBayes (Fig. 4). This tree consists of four major Cariceae clades identical to those recovered in parsimony analyses: (1) A strongly supported “Core Carex Clade” (97% BS; 100% PP), containing typical multispicate and highly compound (C. fecunda) members of Carex subg. Carex, a dioecious unispicate member (C. scirpoidea) of Carex subg. Psyllophora, and species of Carex subg. Vigneastra (C. echinochloe, C. filicina, and C. polystachya) that are either variously placed within the clade or successively positioned as sister to all other clade members (C. cruciata and C. baccans); (2) a strongly supported “Vignea Clade” (98% BS; 100% PP) consisting of typical multispicate and highly compound (C. crus-corvi, C. decomposita, C. paniculata) members of Carex subg. Vignea, a dioecious unispicate species of Carex subg. Psyllophora (C. dioica) and C. gibba (subg. Vignea), a tristigmatic species with cladoprophylls that is sister to the remainder of the clade; (3) a moderately supported “Schoenoxiphium Clade” (73% BS; 100% PP), consisting of a poorly supported but monophyletic Schoenoxiphium (<50% BS; 40% PP) and androgynous unispicate members of Carex subg. Psyllophora from Europe and South America; and (4) a poorly supported “Core Unispicate Clade” (<50% BS; 100% PP) that consists of the androgynous unispicate species of Uncinia, Kobresia, Cymophyllus, Carex subgenera Carex (i.e., sect. Phyllostachyae) and Psyllophora and multispicate species of Carex subg. Vignea (i.e., Carex baldensis) and Kobresia. Within the Core Unispicate Clade notable relationships include the strong support for an Uncinia s.str. clade (100% BS, 100% PP; Fig. 4) that is sister to Uncinia kingii (i.e., Uncinia s.l.; 98% BS, 100% PP); the nested position of Cymophyllus as sister to Carex sect. Phyllostachyae (C. cordillerana and C. backii; 90% BS, 100% PP); an unnatural Kobresia, but a strongly supported unispicate Kobresia clade (100% BS, 100% PP; Fig. 4), and a nested C. baldensis sister to another subg. Vignea species, C. curvula (93% BS, 100% PP). Notably, Ecuadorian and Scottish samples of C. microglochin are positioned in separate clades (Fig. 4).
Fifty percent majority-rule consensus with all compatible groups of 33,157 trees sampled from the posterior distribution under a GTR + G + I model of sequence evolution. Numbers above branches are posterior probabilities; numbers below branches are bootstrap values ≥ 50%. Numbers in parentheses after species epithets correspond to specific vouchers in Table 1. Except for Cymophyllus, generic names are abbreviated as follows: C. = Carex, K. = Kobresia, S. = Schoenoxiphium, U. = Uncinia
The only potentially important differences between parsimony and Bayesian analyses lie in the relationships portrayed for the major Cariceae clades (cf. Figs. 3 and 4) and in the fact that Schoenoxiphium is paraphyletic in parsimony analyses but monophyletic in Bayesian analyses. These two topological differences are typical of comparisons between parsimony and model-based methods (i.e., ML/Bayesian analyses), although they have never been shown to be statistically significant (Starr et al., 2004, 2008; Waterway & Starr, 2007).
Character Evolution
The generalized topologies of the six trees used in character reconstructions are given in Fig. 3. Character 1, Gross Inflorescence Structure, had a ci of 0.11. For all topologies and reconstruction methods the ancestral character for the Vignea Clade is a multispicate inflorescence (*pL = 0.96). Within the Vignea Clade, the highly compound inflorescences of Carex decomposita, C. crus-corvi, and C. paniculata have all evolved independently. The dioecious unispicate condition seen in C. dioica and the often unispicate condition seen in C. exilis have evolved independently from monoecious multispicate ancestors. Either multispicate or highly compound inflorescences are ancestral for the Core Carex Clade, although ML reconstructions favour multispicate inflorescences as the more likely state for this node (*pL = 0.79 vs. *pL = 0.15). The dioecious unispicate C. scirpoidea evolved by reduction from a monoecious multispicate ancestor. The ancestral condition of the Caricoid Clade is most parsimoniously inferred to be unispicate on the topologies of Roalson et al. (2001), Starr et al. (2004), and in the present Bayesian analysis (PBA). ML reconstructions also suggest that unispicate inflorescences are the most likely ancestral state for this clade (unispicate *pL = 0.83 vs. multispicate *pL = 0.16). In those analyses where the Schoenoxiphium and Core Unispicate Clades are separate (Yen & Olmstead, 2000a, b; Waterway & Starr, 2007; present parsimony analysis or PPA), the ancestral state for both clades is ambiguous (multispicate/unispicate). The root node for Cariceae is ambiguous in all topologies (multispicate/unispicate or multispicate/highly compound for PPA) except those of the PBA and the parsimony analysis of Starr et al. (2004) where it is multispicate. However, all studies have the ancestral state of multispicate at the Cariceae root node if the genus Schoenoxiphium is placed as sister to the androgynous unispicate species in its clade. Moreover, when this is done, all those studies that have a Core Carex + Vignea clade (Yen & Olmstead, 2000a, b; Starr et al., 2004; Waterway & Starr, 2007; PBA) also infer the multispicate condition as being ancestral for this group. ML reconstructions suggest that multispicate inflorescences are more likely (*pL = 0.67) to be ancestral than unispicate inflorescences (*pL = 0.31) at the Cariceae root node. Character 2, Utricle Fusion, had a ci of 0.20. Both character reconstruction methods suggest that closed utricles are ancestral at the Cariceae root node and for all of the major clades in the tribe (*pL = 1.00). Character 3, Spikelet Sexuality, had a ci of 0.33. Perfect flowers are ancestral at the outgroup node in both parsimony and ML (*pL = 0.99) reconstructions. Likewise, both methods indicate that unisexual spikelets are ancestral for both the Cariceae and for all the major clades in the tribe (*pL = 1.00). Bisexual spikelets evolved multiple times and in parallel in Schoenoxiphium and Kobresia. Character 4, Stigma Number, had a ci of 0.08, with three stigmas being the ancestral character state for the tribe and all major Cariceae clades in parsimony and ML (*pL = 0.94–1.00) reconstructions. Stigma losses have been frequent and have occurred in all major Cariceae lineages. Within the Vignea Clade the loss of a stigma distinguishes a large clade that includes all Carex subg. Vignea species except Carex gibba (*pL = 0.99). Within this distigmatic clade a single reversion to the ancestral tristigmatic condition has occurred in Carex sect. Macrocephalae (C. macrocephala and C. kobomugi; *pL = 1.00). Character 5, Cladoprophylls, had a ci of 0.06. Four of the six topologies examined place the presence of cladoprophylls as the most parsimonious ancestral state for the Cariceae (Roalson et al., 2001; Starr et al., 2004; PPA and PBA). However, if the genus Schoenoxiphium is placed as sister to the unispicate androgynous species in its clade, all topologies unambiguously place the presence of a cladoprophyll as ancestral for the tribe. In contrast, ML reconstructions suggest that cladoprophyll absence is more likely (pL = 0.61) than presence (pL = 0.39) at the Cariceae root node, although the difference was not significant. All topologies and methods infer cladoprophyll presence (*pL = 0.96) as the most parsimonious ancestral state for the Core Carex Clade. Two topologies (Roalson et al., 2001 and PBA) also unambiguously place the presence of a cladoprophyll as ancestral for a Vignea + Core Carex Clade, although all other topologies are either ambiguous or consider the presence of a cladoprophyll in the Vignea Clade as an autapomorphy for C. gibba and a synapomorphy for Carex sect. Ammoglochin subsect. Herporrhizae. Likewise, ML reconstructions reflect the same ambiguity in the presence/absence of cladoprophylls in the Vignea + Core Carex (presence pL = 0.43; absence pL = 0.57) and Vignea Clades (presence pL = 0.43; absence pL = 0.57), whilst providing strong support for cladoprophyll presence as a synapomorphy for sect. Ammoglochin subsect. Herporrhizae (*pL = 0.99). The scale-like cladoprophylls of subsect. Herporrhizae and the utricle-like prophylls of C. baldensis (Core Unispicate Clade) have evolved independently. The cladoprophylls in Schoenoxiphium have also evolved independently. Character 6, Inflorescence Prophylls, had a ci of 0.20. All topologies suggest that inflorescence prophylls evolved from species that lacked them; the ancestral state at the Cariceae root node is the absence of inflorescence prophylls for both parsimony and ML (*pL = 1.00) reconstructions. All topologies demonstrate that the inflorescence prophylls in Schoenoxiphium have evolved independently of those in Carex subg. Vigneastra. Although several species of subg. Vigneastra are seen near the base of the Core Carex Clade, parsimony reconstructions cannot resolve the ancestral state for this clade. In contrast, ML reconstructions indicate that the absence of inflorescence prophylls (*pL = 0.92) is the ancestral state for this clade. Analyses suggest that the inflorescence prophylls of C. echinochloe, C. filicina and C. polystachya are examples of parallelism.
Discussion
The Major Clades of Tribe Cariceae: Circumscription and Relationships
Although Cariceae studies have differed considerably in their taxonomic (27 taxa in Yen & Olmstead, 2000b vs. 101 in Roalson et al., 2001) and geographic sampling (36.1% of taxa were of North American or European origin in Starr et al., 2008 vs. 82.5% in Waterway & Starr, 2007), all analyses have recovered the same four major clades recognized by Starr et al. (2004). To facilitate discussions on tribal phylogeny and to avoid the inconsistent practice of characterising clades by numbers or letters (e.g., Roalson et al., 2001; Starr et al., 2004, 2008), Waterway and Starr (2007) named these four clades according to the most distinctive element in each group; i.e., the “Core Carex”, “Vignea”, “Core Unispicate”, and “Schoenoxiphium” Clades (see Results for detailed clade composition). The latter two clades were further described as the “Caricoid” Clade (Waterway & Starr, 2007) to convey the idea that their morphology was different from the “typical” Carex-like features seen in the Core Carex and Vignea Clades.
In agreement with traditional views (e.g., Koyama 1962; Smith & Faulkner, 1976; Reznicek, 1990), molecular phylogenies strongly indicate that the Core Carex and Vignea Clades are monophyletic (Fig. 3). In contrast, the Core Unispicate and Schoenoxiphium Clades have received only moderate support from molecular data (Fig. 3), and the seemingly odd placement of both highly compound and multispicate Kobresia and Schoenoxiphium amongst androgynous unispicate taxa (Uncinia, Kobresia, Cymophyllus, Carex subgenera Psyllophora and Carex p.p.) appears to defy a morphological definition for these groups. The significant morphological gap that separates the multispicate and unispicate taxa of these clades could be due to an ancient origin followed by extinction, although a clearer explanation may appear when relationships are better resolved by increased character and taxonomic sampling.
Although all four major Cariceae clades have appeared in those analyses that have sampled at least one individual from each group, relationships amongst these clades have not been stable. Six different topologies have been presented in molecular studies, and no group consisting of two or more major Cariceae clades has received more than 65% BS (Fig. 3). Moreover, differences in topology are often seen between parsimony and ML/Bayesian analyses within the same study (Yen & Olmstead, 2000a; Starr et al., 2004; Waterway & Starr, 2007). The majority of topologies favour a Caricoid Clade sister to a Core Carex + Vignea Clade (Fig. 3), but until character sampling is increased, the relationships of the major Cariceae clades should be treated as unresolved.
There is also evidence to indicate that a fifth major clade may exist within the tribe. Recent work by Waterway et al. (2008) suggests that the East Asian Carex sect. Siderostictae Franch. ex Ohwi (subg. Carex), which includes species such as C. siderosticta Hance and C. pachygyna Franch. et Savat., may be sister to all remaining Cariceae. The implications of this discovery to our conclusions on tribal character evolution and to future tribal analyses are discussed in “Future work”.
Taxonomic Implications of Molecular Phylogenies
Generic Circumscription
The most significant molecular result for generic circumscription is that Carex as presently circumscribed is either paraphyletic with respect to all Cariceae genera (Roalson et al., 2001; Starr et al., 2004, 2008; Waterway & Starr, 2007; present analyses) or to all genera except Schoenoxiphium (Yen & Olmstead, 2000a, b). The implications of this result to future infratribal taxonomic name changes should be considered while assessing the conclusions drawn below.
Although no analysis has ever recovered a monophyletic Kobresia, Schoenoxiphium has been strongly supported (100% BS) in the analyses of Yen and Olmstead (2000a, b) and it has been grouped as monophyletic in several ML/Bayesian analyses (Starr et al., 2004, 2008; Waterway & Starr, 2007; PBA). Statistical tests conducted by Starr et al. (2004, 2008; nrDNA data only) suggested that the branch supporting Schoenoxiphium was not significantly different from zero. However, Waterway and Starr (2007), whose data also included cpDNA sequences like those of Yen and Olmstead (2000a, b), did find a significant difference and a >95% PP support for the clade. To date, only seven of the ca. 17 species of Schoenoxiphium have been sampled, and no study has included more than five taxa in a single analysis. Further taxonomic sampling is warranted, but current evidence suggests that Schoenoxiphium is a clade.
Molecular analyses have also rejected two long-held hypotheses of homology that would support a Schoenoxiphium + Kobresia clade (e.g., Koyama 1961; Kern 1974; Smith & Faulkner 1976), or a phylogenetic link between Carex subg. Vigneastra and Schoenoxiphium (Haines & Lye 1972, 1983; Smith & Faulkner, 1976; Reznicek 1990). The clear separation of Schoenoxiphium and Kobresia in all analyses is particularly interesting because of the strong conviction of numerous authors that these genera were morphologically indistinct (e.g., Nelmes 1951; Kern 1958, 1974; Koyama 1961; Smith & Faulkner, 1976). This example illustrates why Cariceae taxonomy can be so difficult because even these distantly related taxa cannot be easily separated on the basis of morphology. As with exemplary cases in Uncinia and major Cariceae groups like the Core Unispicate Clade, the separation of Schoenoxiphium and Kobresia represents one of several problems of cryptic morphology that have been identified by molecular analyses (Starr et al., 2004, 2008).
Uncinia s.str. (sensu Reznicek 1990) is a monophyletic group based on the strong support (99–100% BS) found in all previous molecular analyses (two to three spp.; Yen & Olmstead, 2000a, b; Roalson et al., 2001; Waterway & Starr, 2007) including the recent study of Starr et al. (2008) that sampled over a third of the genus (24 spp.). This supports strong morphological evidence for the monophyly of this group (see Reznicek, 1990). However, molecular analyses also strongly indicate that U. kingii is sister to all other Uncinia (i.e., Uncinia s.l.), which contradicts its recent transfer to Carex sect. Leucoglochin (Reznicek, 1990) based partly on evidence that its hooks (formed by a bend in the rachilla) were not homologous to the hooks in Uncinia s.str. (formed by a retrorse inrolled scale). We suggest that while the hooks may be analogous, the bend in the rachilla where the hooks begin could provide a homologue for Uncinia s.l. (cf. Figs. 18, 20, 21 in Reznicek, 1990, and Figs. 5, 6 in Kukkonen, 1967).
The monotypic genus Cymophyllus with its closed white utricles, pendulous stamens, clavate stigmas, condensed, solitary spikes and unique strap-like leaves suggested to numerous authors that its origins were enigmatic (Kükenthal, 1909; Mackenzie, 1935; Kreczetovicz, 1936; Nelmes, 1952). Given that molecular analyses always place Cymophyllus in the Core Unispicate Clade, typically sister to androgynous unispicate Carex, and that all of its characteristics are seen in that clade except its strap-like leaves, we recommend that future systematic studies should refer to this taxon as Carex fraseriana Ker-Gawler.
Subgeneric and Sectional Circumscription
Of the four subgenera that are currently recognized in Carex, molecular analyses indicate that only subg. Vignea is largely monophyletic as traditionally circumscribed (Yen & Olmstead, 2000a, b; Roalson et al., 2001; Starr et al., 2004, 2008; Waterway & Starr, 2007; Ford et al., 2006; present analyses). This confirms the strong traditional evidence from morphology (e.g., Bailey, 1886; Koyama, 1962), anatomy (Le Cohu, 1967), and even smut host-parasite relationships (e.g., Nannfeldt, 1977) that Carex subg. Vignea is natural.
In contrast, there has been considerable debate over whether the large, and highly variable subg. Carex (ca. 1,400 spp.) and the small, mainly tropical subg. Vigneastra (ca. 100 spp.) are natural or should be merged due to seemingly transitional East Asian species from sections Hymenochlaenae (Holm, 1900; Ohwi, 1936; Koyama, 1962) and Decorae (Kük.) Ohwi (Raymond, 1959; Koyama, 1957, 1962). Molecular analyses indicate that these subgenera cannot be separated (Starr et al., 1999, 2004, 2008; Yen & Olmstead, 2000a; Roalson et al., 2001; Waterway & Starr, 2007; present analyses) and that neither group forms a clade as presently circumscribed (Starr et al., 1999, 2004, 2008; Yen & Olmstead, 2000a; Waterway & Starr, 2007; present analyses). Interestingly, most analyses position species of Carex subg. Vigneastra near the base of the Core Carex Clade (Starr et al., 1999, 2004, 2008; Waterway & Starr, 2007; present analyses), which is compatible with past arguments based on phytogeography (Kreczetovicz, 1936; Nelmes, 1951; Ball, 1990) and inflorescence structure (Kreczetovicz, 1936; Koyama, 1962; Smith & Faulkner, 1976) that subg. Vigneastra may retain the plesiomorphic characteristics of a wider subgenera Vigneastra + Carex + Psyllophora line. Although molecular analyses would indicate that subgenera Carex and Vigneastra are unnatural as circumscribed, no analysis has ever sampled more than five subg. Vigneastra species. Moreover, only the recent analyses of Waterway and Starr (2007), which placed C. cruciata (subg. Vigneastra) as sister to all other Core Carex taxa, provides strong statistical evidence (92% BS) for merging the two. Like many of the results generated by molecular data, their final impact on tribal classification will depend upon further taxonomic and character sampling (see “Future work”).
As expected, all molecular analyses demonstrate that the unispicate Carex subg. Psyllophora is polyphyletic, a result that confirms the common belief that reduction has occurred along several different evolutionary lines in Cariceae (e.g., Kreczetovicz, 1936; Nelmes, 1952; Smith & Faulkner, 1976). Analyses indicate that dioecious unispicate Carex sections, such as Physoglochin and Scirpinae, which are traditionally placed in subg. Psyllophora (e.g., Kükenthal, 1909; Egorova, 1999; Dai & Liang, 2000), should now be distributed amongst the multispicate sections of subgenera Carex and Vignea. Likewise, those androgynous unispicate sections such as the Capituligerae, Phyllostachyae, Leptocephalae, etc., which have been variously attributed to subgenera Carex and Vignea in some treatments (e.g., Marie-Victorin, 1935; Koyama, 1962; Aiken, et al., 1999), should no longer be placed in these groups since it is clear that they are more closely related to Uncinia, Kobresia, and Schoenoxiphium. Because there is no satisfactory classification at present to recognize the Core Unispicate and Schoenoxiphium Clades, and support for these groups is still weak, we suggest that a practical solution for the interim would be to treat these sections as elements of Carex subg. Psyllophora. Although this subgenus remains unnatural, the placement of the androgynous unispicate sections in this group better reflects their true relationships than their assignment to either subg. Carex or Vignea.
Sections are the principle category used to organize the enormous diversity of Carex. When molecular studies have included multiple members of a section they have generally found that morphologically cohesive groups such as sections Phyllostachyae, Stellulatae and Ovales (Starr et al., 1999; Ford et al., 2006) have been monophyletic, whereas large groups suspected of being heterogeneous such as sections Phacocystis or Phaestoglochin (Waterway & Starr, 2007; Ford et al., 2006) are unnatural. Carex is divided into more than 130 sections worldwide (Egorova, 1999), and determining whether these groups are natural will constitute one of the more difficult challenges of the future (see “Future work”).
In Uncinia, molecular analyses suggest that the subgeneric and sectional classification of the genus is largely natural. Kükenthal’s (1909) division of the genus into subg. Hemihamatae (=subg. Pseudocarex Kük. nom. illegit.) for U. kingii and subg. Uncinia (=Eu-Uncinia) for Clarke’s (1883) sections Platyandrae and Uncinia almost directly reflects the pattern of relationships seen in molecular phylogenies (Starr et al., 2008). The only major difference is seen in the placement (72–86% BS; Starr et al., 2008; present analyses) of sect. Uncinia series Macrolepidae as sister to sect. Platyandrae. Even though this may seem like a straightforward result it creates a philosophical problem because the three discrete characters used by Clarke (1883) and Hamlin (1958, 1959) to distinguish section Uncinia from Platyandrae are plesiomorphic (respectively, filiform vs. dilated filaments, glabrous vs. hispid utricles, deciduous vs. persistent scales). A re-evaluation of morphology may reveal previously undetected homologies for sect. Uncinia, but this example highlights the general lack of discrete morphological apomorphies for tribal classification.
Recently Zhang (2001) proposed a new subgeneric and sectional classification for Kobresia. Although taxonomic sampling of Kobresia in all molecular studies has been poor (≤7 spp. per analysis), one consistent result is the strong support (100% BS) for the monophyly of an androgynous unispicate clade (Roalson et al., 2001; Starr et al., 2004, 2008; Waterway & Starr, 2007; Fig. 4). Molecular studies suggest that at least the unispicate species of subg. Kobresia sections Kobresia [e.g., K. capillifolia (Decne.) C. B. Clarke, K. sibirica (Turcz. Ex Ledeb.) Boeck., K. myosuroides, K. schoenoides] and Hemicarex (e.g., K. esenbeckii, K. nepalensis) should be recognized as a distinct taxonomic category. Other unispicate species such as K. robusta Maxim. (subg. Kobresia sect. Psammostachys Ivanova) and K. macrantha Boeck. [subg. Blysmocarex (N. A. Ivanova) S. R. Zhang] may also be a part of this clade.
The small African genus Schoenoxiphium remains a poorly studied group (Kukkonen, 1978, 1983). Although no infrageneric classification exists for the genus, its species can be divided into two distinct groups based on bracts short and sheathless vs. elongate and long-sheathing. Species in the latter group also tend to possess inflorescences that are lax and elongated (A. A. Reznicek, personal communication). Further study is required to determine whether these two groups could represent distinct lineages.
Character Evolution in Cariceae
The Origin of the Caricoid Unisexual Spikelet
Because of its distinctive utricle and unisexual flowers, circumscribing Cariceae has always been straightforward. In contrast, deciphering the phylogenetic origins of its atypical floral morphology has not been easy. Some authors have hypothesized that the Cariceae is a direct descendant of the unisexually-flowered Sclerieae Kunth ex Fenzl (Haines & Lye, 1972; Smith & Faulkner, 1976), the bisexually-flowered Hypolytreae Presl ex Fenzl (Smith & Faulkner, 1976), or that the similarities between Cariceae and Sclerieae are due to an independent descent from the bisexually-flowered Rhynchosporeae Nees (Koyama, 1961). Molecular rbcL analyses (Muasya et al., 1998; Simpson et al., 2007) have consistently placed the Cariceae in a Dulichieae Rchb. ex J. Schultze-Motel + Scirpeae Kunth ex Dumort. p.p. + Cariceae clade and sister to the small Scirpeae genera Eriophorum (ca. 20 spp.) and Scirpus s.str. (ca. 20 spp.). However, statistical support for these relationships has been lacking and there is little traditional evidence in favour of such an alignment (anatomy, Kukkonen, 1967; smut host–parasite data, Savile, 1979, Kukkonen & Timonen, 1979). Nevertheless, recent chloroplast (matK, ndhF, rbcL) and nuclear (arginine decarboxylase) data strongly support a Dulichieae + Scirpeae p.p. + Cariceae clade (94% BS) with Cariceae sister to Scirpus s.str. and Eriophorum (90% BS; Starr et al., 2006). Our character analyses and the nested position of Cariceae amongst bisexually-flowered tribes (Dulicheae, Scirpeae) indicate that caricoid unisexual flowers evolved from perfect flowers. In addition, the close relationship of Scirpus s.str. and Eriophorum to Cariceae suggests that the tribe has undergone a relatively high diversification rate relative to its sister. Whether this could be due to the tribe’s defining features (utricles, a separation of the sexes) or to other factors can only be speculated.
The Origin of the Major Groups in Cariceae
In general, Cyperaceae and Cariceae authors have focused on reduction as the principle means by which taxa evolve (e.g., Nelmes, 1952; Dahlgren et al., 1985). The broad consensus over the past 100 years is that starting from a Schoenoxiphium- or Kobresia-like ancestor with highly compound inflorescences subtended by cladoprophylls and composed of bisexual spikelets, open utricles, and tristigmatic pistils, each of the remaining Cariceae genera could be derived by reduction (see Reznicek, 1990; Starr et al., 2004). Likewise for Carex, the search for an ancestral group has centred on those taxa that have the most compound inflorescences. The most promising candidate has usually been Carex subg. Vigneastra (Reznicek, 1990), whose highly compound inflorescences could have generated subgenera Carex or Vignea by a series of simple losses (Carex = loss of inflorescence prophylls, specialization in sex of spikes, Vignea = loss of one stigma, prophylls, peduncles; e.g., Nelmes, 1951; Hamlin, 1959; Smith & Faulkner, 1976). In contrast, the ultimate example of reduction, the unispicate Carex subg. Psyllophora, was considered to be an unnatural collection of species derived independently from multiple Carex subgenera and/or Cariceae genera (e.g., Kreczetovicz, 1936; Nelmes, 1952; Smith & Faulkner, 1976; but see Savile & Calder, 1953). Alternatively, Reznicek (1990) proposed that the highly compound inflorescences of some Carex subg. Vignea or Carex sect. Fecundae species (the latter group with an admixture of subg. Carex/Vignea features; Reznicek, 1990, 1992) were the ancestral type from which subg. Carex was derived. The origin of subg. Vigneastra was uncertain.
Our analyses, which take into account all previous topologies seen in molecular analyses, suggest that for the characters examined, the ancestral condition in Cariceae is most similar to that seen in multispicate Carex subg. Carex species. In general, all the major Cariceae clades displayed both inflorescence proliferation and extreme reduction to the unispicate state, but the highly compound condition was consistently derived. Within the Vignea Clade, multispicate inflorescences were ancestral, with both the highly compound (e.g., Carex decomposita) and unispicate (e.g., C. dioica) conditions representing derived character states. In the Core Carex Clade, ambiguity was seen at the root node, but the position of C. fecunda and C. scirpoidea demonstrate that proliferation and reduction have also occurred in this clade. For the Caricoid Clades, the root node was either ambiguous or suggested that the ancestral condition was a unispicate inflorescence. Within these clades, analyses suggested that the multispicate or highly compound inflorescences of Schoenoxiphium, Kobresia, and C. baldensis have evolved in parallel from unispicate ancestors.
The phylogenetic position of Carex baldensis is particularly significant because it suggests that rachillae axes in fertile utricles have evolved into complete androgynous lateral spikes (Reznicek, 1990; Starr et al., 2004). In Carex, rachilla proliferation often produces androgynous lateral spikes in teratological specimens (Snell, 1936; Smith & Faulkner, 1976), and presumably these spikes could become genetically fixed if there was a selective advantage. In the case of C. baldensis this advantage would appear to be entomophily. The white, congested inflorescences of C. baldensis are strongly analogous to those seen in the entomophilous Rhynchospora Vahl sect. Dichromena (Michx.) Griseb. (see Thomas, 1984).
The implication that inflorescence proliferation and reduction could be a common and frequent mode of evolution in Cariceae largely explains the difficulty of determining relationships and homology across the tribe. Cariceae inflorescences are essentially repeated structures where all prophyll types are homologous (i.e., inflorescence prophylls, cladoprophylls, utricles), and scales are homologous to the bracts subtending lateral spikes (Snell, 1936; Smith & Faulkner, 1976). The lack of inflorescence prophylls and bracts in unispicate species is thus explained by the absence of more than one inflorescence axis. Because the strict sequence of bract → prophyll → female flower → axis (rachilla) is normally followed during development, proliferation or reduction across widely divergent clades is likely to produce similar structures that could be misinterpreted as homology.
All topologies suggest that the ancestral pistillate spikelet in Cariceae had three stigmas and that the loss of a stigma has occurred frequently during the evolution of the tribe. Other sedge genera such as Schoenoplectus (Rchb.) Palla and Eleocharis R. Br. also possess distigmatic and tristigmatic species (e.g., Ball et al., 2002) suggesting that stigma losses may be common throughout the family. In Carex subg. Vignea, only three species are tristigmatic. Whilst C. gibba retains the ancestral tristigmatic condition as sister to all other subg. Vignea taxa, the three stigmas in C. macrocephala and C. kobomugi (sect. Macrocephalae) appear to be the result of a reversal.
Most topologies suggest that cladoprophylls are ancestral for the Cariceae as would be expected given their presence in the outgroup and throughout the family (Blaser, 1944). All topologies resolve cladoprophylls as ancestral to the Core Carex Clade, but only two topologies unambiguously do so for the Vignea Clade; other topologies suggest ambiguity or consider cladoprophylls as an autapomorphy for Carex gibba. Given the position of C. gibba as sister to all other Vignea species, and its retention of the ancestral tristigmatic condition, we believe that the presence of cladoprophylls in this species is plesiomorphic. The nested positions of Carex sect. Ammoglochin subsect. Herporrhizae (Vignea Clade), C. baldensis (Core Unispicate Clade), and the genera Schoenoxiphium and Kobresia suggest that their cladoprophylls have evolved independently.
Not surprisingly, the absence of inflorescence prophylls appears to be the ancestral state for Cariceae. All topologies demonstrate that the inflorescence prophylls seen in Schoenoxiphium are not homologous to the inflorescence prophylls in Carex subg. Vigneastra. Although several species of subg. Vigneastra are seen at the base of the Core Carex Clade, the ancestral character state for this clade is unresolved. Nonetheless, the nested positions of Carex echinochloe, C. filicina and C. polystachya within the Core Carex Clade suggest that their inflorescence prophylls are not homologous to those of C. cruciata or C. baccans.
In summary, our analyses would suggest that cladoprophylls subtending multispicate inflorescences of strictly unisexual, tristigmatic pistillate spikelets with closed utricles are the ancestral characters for Cariceae. In extant groups, this combination of characters most closely resembles the morphology of multispicate species from Carex subg. Carex. Moreover, if sect. Siderostictae, which is currently placed in Carex subg. Carex and possesses this suite of characters, is confirmed as sister to all remaining Cariceae as Waterway et al. (2008) have proposed, it would strengthen this hypothesis.
Future Work
All analyses to date have recognized three (Core Carex, Vignea, Caricoid) or four (Caricoid = Core Unispicate + Schoenoxiphium) major clades within Cariceae. Our current level of understanding suggests that a sectional or species-level revision is possible for at least the Vignea Clade (ca. 300 spp.) because it would be easy to sample (17 of the 26 sections and more than half of all species are found in North America) and nearly a third of all Vignea species are found in sect. Ovales (ca. 90 spp.), a taxon that is clearly monophyletic (Hendrichs et al., 2004; Ford et al., 2006; Hipp, 2008). On the other hand, a revision of the Caricoid Clade will be faced with the problem of its phytogeography. Uncinia has a Gondwanan distribution, Kobresia ranges from North America through Eurasia, and the species of Carex subg. Psyllophora are widely scattered in high latitudinal or altitudinal habitats across six different continents. Although the total number of species in the Caricoid Clade is relatively small (ca. 215), such a distributional pattern will require considerable effort to clarify its systematics. Understanding phylogenetic relationships within the Core Carex Clade will continue to be our greatest challenge because of the large number of taxa (ca. 1,400 spp.) and cosmopolitan distribution of its species. The studies of Roalson et al. (2001) and Waterway and Starr (2007) represent strong beginnings. Also, the conclusion of Waterway et al. (2008) that Carex sect. Siderostictae is sister to all other Cariceae could have significant implications for polarizing characters and understanding evolution in the tribe.
The conclusion of Waterway et al. (2008) that the East Asian Carex sect. Siderostictae is sister to all remaining Cariceae not only has significant implications for polarizing characters and understanding evolution in the tribe, but it highlights the need for a more balanced sampling regime in future studies. No analysis has included more than 5% of Cariceae diversity; no Cariceae genus has been sampled for more than 40% of its species (i.e., Uncinia, 24 of ca. 60 spp.; Starr et al., 2008), and no Carex subgenus has been sampled for more than 7% of its diversity except subgenera Psyllophora (31% or 20 of ca. 65 spp.; present analyses) and Vignea (30% or 100 of ca. 300 spp.; Ford et al., 2006). On average, 60% of the taxa that have been sampled in molecular studies are from Europe or North America, whereas only 6% are from Eastern Asia. Although Cariceae diversity is high in Europe (182 spp., Chater, 1980) and North America (484 spp.; Ball et al., 2002), it is as high or higher in Eastern Asia (e.g., 203 spp. for Japan, Ohwi, 1965; 488 spp. of Carex for China, Dai & Liang, 2000). This is particularly significant when we consider that our analyses suggest that at least two of the four major Cariceae clades (i.e., Vignea and Core Carex) have East Asian species at their base. Asia contains many poorly studied and enigmatic groups including Carex sect. Hemiscaposae C. B. Clarke (subg. Vigneastra), which shares many of the features found in sect. Siderostictae (e.g., androgynous spikes, broad lanceolate leaves, lateral culms; Waterway et al., 2008), and Carex sect. Hypolytroides Nelmes (subg. Vigneastra) with its Scleria-like stems and corymbiform panicles similar to narrow-leaved Hypolytrum Rich. ex Pers. species (Nelmes, 1951, 1955; Raymond, 1959). Moreover, some East Asian sections, such as the Hymenochlaenae p.p. and Decorae, contain problematic species that blur the boundaries between traditionally recognized Carex subgenera (Holm, 1900; Ohwi, 1936; Koyama, 1957, 1962; Raymond, 1959). Knowing the phylogenetic position of any of the above mentioned taxa could have important implications for our understanding of tribal classification and character evolution.
Central and South America also represent a region of great diversity (Mexico, ca. 100 spp., Reznicek, 1993; Mesoamerica, 44 spp., Chater, 1994; South America, ca. 200 spp., Wheeler, 1996) that has been very poorly sampled (but see Starr et al., 2004, 2008). Species such as Carex david-smithii Reznicek, C. catamarcensis Kük., and other undescribed taxa from the Andes, have all been considered critical to our understanding of evolutionary relationships in Carex but have yet to be included in phylogenetic analyses (see Reznicek, 1990, 1992; Ford et al., 2006). Just as the position of the New Caledonian genus Amborella Baill. as sister to all other angiosperms (Qiu et al., 1999; Mathews & Donoghue, 1999) came as a surprise after it was added to molecular analyses, it is clear that many further surprises await Cariceae analyses when taxonomic sampling becomes reflective not only of perceived morphological and taxonomic diversity, but also of geographic distribution.
Considerable effort has been made to understand Cariceae relationships and floral structures through the study of teratology, positional homology and inflorescence development (e.g., Smith, 1966; Smith & Faulkner, 1976; Timonen, 1998; Reznicek, 1990). These studies have focused on species that were meant to represent the structural diversity of Cariceae as reflected by Kükenthal’s (1909) traditional generic and subgeneric classification. Unfortunately, this approach has been largely uninformative on the taxonomic limits and relationships of major Cariceae groups. A new and potentially more profitable method would be to select species on the basis of recent developments in phylogenetic research (Starr et al., 2004). For example, comparisons of the morphology and development of Carex sect. Siderostictae, C. gibba and C. cruciata to typical members of their sister groups could potentially clarify many questions on tribal homology and evolution. Moreover, a comparison of the morphology and development of the multispicate C. curvula, C. baldensis, and C. supina Wild. ex Wahlenb. to the strictly unispicate and highly compound Kobresia species of their clade (Roalson et al., 2001; Starr et al., 2004; present study) could also be informative. We believe that the clear separation of unispicate dioecious, paradioecious (see Starr et al., 2004) and gynaecandrous species (e.g., Carex squarrosa, Carex exilis, often unispicate; Ford et al., 2006; Waterway & Starr, 2007; present analyses) from androgynous unispicate taxa in all molecular analyses may signal a fundamental difference in inflorescence development between the Core Carex and Vignea Clades and the Core Unispicate and Schoenoxiphium Clades. It is notable that although androgyny is present throughout Cariceae, gynaecandry is only seen in the Core Carex and Vignea Clades.
Although we consider the four major clades described in this paper as “real”, it cannot be discounted that the perceived congruence amongst studies may be due more to marker choice than accuracy. All five Cariceae analyses that have sequenced a nuclear marker have used the ITS region and four of these have also employed the linked ETS 1f region of nrDNA (Fig. 3). For analyses that have used chloroplast markers, whether alone or in combination with nrDNA data, all four have sequenced portions of the non-coding trnT-L-F region and two have sequenced the ndhF gene (Fig. 3). In addition, incongruence length difference tests (ILD; Farris et al., 1994) suggest that there may be some concerns with phylogenetic accuracy. With the exception of Yen and Olmstead (2000a, b) who compared chloroplast regions, all Cariceae studies that have conducted ILD tests have found incongruence either between nuclear and chloroplast data (Roalson et al., 2001; Waterway & Starr, 2007) or between nrDNA partitions (Ford et al., 2006; Starr et al., 2004, 2008). Whether this may be due to paralogues, hybridization, or other sources of systematic error (e.g., G/C bias, long-branch attraction) is unclear, but the unexpected placement of multiple samples of Carex microglochin in separate nrDNA clades suggests that some form of error may be present (see Starr et al., 2008; Fig. 4). New genetic markers are needed, not simply from the linked and conserved chloroplast genome or the potentially problematic nrDNA locus (Alvarez & Wendel, 2003), but from low-copy nuclear genes that can provide independent phylogenetic estimates. Given the lack of a robust topology and the consistently low statistical support for many clades, future studies should not focus on trying to add more data from these loci, but to develop new nuclear markers.
Literature Cited
Aiken, S.G., R.L. Boles & M. J. Dallwitz. 1999. Carex (Dill.) L. Cyperaceae of the Canadian Arctic Archipelago: Descriptions, Illustrations, Identification, and Information Retrieval. Version: 6th November 2000. http://www.mun.ca/biology/delta/arcticf/cyp/www/cyca.htm.
Alvarez, I. & J. F. Wendel. 2003. Ribosomal ITS sequences and plant phylogenetic inference. Molec. Phylogenet. Evol. 29: 417–434.
Bailey, L. H. 1886. A preliminary synopsis of North American carices. Proc. Am. Acad. Arts 3: 59–157.
Ball, P. W. 1990. Some aspects of the phytogeography of Carex. Can. J. Bot. 68: 1462–1472.
————, A. A. Reznicek & D. A. Murray. 2002. Cyperaceae Jussieu. Pp. 1–608 in Flora of North America Editorial Committee (ed.), Flora of North America, North of Mexico, Vol. 23. Oxford University Press, Oxford.
Blaser, H.W. 1944. Studies in the morphology of the Cyperaceae. II. The prophyll. Am. J. Bot. 31: 53–64.
Chater, A. O. 1980. Carex. Pp. 290–323 in T. G. Tutin, V. H. Heywood, N. A. Burges, D. M. Moore, S. M. Waters & D. A. Webb (eds.), Flora Europaea, Vol. 5. Cambridge University Press, Cambridge.
————. 1994. Carex. Pp. 464–473 in G. Davidse, M. Sousa. S. & A. O. Chater (eds.), Flora Mesoamericana. Vol. 6. Alismataceae a Cyperaceae. Universidad Nacional Autonoma de Mexico, Instituto Biologia, Missouri Botanical Garden, The Natural History Museum, London.
Clarke, C. B. 1883. On Hemicarex, Benth., and its allies. J. Linn. Soc., Bot. 20: 374–403.
Croizat, L. 1952. Manual of phytogeography or an account of plant-dispersal throughout the world. Uitgeverij Dr. W. Junk, The Hague.
Dai, L. K. & S. Y. Liang, eds. 2000. Flora Reipublicae Popularis Sinicae: delectis florae Reipublicae Popularis Sinicae. Tomus 12. Angiospermae, Monocotyledoneae, Cyperaceae (2), Caricoideae. Science Press, Beijing.
Dahlgren, R. M. T., H. T. Clifford & P. F. Yeo. 1985. Cyperaceae A. L. Jussieu. Pp. 407–418 in The families of the monocotyledons: structure, evolution, and taxonomy. Springer, Berlin.
Davies, E. W. 1956. Cytology, evolution and the origin of the aneuploid series in the genus Carex. Hereditas 42:349–365.
DeBry, R. W. & R. G. Olmstead. 2000. A simulation study of reduced tree-search effort in bootstrap resampling analysis. Syst. Biol. 49: 171–179.
Edwards, A. W. F. 1972. Likelihood: an account of the statistical concept of likelihood and its application to scientific inference. Cambridge University Press, Cambridge.
Egorova, T. V. 1999. The sedges (Carex L.) of Russia and adjacent states (within the limits of the former USSR). St. Petersburg State Chemical-Pharmaceutical Academy, St. Petersburg; Missouri Botanical Garden Press, St. Louis, MO.
Farris, J. S., M. Källersjö, A. G. Kluge & C. Bult. 1994. Testing significance of incongruence. Cladistics 10: 315–319.
Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791.
Ford, B. A., M. Iranpour, R. F. C. Naczi, J. R. Starr & C. A. Jerome. 2006. Phylogeny of Carex subg. Vignea (Cyperaceae) based on non-coding nrDNA sequence data. Syst. Bot. 31: 70–82.
Goetghebeur, P. 1986. Genera Cyperacearum. Een bijdrage tot de kennis van de morfologie, systematiek enfylogenese van de Cyperaceae-genera. Doctoral Thesis, Rijksuniversiteit Gent.
————. 1998. Cyperaceae. Pp. 141–190 in K. Kubitzki (ed.), The families and genera of vascular plants IV, flowering plants—monocotyledons, Alismatanae and Commelinanae (except Gramineae). Springer, Berlin.
Haines, R. W. & K. A. Lye. 1972. Studies in African Cyperaceae VII: panicle morphology and possible relationships in Sclerieae and Cariceae. Bot. Not. 125: 331–343.
———— & ————. 1983. The sedges and rushes of East Africa. East African Natural History Society, Nairobi.
Hamlin, B. G. 1958. A new classification of Uncinia (Cyperaceae-Caricoideae). Rec. Domin. Mus. 3: 85–88.
————. 1959. A revision of the genus Uncinia (Cyperaceae–Caricoideae) in New Zealand. Dominion Museum Bulletin No. 19. Dominion Museum, Wellington.
Hendrichs, M., S. Michalski, D. Begerow, F. Oberwinkler & F. H. Hellwig. 2004. Phylogenetic relationships in Carex, subgenus Vignea (Cyperaceae), based on ITS sequences. Pl. Syst. Evol. 246: 109–125.
Hipp, A. L. 2008. Phylogeny and patterns of convergence in Carex Sect. Ovales (Cyperaceae): evidence from ITS and 5.8S sequences. In R. F. C. Naczi and B. A. Ford (eds.), Sedges: uses, diversity, and systematics of the Cyperaceae. Monogr. Syst. Bot. Missouri Bot. Gard. 108:197–214.
Holm, T. 1900. Studies in the Cyperaceae XIII. Carex willdenowii and its allies. Amer. J. Sci. 10: 33–47.
Holmgren, P. K., N. H. Holmgren & L. C. Barnett. 1990. Index Herbariorum, Part I: The Herbaria of the World, 8th edn. New York Botanical Garden, New York.
Huelsenbeck, J. P. & F. Ronquist. 2001. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755.
Jermy, A. C., A. O. Chater & R. W. David. 1982. Sedges of the British Isles. Handbook No. 1 (2nd ed.). Botanical Society of the British Isles, London.
Jones, E., D. A. Simpson, T. R. Hodkinson, M. W. Chase & J. A. N. Parnell. 2007. The Juncaceae–Cyperaceae interface: a combined plastid sequence analysis. Aliso 23:55–61.
Kern, J. H. 1958. Florae Malesianae precursores XXI: notes on Malaysian and some S. E. Asian Cyperaceae VII. Acta Bot. Neerl. 7: 786–800.
————. 1974. Cyperaceae. Flora Malesiana 7: 435–753.
Koyama, T. 1957. Taxonomic study of Cyperaceae 7: the systematic position of Carex sect. Decorae with a taxonomic treatment of the Japanese species. Bot. Mag. (Tokyo) 70: 347–357.
————. 1961. Classification of the family Cyperaceae (1). J. Fac. Sci. Univ. Tokyo, Sect. 3, Bot. 8: 37–141.
————. 1962. Classification of the family Cyperaceae (2). J. Fac. Sci. Univ. Tokyo, Sect. 3, Bot. 8: 149–278.
Kreczetovicz, V. I. 1936. Are the sedges of subgenus Primocarex Kük. primitive? Bot. Zhurn. S.S.S.R. 21: 395–425.
Kükenthal, G. 1909. Cyperaceae–Caricoideae. Pp. 1–824 in A. Engler (ed.), Das Pflanzenreich, IV. 20 (Heft 38). Wilhelm Englemann, Leipzig.
Kukkonen, I. 1967. Spikelet morphology and anatomy of Uncinia Pers. (Cyperaceae). Kew Bull. 21: 93–97.
————. 1978. Two new species of Schoenoxiphium (Cyperaceae). Bot. Not. 14: 819–823.
————. 1983. The genus Schoenoxiphium (Cyperaceae). A preliminary account. Bothalia 14: 819–823.
———— & T. Timonen. 1979. Species of Ustilaginales, especially of the genus Anthracoidea, as tools in plant taxonomy. Symb. Bot. Upsal. 22: 166–179.
———— & H. Toivonen. 1988. Taxonomy of wetland carices. Aquatic Bot. 30: 5–22.
Le Cohu, M.-C. 1967. Recherches taxinomiques sur les Carex du Massif Armoricain. Bot. Rhedon., série A, n°3, 1–213.
Mackenzie, K. K. 1935. Cyperaceae - Cariceae. N. Amer. Fl. 18: 1–478.
Maddison, W. P. & D.R. Maddison. 2005. Mesquite: a modular system for evolutionary analysis. Version 1.06. http://mesquiteproject.org.
Marie-Victorin, F. É. C. 1935. Flore Laurentienne. Les presses de l’Université de Montréal, Montréal, Canada.
Mathews, S. & M. J. Donoghue. 1999. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286: 947–950.
Muasya, A. M., D. A. Simpson, M. W. Chase & A. Culham. 1998. An assessment of suprageneric phylogeny in Cyperaceae using rbcL DNA sequences. Pl. Syst. Evol. 211: 257–271.
————, J. J. Bruhl, D. A. Simpson & M. W. Chase. 2000. Supragenic phylogeny of Cyperaceae: a combined analysis. Pp. 593–600 in K. L. Wilson & D. A. Morrison (eds.), Monocots: Systematics and Evolution. CSIRO, Melbourne.
Nannfeldt, J. A. 1977. The species of Anthracoidea (Ustilaginales) on Carex subgen. Vignea with special regard to the Nordic species. Bot. Not. 130: 351–375.
Nelmes, E. 1951. The genus Carex in Malaysia. Reinwardtia 1: 221–450.
————. 1952. Facts and speculations on phylogeny in the tribe Cariceae of the Cyperaceae. Kew Bull. 1951: 427–436.
————. 1955. The genus Carex in Indo-China, including Thailand and lower Burma. Mém. Mus. Natl. Hist. Nat., Ser. B, Bot. 4: 83–182.
Nicolson, D. H. 1992. Seventy-two proposals for the conservation of types of selected Linnaean generic names, the report of Subcommittee 3C on the lectotypification of Linnaean generic names. Taxon 41: 552–583.
Noltie, H. J. 1994. Kobresia Willdenow. Pp. 333–352 in H. J. Noltie (ed.), Flora of Bhutan (vol. 3, part 1). Royal Botanic Garden, Edinburgh.
Ohwi, J. 1936. Cyperaceae Japonicae. I. A synopsis of the Caricoideae of Japan, including the Kuriles, Saghalin, Korea, and Formosa. Mem. Coll. Sci. Kyoto Imp. Univ., Ser. B, Biol. 11: 229–530.
————. 1965. Flora of Japan (in English). Smithsonian Institution, Washington, DC.
Pagel, M. 1999. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 48: 612–622.
Qiu, Y.-L., J. Lee, F. Bernasconi-Quadroni, D. E. Soltis, P. S. Soltis, M. Zanis, E. A. Zimmer, Z. Chen, V. Savolainen & M. W. Chase. 1999. The earliest angiosperms: Evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404–407.
Raymond, M. 1951. Sedges as material for phytogeographical studies. Mém. Jard. Bot. Montréal 20:1–23.
————. 1959. Carices Indochinenses necnon Siamenses. Mém. Jard. Bot. Montréal 53: 1–125.
Reznicek, A. A. 1990. Evolution in sedges (Carex, Cyperaceae). Canad. J. Bot. 68: 1409–1432.
————. 1992. Carex david-smithii (Cyperaceae), a new species from high Andean Peru. Novon 2: 433–436.
————. 1993. Carex. Pp. 243–267 in R. McVaugh, Flora Novo-Galiciana: a descriptive account of the vascular plants of western Mexico. Vol. 13. Limnocharitaceae to Typhaceae. The University of Michigan, Ann Arbor.
Roalson, E. H., J. T. Columbus & E. A. Friar. 2001. Phylogenetic relationships in Cariceae (Cyperaceae) based on ITS (nrDNA) and trnT-L-F (cpDNA) region sequences: assessment of subgeneric and sectional relationships in Carex with emphasis on section Acrocystis. Syst. Bot. 26: 318–341.
Savile, D. B. O. 1979. Fungi as aids in higher plant classification. Bot. Rev. (Lancaster) 43: 377–503.
———— & J. A. Calder. 1953. Phylogeny of Carex in the light of parasitism by the smut fungi. Can. J. Bot. 31: 164–174.
Simmons, M. P. & H. Ochoterena. 2000. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 49:369–381.
Simpson, D. A., A. M. Muasya, M. V. Alves, J. J. Bruhl, M. W. Chase, C. A. Furness, K. Ghamkhar, P. Goetghebeur, T. R. Hodkinson, A. D. Marchant, A. A. Reznicek, E. H. Roalson, E. Smets, J. R. Starr, W. W. Thomas, K. L. Wilson & X. Zhang. 2007. Phylogeny of Cyperaceae based on DNA sequence data—a new rbcL analysis. Aliso 23: 72–83.
Smith, D. L. 1966. Development of the inflorescence in Carex. Ann. Bot. (Oxford) 30: 475–486.
———— & J. S. Faulkner. 1976. The inflorescence of Carex and related genera. Bot. Rev. (Lancaster) 42: 53–81.
Snell, R. S. 1936. Anatomy of the spikelets and flowers of Carex, Kobresia, and Uncinia. Bull. Torrey Bot. Club 63: 277–295.
Starr, J. R., R. J. Bayer & B. A. Ford. 1999. The phylogenetic position of Carex section Phyllostachys and its implications for phylogeny and subgeneric circumscription in Carex (Cyperaceae). Am. J. Bot. 86: 563–577.
————, S. A. Harris & D. A. Simpson. 2003. Potential of the 5′ and 3′ ends of the intergenic spacer (IGS) of rDNA in the Cyperaceae: new sequences for lower-level phylogenies in sedges with an example from Uncinia Pers. Int. J. Plant Sci. 164: 213–227.
————, ———— & ————. 2004. Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae I: generic relationships and evolutionary scenarios. Syst. Bot. 29:528–544.
————, V. Teoh, E. H. Roalson, A. M. Muasya & D. A. Simpson. 2006. Towards a phylogenetic classification of sedges (Cyperaceae): chloroplast (rbcL, matK, ndhF) and nuclear (ADC) data. Abstract 159, Botany 2006 “Looking to the future—conserving the past”, Chico, California. http://www.2006.botanyconference.org/engine/search/index.php?func=detail&aid=159.
————, S. A. Harris & D. A. Simpson. 2008. Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae II: the limits of Uncinia. In R. F. C. Naczi and B. A. Ford (eds.), Sedges: uses, diversity, and systematics of the Cyperaceae. Monogr. Syst. Bot. Missouri Bot. Gard. 108:243–267.
Swofford, D. L. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer, Sunderland, MA.
Thomas, W. W. 1984. The systematics of Rhynchospora section Dichromena. Mem. New York Bot. Gard. 37: 1–116.
Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin & D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24: 4876–4882.
Timonen, T. 1998. Inflorescence structure in the sedge tribe Cariceae (Cyperaceae). Publ. Bot. Univ. Helsinki 26:1–35.
Waterway, M. J. & J. R. Starr. 2007. Phylogenetic relationships in the tribe Cariceae (Cyperaceae) based on nested analyses of three molecular data sets. Aliso 23: 165–192.
————, T. Hoshino & T. Masaki. 2008. Phylogeny, species richness, and ecological specialization in Cyperaceae tribe Cariceae. Bot. Rev. (Lancaster) (this issue).
Wheeler, G. A. 1989. A new species of Carex sect. Abditispicae (Cyperaceae) from northern Argentina and the status of Vesicarex collumanthus in South America. Syst. Bot. 14: 37–42.
————. 1996. Three new species of Carex (Cyperaceae) from Argentina and a range extension for C. ecuadorica. Hickenia 2: 189–200.
Yen, A. C. & R. G. Olmstead. 2000a. Molecular systematics of Cyperaceae tribe Cariceae based on two chloroplast DNA regions: ndhF and trnL intron-intergenic spacer. Syst. Bot. 25: 479–494.
———— & ————. 2000b. Phylogenetic analysis of Carex (Cyperaceae): generic and subgeneric relationships based on chloroplast DNA. Pp. 602–609 in K. L. Wilson & D. A. Morrison (eds.), Monocots: systematics and evolution. CSIRO, Collingwood, Australia.
Young, N. D. & J. Healy. 2003. GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4: 6.
Zhang, S. R. 2001. A preliminary revision of the supraspecific classification of Kobresia Willd. (Cyperaceae). Bot. J. Linn. Soc. 135: 289–294.
Acknowledgements
The authors would like to thank Tony Reznicek for detailed discussions on Cariceae evolution and a live sample of Carex baldensis. We also thank Paul Goetghebeur for helpful discussions on inflorescence morphology and development, and two anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starr, J.R., Ford, B.A. Phylogeny and Evolution in Cariceae (Cyperaceae): Current Knowledge and Future Directions. Bot. Rev 75, 110–137 (2009). https://doi.org/10.1007/s12229-008-9020-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-008-9020-x