Abstract
Ubiquitously distributed multifunctional superfamily of Glutathione S-transferases (GST) generally constitute a dimeric enzymes and catalyse the conjugation of the thiol group of the glutathione (GSH) to diverse electrophilic centres on lipophilic molecules with the formation of rather less active end products. Besides their well investigated conjugation reaction for the detoxification of endogenous and xenobiotic compounds, they can also be involved in both GSH dependent peroxidation or isomerization reactions, and several other non-catalytic functions, like binding of non-substrate ligands, stress-induced signalling processes and preventing of apoptosis. Plant GSTs have been a focus of attention because of their roles in herbicide detoxification and today seven distinct classes of soluble (cytosolic) GSTs are presented as Phi, Tau, Theta, Zeta, Lambda, Dehydroascorbate reductases (DHARs) and Tetrachlorohydroquinone dehalogenase (TCHQD). While GSTs show overall sequence diversification within and between classes, they retain a high level of three-dimensional structure conservation over long evolutionary periods. In this review mainly the soluble plant GSTs will be considered by giving attention to their structures, subcellular localizations, genomic organizations, catalytic/noncatalytic functions, and comparisons given with respect to their mammalian counterparts where necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutathione S-Transferases (GSTs, EC.2.5.1.18) are a superfamily of multifunctional enzymes that detoxify endobiotic and xenobiotic compounds by conjugating glutathione (GSH) to a hydrophobic substrate. In plants, the resulting water soluble and less toxic glutathione S-conjugates are coupled to internal compartmentation due to the lack of effective excretion pathways (Sandermann, 1992; Rea, 1999). However, the conjugate subsequently gets catabolized and excreted in animals (Boyland & Chasseaud, 1969).
GSTs have been found in all living species, including plants, animals, fungi and bacteria. Most GSTs exist as soluble enzymes, however in addition to them, three other GST protein families have been identified; the mitochondrial family (also known as class Kappa), the microsomal (MAPEG) family, and the bacterial antibiotic resistance proteins (For a more detailed discussion for these GST protein families readers are referred to recent reviews by Sheehan et al., 2001; Pearson, 2005; Frova, 2006).
Even those active monomers have been reported recently, as a general rule, most of the soluble GSTs exist as dimeric proteins, with subunit molecular weights of approximately 25 kDa. The subunits appear to be only capable of hybridizing within the same class (Hayes & Pulford, 1995). As both homo- or hetero-dimers’ subunits are the products of independent genes, dimerization definitely increases the number and diversity of GSTs in all species (Edwards et al., 2000).
Soluble GSTs have been grouped into different classes according to their sequence relatedness, immunological cross-reactivities, kinetic properties and genome organizations. In plants, GSTs can compose up to 2% of soluble proteins (Scalla & Roulet, 2002) and seven distinct classes are presently recognized, those are namely; Phi, Tau, Lambda, Dehydroascorbate reductases (DHARs), Theta, Zeta, and Tetrachlorohydroquinone dehalogenase (TCHQD), with the exception of functional characterization reports on TCHQD. The first four of those classes are specific to plants (Edwards & Dixon, 2005). In plants, there are also reports from the other GST subfamilies, like microsomal GSTs which are members of the MAPEG (membrane-associated proteins in eicosanoid and glutathione metabolism) family (Diesperger & Sandermann, 1979; Zettl et al., 1994) and apoplast-localized enzymes (Flury et al., 1996). However, these enzymes are infrequently seen in plants.
The overall sequence of GSTs diverge significantly even within a class. In mammals, generally the amino acid sequence identity within class is greater than 70% and interclass identity is usually less than 30% (Mannervik & Danielson, 1988; Rossjhon et al., 1996, 1998), whereas in plants, identity between classes is usually less than 20%, but within classes identity can be as low as 40% as gathered from the full genome sequence of Oryza sativa (L) (Soranzo et al., 2004).
The plant GSTs, in addition to their enzymatic activities, have less well characterized roles in endogenous metabolism including functioning as GSH-dependent peroxidases counteracting oxidative stress (Bartling et al., 1993; Cummins et al., 1999; Roxas et al., 1997), or GSH-dependent isomerases (Dixon et al., 2000, Thom et al., 2001), and noncatalytically acting as flavonoid-binding proteins (Mueller et al., 2000), stress signalling proteins (Loyall et al., 2000), regulators of apoptosis (Kampranis et al., 2000).
Even though GSTs are distributed in a wide range of organisms, their existence in plants was first shown with the discovery of the relationship between the GST from maize and it’s protective activity against injury of herbicide atrazine, approximately four decades ago (Frear & Swanson, 1970). Since then GSTs have been identified and characterized with differential and overlapping substrate specifities in many plants such as maize, wheat, tobacco, dwarf pine, soybean, A.thaliana, barley, Setaria spp., carnation, potato, chickpea, sorghum, velvetleaf and sugarcane (Karam, 1998 and references herein). However, major progress on their characterization, cloning, and classsification has occurred by the following years (Dixon et al., 2002a, for reviews Marrs, 1996; Edwards et al., 2000; Dixon et al., 2002b).
Nomenclature and Classification of GSTs in Plants
Originally, most of the non-mammalian GSTs were collected into the Theta class, because they were known as very ubiquitous and heterogeneous GST classes by that time. However, with the increasing number of plant GST studies revealing the significant differences between plant and mammalian GSTs from various research groups, this view has definitely changed today.
Initially three distinct types of plant GSTs were recognized by Marrs (1996). This scheme was revised by Droog (1997) and Edwards et al. (2000) in the following years. According to that scheme four principal classes were classified, as two of which are plant-specific (Phi class; previously Type I and Tau class; previously Type III) and two of which are more common phylogenetically (Theta class; previously Type IV and Zeta class; previously Type II; Edwards et al., 2000).
Two outlying classes of the GST superfamily in A. thaliana which differed from all other plant GSTs by containing a cysteine in place of serine at the active site have been identified by Dixon et al. (2002a). Those are namely; glutathione-dependent DHARs and the Lambda GSTs and both are plant-specific as Phi and Tau classes.
Very recently, a further gene similar to GSTs has been identified in the genome of Arabidopsis thaliana (At1g77290). Even though the functional characterization of this protein has not been reported yet, but the predicted protein most closely resembles the TCHQD enzymes from prokaryotes (Edwards & Dixon, 2005).
According to the recommendations of the Committee for Human Gene Nomenclature, italic letters are denoting the source organism, followed by GST, and a letter indicating the class (Z for Zeta, F for Phi, U for Tau, T for Theta, L for Lambda, so on). The subsequent numbers at the end are indicating the order of gene discovery in each class in that relevant species followed by the subunit composition at the protein level. For example, while At GST U1-1 is a homodimer of AtGSTU1, At GST U1-2 is a heterodimer of A. thaliana U1 and U2 subunits. However, some variations of this scheme are in usage as the species is frequently indicated by single letter (h for human, m for mouse, etc.) for mammalian GSTs (Edwards et al., 2000).
Structures of GSTs in Plants
Since the beginning of 90’s, X-ray crysallographic studies has fairly gained a success and today three-dimensional structures are available for nearly all cytosolic classes of GSTs. In plants, for example, the structural information is available for Phi GSTs from Arabidopsis (Reinemer et al., 1996) and maize (Neuefeind et al., 1997a, b), Zeta class GST from Arabidopsis (Thom et al., 2001) and for Tau GSTs from wheat (Thom et al., 2002).
Here, the detailed information of the structural features of GSTs will be avoided, but readers are addressed to the relevant literature (Armstrong, 1997; Sheehan et al., 2001). However, the main structural characteristics of plant GSTs and the differences with their mammalian counterparts will be mentioned.
Despite the overall low sequence similarity between the GST classes, the 3-D structures of the enzymes are very similar. It is suggestive of a strong evolutionary pressure for conservation of some structural motifs involved at the active site (Dirr et al., 1994; Armstrong, 1997; Sheehan et al., 2001) and a dimeric composition.
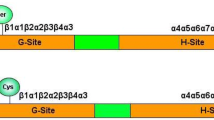
Generally, soluble GSTs are active as either homo- or hetero-dimers of subunits of approx 25 kDa in size. Each subunit of the dimer contains an independent catalytic site composed of two components (domains I and II) and between the two domains is a short variable linker region of 5–10 residues.
Domain I is a binding site specific for GSH (G-site), formed from a group of amino-acid residues in the amino-terminal domain of the polypeptide. Domain II is a site that binds the hydrophobic substrate (the H site), which is formed from residues in the carboxy-terminal domain. The G- and H- sites together constitute the catalytically active site. While domain II is very variable both in sequence and structure, which determines the numerous and diverse hydrophobic substrate specifities observed among different GST classes, domain I is highly conserved and provides specific residues for GSH binding and catalytic activity. So, in the catalytic activation of GSH the Ser residue of the plant specific Phi and Tau, and of more common Zeta and Theta classes have a crucial role (like Tyr residue of the Alpha/Mu/Pi classes in mammals). The suggested role for these residues is to achieve the formation and stabilization of the reactive thiolate anion of GSH (Armstrong, 1997). By site-directed mutagenesis, the Ser (or Tyr) residues have been proven to be catalytically essential in GST catalysis in different organisms (Dirr et al., 1994; Tan et al., 1996; Caccuri et al., 1997; Thom et al., 2001).
However, rather chronologically new plant-specific GST classes; DHARs and Lambda GSTs possess a catalytic Cys residue rather than Ser in the active site. This residue achieves the formation of mixed disulphides with GSH, but not the formation of thilolate anion. This is also common by Beta, Omega, glutaredoxins (GRX) and chloride intracellular channels (CLICs) (Frova, 2006).
The subunits are related by two-fold symmetry in all dimeric GSTs, and intersubunit interactions occur between domain I of one subunit and domain II of its partner. The dimer interface has a large V-shaped cleft, with generally a buried surface area of between 2,700 and 3,400 Å, and this cleft having an auxiliary role in the functional specifities of GSTs. As indicated by Armstrong (1997), GSTs have one of two types of subunit interface, either a hydrophobic ball-and-socket interface (as Alpha, Mu, Pi, Omega and Phi classes), or a hydrophilic interface (as Theta, Sigma, Beta and Tau classes). In nature, all catalytically active GSTs found (with few exceptions, those will be considered later) are dimers, but the subunits from different GST classes are not able to dimerize. The reason behind that should probably be either the hydrophobic/hydrophilic characteristics of the interfaces or the incompatibility of the interfacial residues due to domains orientation. In a dimer construction each subunit has a catalytically independent active site. Therefore, the expectation for GSTs to show their functions as monomers. Erhardt and Dirr (1995), have published a report on explaining the molecular basis of dimerization through conserved evolution and suggested that dimerization stabilizes the tertiary structure of each subunit in mammalian GSTP1-1. In 2004, Hegazy et al. showed the functional role of the lock and key motif at the subunit interface of GSTP1-1, and reported that the two active sites of GSTP1-1 work synergistically, therefore a dimeric organization increases the efficiency of this enzyme.
Today, as reported by Dixon et al. (2002a), Lambda and DHAR classes are the only GSTs shown to be active as monomers. As mentioned previously above, these plant-specific classes are bearing a cysteine at the active site, in place of serine. However, these enzymes have poor conjugation activity toward GSH and are rather implicated in oxidoreductions reactions.
By comparison the crystal structure of the plant GSTs with that of their mammalian counterparts, the inferences are as follows;
-
1.
The G site in plant GSTs is capable of carrying over the thiol binding without any cooperation from the dimer’s other subunit as in mammalians (Prade et al., 1997). This is in good accordance with considerable sequence diversity found between the amino acid residues which form the dimer interface.
-
2.
Serine (in Phi, Tau, Theta, and Zeta) or cysteine (in Lambda and DHAR) is the residue in place of a tyrosine at the active site to activate GSH in plant GSTs
-
3.
The plant GSTs have a larger cleft, therefore could probably accept larger and more divers substrates (Dixon et al., 1998b).
Subcellular Localization, Regulation of Gene Expression and Genome Organization in Plants
In the literature, there are few reports about expression of GSTs in the nucleus and extracellular localization. Rather, most of the investigations show largely cytosolic localization for soluble GSTs in plants. For example, GSTs collectively constitute >1% of the soluble protein in maize leaves (Marrs, 1996; Edwards et al., 2000).
GSTs are present at every stage of plant development from early embryogenesis to senescence and in every tissue type examined. In the study of Sari-Gorla et al. (1993), by using in-bred maize lines, different GST isoenzymes were seen to be expressed in different tissues. Recently, Soranzo et al. (2004) show the expression patterns of rice GST genes with respect to different plant tissues, developmental stages and stress conditions. The results indicated that while the Tau class was the most abundant and expressed especially in callus and shoot tissue, the Phi genes were mainly expressed in vegetative tissues and the Zeta genes show rather low but ubiquitous expression under all stress conditions examined. Even though the low GST expression was observed in leaves, this result was in good agreement with soybean and maize data (McGonigle et al., 2000). However, this tissue-specific expression can be changed by treating plants with some chemicals, for example maize (Zea mays) ZmGSTF2 is normally expressed only in the roots, but will appear in the foliage only after exposure to herbicide safeners or chemical treatments (Dixon et al., 1997). Chemical treatment with herbiside safeners is not the only way of effecting inducibility, there are also some other specific factors present for induction, like plant hormones (such as auxins, ethylene, cytokinin), different chemicals including xenobiotics, and a range of biotic and abiotic stresses (Marrs, 1996).
Table 1 shows a comparison between expression levels by gene classes of maize, soybean and rice. These species indicate that the most expressed GST class is the Phi class in maize and the Tau class in soybean and rice, indicating a variation across species.
Although a few examples are known of posttranscriptional regulation, GST gene expression is regulated predominantly at the transcriptional level (Marrs, 1996; Davies & Caseley, 1999). The transcriptional regulation of individual subunits influences the range of GST homodimers and heterodimers formed. In maize, for example, safeners induce the synthesis of the ZmGSTF2 subunit, which then associates with the constitutively expressed ZmGSTF1 subunit to form the ZmGSTF1-2 heterodimer (Dixon et al., 1997).
By comparing to animal GST promoters, plant GST promoters have not been found to contain Xenobiotic Regulatory Elements (XRE), Antioxidant Regulatory Elements (ARE) or Electrophilic Regulatory Elements (EpRE). On the other hand, the ocs (octopine synthase) elements are the only plant gene promoters found in GSTs from soybean, wheat, tobacco, A. thaliana and Silene. The ocs elements in plant GST promoters also appear to be stress-induced elements, because the ocs elements have not only respond to strong auxins and salicylic acid, but also with their weak analogues and other agents like heavy metals in Arabidopsis, tobacco and soybean (Ulmasov et al., 1994; Zhang & Singh, 1994; Cheng & Singh, 1999). The best characterized promoter in plants belong to a carnation Zeta GST which is induced by ethylene. This promoter has an Ethylene Regulatory Element (ERE; Maxon & Woodson, 1998).
The genome distributions of GST genes in different organisms are lightened by increasing number of genome sequencing projects. The mammalian (human and mouse) and the insect GST genes are grouped in limited specific regions known as clusters (Morel et al., 2002; Henderson et al., 1998; Ranson et al., 1998 and refs within). In plants, the whole genome sequences of Arabidopsis thaliana and rice species provide a general clustering tendency of GST genes. The 52 Arabidopsis GST genes belong to six classes: 28 Tau (U), 13 Phi (F), 3 Theta (T), 2 Lambda (L), 2 Zeta (Z) and 4 DHAR (Dixon et al., 2002a; Wagner et al., 2002). Chromosome 1 is found as the richest with 23 sequences (out of 52), followed by chromosomes 2 with 13 seq., 5 with 6 seq., 3 with 5 seq. and 4 with 1 sequences. The Tau and Phiclass GSTs are present in a series of clusters (between 2 and 7 members), presumably as a result of multiple duplication events with considerable sequence variability. In contrast all Zeta GSTs and Theta GSTs are located in single clusters, lay in tandem on chromosomes 2 and 5, respectively, and are well conserved. Within the larger clusters, the amino acid sequence of the most divergent Phi and Tau GSTs shows only between 50% and 60% identity within each group (Wagner et al., 2002).
In 2004, by in-silico screening of the EST and genome divisions of the Genbank/EMBL/DDBJ database, Soranzo et al. (2004) have isolated 61 genes (including two pseudogenes) in rice: 40 Tau, 16 Phi, 3 Zeta, 2 Theta, making this the largest plant GST family characterized to date. All GST genes isolated during this study were mapped onto the genome. Of the 16 Phi class genes, 11 are on chromosome 1 and 29 out of 40 Tau sequences map to chromosome 10. A DHAR coding gene has also been cloned by another group (Urano et al., 2000).
The presence of clusters in the fully sequenced Arabidopsis and rice genomes suggest a common organizational genome structure within the GST gene family. However, detailed sequence information from other species will definitely brighten this situation in the future.
Catalytic Mechanism and Cellular Functions of GSTs in Plants
Catalytic Mechanism
GSTs catalyze the nucleophilic addition of the thiol of reduced glutathione to electrophilic centers in endogenous and exogenous compounds. Electrophilic centers can be found in environmental carcinogens/toxicants, pesticides (herbicides, insecticides), drugs, and few endogenous molecules. However, this list can be extensible with additional substrates. In plants, GSH conjugation by nucleophilic addition has been reported less frequently than GSH conjugation by nucleophilic displacement (or substitution).
As a result of the conjugation reaction between electrophiles and GSH, a conjugate that is generally less reactive than the parental compound is formed and the solubility of hydrophobic xenobiotics is increased. However, in some cases toxic products can be produced as a result of GSH conjugation. In spite of such exceptions, GSH conjugation usually results in the production of relatively nontoxic products.
Most of the enzyme kinetics studies suggest that the GSH conjugation reactions would be prevailed by a random sequential two-substrate, two-product mechanism. However, under physiological conditions GSH addition occurs first, because the concentration of GSH in normal cells (1–10 mM) is about three orders of magnitude higher than the dissociation constant between GSH and enzyme (Wilce & Parker, 1994)
The main aspect of the catalytic mechanism is the lowering of the pKa of the glutathione thiol group from 9 in aqueous solution to 6–7 when bound to the protein (Graminski et al., 1989), which is achieved in ZmGSTF1–1 (dissociation constant of the thiol is lowered from 8.7 to 6.2) with the effect of hydrogen-bonding activation (Labrou et al., 2001). Here, the natures of both the G site, for binding and correct orientation of glutathione and H site, for accepting numerous different co-substrates are responsible for carrying out a range of reactions. Finally, as a result of reaction, the conjugation product could be followed by a change in absorbance at 340 nm with spectrophotometric assay (Habig et al., 1974).
Cellular Functions
Detoxification and Toxification Reactions of GSTs with Xenobiotics in Plants
Detoxification Reactions.
Xenobiotics usually contain strong electrophilic centers and that electrophilic functional center of the substrates can be provided by a carbon, nitrogen or sulfur atom (Eaton & Bammler, 1999). Generally, GST reactions with xenobiotics results in the formation of an S-glutathionylated reaction product as a consequence of the conjugation of the toxic substrate. The GSH conjugates so-formed are rendered less reactive and more water-soluble, thus facilitating their eventual elimination. Therefore, GSTs are usually detoxification reactions.
In plants, the most commonly observed GSH conjugation reaction has been the nucleophilic displacement of a halogen from an electrophilic site on an aromatic ring, a heterocyclic ring, or an alkyl group. Conjugations of the herbicides atrazine, fluorodifen, pentachloronitrobenzene (PCNB), propachlor, chlorimuron ethyl or insecticide, methidathion are examples for this type of reaction. (Lamoureux & Rusness, 1986; Brown, 1990). A few of those compounds known to be metabolized by conjugation with GSH in plants are presented in Fig. 1.
In plants, the S-glutathionylated conjugates are tagged and transported from the cytosol into the vacuole via the ATP-binding cassette (ABC) transporters (glutathione pump) for storage excretion (Coleman et al., 1997).
Toxification Reactions.
Generally most of the GSH conjugates turn into detoxification products during the reaction catalyzed by GSTs. However, in some cases the toxic products can also be produced as a result of GSH conjugation. A more serious situation can arise with a small number of GST substrates which yield a GSH conjugate, or a metabolite of the conjugate that is more reactive than the master compound. As those with some haloalka(e)nes, glutathione conjugates formed such as episulfonium ion (ethylene dibromide) or formaldehyde (dichloromethane) are generally unstable and thus catalyze rather activation reactions (Eaton & Bammler, 1999). The elimination of GS-conjugates seems to be especially important in cases where the conjugation leads to bioactivation because of genotoxic electrophiles reacting with guanidine in DNA (Ishikawa et al., 1994).
In plants, an example of a toxification reactions has been described in strawberry. A fungicide dichlofluanid is metabolized to a toxic metabolite thiophosgene derivative in a process that appears to involve two GSH conjugation steps (Schuphan et al., 1981).
GSTs in the Conjugation of Endogenous Products in Plants
In plants, the conjugation reactions of xenobiotics by GSTs is very well understood with the help of ever increasing number of studies on the field. However, the recognition, metabolism and transportation of endogenous substrates, in a similar way to that of xenobiotics, is empirical.
By comparison with vast numbers of xenobiotics, few nominees exist as natural metabolites. Among them the GSH conjugate of caftaric acid, a sulphur containing metabolite of gibberellic acid (gibberthione) (Lamoureux & Rusness, 1993), and 4-hydroxynonenals (cytotoxic alkenals produced by oxidative stress damage) (Cummins et al., 1997; Gronwald & Plaisance, 1998) can be counted. The plant secondary metabolites; isoflavonoid medicarpin (Li et al., 1997), and cinnamic acid (Edwards et al., 1991) were also considered as natural substrates, but later found that the phenylpropanoid - GSH conjugates are catalysed by an ascorbate peroxidases rather than GSTs (Dean & Devarenne, 1997).
Other plant secondary metabolites also proposed as endogenous substrates for GSTs are anthocyanins. Because the anthocyanin sequestration in the vacuole requires GSTs in both maize (Bz2 gene) and petunia (An9 gene). The phytotoxic effect could be seen if any mutations occurs in those genes and anthocyanin accumulates in the cytoplasm (Alfenito et al., 1998). Nevertheless, GS-conjugates of anthocyanins have never been isolated and identified from plant cytosol or vacuoles. Today, it is known that both Bz2 and An9 GSTs are flavonoid-binding proteins (see Ligandin functions of GSTs in Plants) which are involved in the intracellular binding and stabilization of flavonoids (Mueller et al., 2000).
The formation of the conjugates in small quantities, even if formed their labile characteristics (sensitivity to pH changes), or reversibility of glutathione conjugation could be a few reasons for the existence of comparatively fewer endogenous substrates for GSTs than xenobiotics (Ishikawa et al., 1994).
Ligandin Functions of GSTs in Plants.
In addition to their well investigated catalytic roles, GSTs also have non-catalytic roles and are called ligandins, because of their ability to bind structurally diverse compounds, such as bilirubin, heme, bile salts, steroids, dyes, carcinogens, and some drugs (Litwack et al., 1971).
Molecules that bind GSTs as nonsubstrate ligands do that at a site other than the catalytic site of the enzyme. The X-ray structures of GSTs from Schistosoma japonica and squid suggest that there might be another (a third, L site) binding site between the subunits, designed as a binding site for those nonsubstrate ligands (McTigue et al., 1995; Ji et al., 1996). In plants, L-site in has also been identified by Reinemer et al. (1996) with the aim of crystallographic studies from A. thaliana.
Bilang and Sturm (1995) and Jones (1994) have reported active GSTs as auxin-binding proteins, but no detection of IAA-GSH conjugates formation. It is known that the binding of nonsubstrate ligands inhibit GST activity toward xenobiotics, but the precise functions of GST binding to non-substrate ligands remain unclear. However this non-enzymatic binding capacity may allow the suggestion that GSTs are involved in the storage and rapid transport of these nonsubstrate ligands in the cell to specific receptors or cellular compartments. In doing so, they prevent cellular damage from cytotoxic and genotoxic compounds which can oxidize protein and insert into DNA (Axarli et al., 2004).
GSTs Catalysing Peroxidase Reactions in Plants.
Beside their ligandin functions, GSTs have another nonconjugating activity, as catalyzing the nucleophilic attack of GSH on electrophilic oxygen and reduction of cytotoxic hydroperoxides to the less-toxic monohydroxy alcohols. By functioning as GPOXs (glutathione peroxidases), GSTs (better terming as GST-GPOXs) protect cells from the effects of active oxygen species (AOS), which are produced during oxidative stress (Marrs, 1996).
GSTs with GST-GPOX activities have been identified in purified isoenzymes from A. thaliana (Bartling et al., 1993; Eshdat et al., 1997), wheat (Cummins et al., 1997), peas (Edwards, 1996), maize (Dixon et al., 1997, 1998a) and soybean (Skipsey et al., 1997). Moreover, relation between the overexpression of Tau and Phi classes with that of high GST-GPOX activities have already been reported, as more tolerance to chilling and salt in transgenic tobacco, while herbicide resistance in black grass, respectively (Roxas et al., 1997; Cummins et al., 1999). Kampranis et al. (2000) have suggested that GSTs functioning as regulators of apoptosis, and able to demonstrate an expression of a tomato Tau GST in yeast which suppresses Bax-controlled apoptosis induced by oxidative stress.
Furthermore, GSTs can also function as stress signaling proteins in plants, as the flavonoid accumulation upon exposure to UV light has been found to be require GSH and the expression of a Tau GST in parsley (Loyall et al., 2000).
Isomerase Activity of GSTs in Plants.
GSTs have an additional isomerization function in plants, animals and fungi. This catalytic role is dependent on GSH, but does not include GSH conjugate as an end product.
Fernandez-Canon and Penalva (1998) have reported the Zeta GSTs in Aspergillus nidulans and humans are identical to an enzyme maleylacetoacetate isomerase (MAAI), which catalyzes the glutathione-dependent cis–trans isomerization of maleylacetoacetate to fumarylacetoacetate in the catabolic pathway of tyrosine/phenylalanine. A complete deficiency of GST Zeta/MAAI may have severe metabolic consequences in mice (Fernandez-Canon et al., 2002; Lim et al., 2004).
The first three-dimensional structure of a Zeta class GST has been characterized from Arabidopsis, which differs catalytically from previously characterised GSTs in that it adds glutathione reversibly to the cis double bond of maleylacetoacetate, allowing bond rotation before elimination of glutathione to yield fumarylacetoacetate, as those with Aspergillus nidulans and human (Thom et al., 2001).
All of the above mentioned roles of plant GSTs in xenobiotic detoxification and endogenous metabolism are summarized in Fig. 2.
Overview of the plant GSTs in xenobiotic detoxification and endogenous metabolism (Dixon et al., 1998b). Most plant GSTs are assumed to be cytosolic, although there is evidence for apoplastic and nuclear isoenzymes. The primary transferase activity of GSTs results in glutathione conjugation of the substrate, usually in a substitution reaction but occasionally as an addition reaction; conjugated molecules (R-SG) are then transported into the vacuole for further processing. There is evidence for alternative activities of GSTs; these include glutathione peroxidase, isomerase and binding activities, which may play additional roles in endogenous metabolism. “Reprinted from Dixon et al. (1998b), with permission from Elsevier”
Model Substrates for the Characterization of GST Isoenzymes
A large number of diverse chemicals serve as substrates for GSTs. However, 1-chloro-2,4-dinitrobenzene (CDNB) is known as the general substrate for nearly all GSTs since it is used for the demonstration of multiple forms of GSTs in all biological organisms from animals to plants. When conjugated with glutathione it gives S-(2,4-dinitrophenyl) glutathione (σ-complex formation) (Armstrong, 1991), which has an absorbance spectrum that allows a simple spectrophotometric assay at 340 nm., is different from the parental compound CDNB (Habig et al., 1974).
Although the presence of activity towards CDNB or other substrates is suggestive of the presence of enzymes from certain GST classes, it can by no means be regarded as proof of the presence of that specific class of GSTs. Likewise, the absence of any detectable CDNB activity does not provide proof of the absence of GSTs, as some GST isoenzymes have very little activity toward CDNB, but very high activity toward other substrates. For example, both class theta 1–1 and theta 2–2 GST isoenzymes were shown not to display any activity with CDNB, but they were characterized by other substrates, namely; 1,2-epoxy-3-(p-nitrophenoxy) propane (EPNP), 4-nitrophenylbromide (4-NPB) and 4-nitrobenzyl-chloride (4-NBC) for class theta 1–1 (Meyer et al., 1991b) and 1-menaphthyl sulfate (MS) for class theta 2–2 (Hussey & Hayes, 1992).
The GST isoenzymes show marked differences in their abilities to conjugate GSH with various electrophiles. The model GST substrates that display selectivity for particular subunits are often used in a ‘diagnostic’sense to identify isoenzymes (Fig. 3). For example, 1,2-dichloro-4-nitrobenzene (DCNB) is selective for M class GSTs and used as a selective marker for this class.
Evolution of the GSTs
As described by Nebert and Deieter (2000) drug-metabolizing enzymes (DME) have existed on the Earth for more than 2.5 billion years. The genes encoding DMEs have functioned in many principal processes in prokaryotes and subsequently in plants and animals.
GSTs, as one of those Phase II DMEs, are thought to have evolved from a thioredoxin-like ancestor in response to the development of oxidative stress (Martin, 1995). They share a thioredoxin-like fold with other GSH- and cysteine-binding proteins. Today, it is clear that GSTs share not only sequence and structural similarities, but also functional similarities with several of stress-related proteins (Rossjohn et al., 1996).
Just like other protein families, to explain the evolution of the GST superfamily by giving priority only to structure, function or sequence alone may lead to obscure consequences. Therefore, it is more important to combine all these elements together with other information like conservation of functionally active site residues, the physical structure of genes and their genomic organizations are necessary. Overall, the best approach is the comparison of all full length sequences where available to avoid misleading outcomes.
Clear evolutionary distinctions between the different enzyme classes could be gathered from their three-dimensional structures; like as the catalytic residues (cysteine or serine or tyrosine) and the dimer interfaces. Despite the generally accepted dimeric forms for GSTs, the plant Lambda and DHAR GSTs conserve monomeric structure. After the dimerization step, Beta and Omega GSTs are found as dimers. Moreover, these four classes bearing the cysteine as active site residue, none of them showing conjugating activity, but share thiol transferase and/or dehydroascorbate reductase activity. Even though the precise order of GST classes is not clear yet, the above mentioned classes predicted to have evolved earlier, as they are structurally and functionally form distinct group from standard conjugating cytosolic GSTs. Afterwards the shift from cysteine to serine at the active site has been occurred (Frova, 2006).
Zeta and Theta classes, which bear a serine as active site residue, are ubiquitous in all eukaryotes and seems to have evolved earlier. As Theta class is also found in bacteria, leading to the suggestion that this class might represent the ancestral class. Even ubiquitous, but poor representation of Zeta and Theta classes in each species is indicative of these genes having undergone one or two duplications and yielding a maximum of two to three members characterised in all plant species analysed (Soranzo et al., 2004). The Zeta GSTs show a high degree of sequence similarity between human—C. elegans and human—D. caryophyllus with 49% and 38% amino acid identity, respectively (Board et al., 1997). Accordingly a monophyletic origin of Zeta and Theta GSTs, preceding the plant-animal split has been suggested by Dixon et al. (1998b, 2002b).
As it is shown in the Fig. 4, the plant specific Phi and Tau classes, and the insect specific Delta classes have evolved later in the lineage. By contrast to Zeta and Theta classes, Phi and Tau GSTs are much more numerous and have many repeated duplications following the divergence of monocots and dicots approx. 200 Myr ago (Wolfe et al., 1989). In plants, the rapid evolutionary rate and presentation with many number of members for plant specific Phi and Tau GST classes by comparing to Zeta and Theta classes are still a mystery. However, a reasonable interpretation would be that the different functionality of the subfamilies may have arisen as an evolutionary strategy to counteract several environmental challenges over a period of time.
Differentiation of cytosolic GSTs. (Modified from Frova, 2006). Thick arrows indicate the likely sequence of critical evolutionary steps, dimerization and changes in active site residues, these last represented by geometric symbols. Circle Cys, diamond Ser, triangle Tyr. (GRX2 stands for Thioredoxins/Glutaredoxins). “Reprinted from Frova (2006), with permission from Elsevier”
Unlike the Alpha, Mu, and Pi classes, Theta and Sigma GSTs share a more hydrophilic interface which lacks the ball-and-socket interaction. However, Theta class have serine at the active region and shows only 7% overall sequence identity with the class Alpha, Mu and Pi classes (Pemble & Taylor, 1992), the Sigma GST uses the same catalytic residue (tyrosine) with those classes. This is another evolutionary step for separation of the cytosolic GSTs, as active site residue changed from serine to tyrosine. It has been suggested that the Sigma class has diverged before the mammalian Alpha, Mu and Pi classes, because of its existence in both vertebrates and invertebrates.
Previously it has been suggested by different study groups the Kappa class as being the progenitor of Theta class and the other soluble GSTs (Pemble et al., 1996; Armstrong, 1997). Today, it is understood that the mitochondrial GSTs were mistakenly named as Kappa class because of the similarities in partial amino acid sequence (Harris et al., 1991), in the active site residue (serine), and the dimeric structure. Nevertheless, the mitochondrial class Kappa proteins do not show statistically significant sequence similarity to any members of the cytoplasmic GST family or vice versa. Accordingly, the complete lack of overlap between mitochondrial GSTs and the cytoplasmic GSTs strongly suggests an independent evolutionary origin as discussed by Pearson (2005). This model substitutes two previous ones which were suggested by Armstrong (1997), and Sheehan et al. (2001), in which Kappa GSTs were placed on the same evolutionary pathways as Theta enzymes, likely preceding them. Figure 4 summarizing a possible pattern of divergence in GST superfamily.
Concluding Remarks
Since the 1960’s GSTs in mammalian and other species have been intensively studied. Plant GSTs had this chance after the 1970’s, as their role in herbicide detoxification was discovered. Today, the availability of whole genome sequences from model plant species, revealing their noticeable feature for combination of phylogenetic and functional diversity, which is not only limited to plant GSTs. From this point of view, it will not be surprising to find a growing number of functions in the future for the newly discovered GST classes, as structural genomics also commits to disclose the functions of protein domains. Surely, with the aid of complementary forced evolution for protein/enzyme engineering and structural/functional genomics, many more crop species that are more tolerant to extreme environmental conditions, herbicides and pesticides could be developed with higher success in the near future.
The field of plant GSTs has been a challenging area for researchers since their discovery and most of the studies have focused on agricultural crop species. The reasons behind this are mainly economic and academic interests. However, there is almost no information about molecular characterization of this superfamily in gymnosperms, except the very recent study from Pinus tabulaeformis (Zeng et al., 2005). As conifers have wide distributions and have to cope with several environmental stresses, the definitions of detoxification enzymes like GSTs in conifers is very important for their adaptations. For this reason, the molecular characterization of GSTs from Pinaceae is in progress in our laboratory.
Literature Cited
Alfenito, M. R., E. Souer, C. D. Goodman, R. Buell, J. Mol, R. Koes & V. Walbot. 1998. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent Glutathione S-transferases. The Plant Cell 10: 1135–1149.
Allocati, N., E. Casalone, M. Masulli, I. Ceccarelli, E. Carletti, M. W. Parker & C. Di Ilio. 1999. Functional analysis of the evolutionarily conserved praline 53 residue in Proteus mirabilis Glutathione S-transferase B1-1. FEBS Lett. 445: 347–350.
Armstrong, R. N. 1991. Glutathione S-transferases: reaction mechanism, structure, and function. Chem. Res. Toxicol. 4: 131–140.
——— 1997. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 10: 2–18.
——— 1998. Mechanistic imperatives for the evolution of glutathione S-transferases. Curr. Opin. Chem. Biol. 2: 618–623.
Axarli, I. A., D. J. Rigden & N. E. Labrou. 2004. Characterization of the ligandin site of maize glutathione S-transferase I. Biochem. J. 382: 885–893.
Bartling, D., R. Radzio, U. Steiner, & E. W. Weiler. 1993. A glutathione S-transferase with glutathione-peroxidase activity from Arabidopsis thaliana Eur. J. Biochem. 216: 579–586.
Bilang, J., & A. Sturm. 1995. Cloning a characterization of a GST that can be photolabelled with 5-azido-indole-3-acetic acid. Plant Physiol. 109: 253–260.
Board, P. G., R. T. Baker, G. Chelvanayagam & L. S. Jermiin. 1997. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem. J. 238: 929–935.
Boyland, E. & L. F. Chasseaud. 1969. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Rel. Areas Mol. Biol. 32: 173–219.
Brown, H. M. 1990. Mode of action, crop selectivity, and soil reactions of the sulfonylurea herbicides. Pestic. Sci. 29: 263–281.
Caccuri, A. M., G. Antonini, M. Nicotra, A. Battistoni, M. Lo Bello, P. G. Board, M. W. Parker & G. Ricci. 1997. Catalytic mechanism and role of hydroxyl residues in the active site of theta class glutathione S-transferases. Investigation of Ser-9 and Tyr-113 in a glutathione S-transferase from the Australian sheep bowfly, Lucilia cuprina. J. Biol. Chem. 272: 29681–29686.
———, G. Antonini, P. Ascenzi, M. Nicotra, M. Nuccetelli, A. P. Mazzetti, G. Federici, M. Lo Bello, & G. Ricci. 1999. Temperature adaptation of glutathione S-transferase P1-1. A case of homotropic regulation of substrate binding. J. Biol. Chem. 274: 19276–19280.
Cheng, W. & K. B. Singh. 1999. The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element. Plant J. 19: 667–677.
Coleman, J. O. D., M. M. A. Blake-Kalff & T. G. E. Davies. 1997. Detoxification of xenobiotics by plants:chemical modification and vascular compartmentation. Trends. Plant Sci. 2: 144–151.
Cummins, I., D. J. Cole & R. Edwards. 1997. Purification of multiple glutathione transferases involved in herbicide detoxification from wheat (Triticum aestivum L.) treated with the sanefer fenchlorazole-ethyl. Pestic. Biochem. Physiol. 59: 35–49.
———, D. J. Cole & R. Edwards. 1999. A role for glutathione S-transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 18: 285–292.
Davies, J. & D. C. Caseley. 1999. Herbicide safeners: a review. Pestic. Sci. 55: 1043–1058.
Dean, J. V. & T. P. Devarenne. 1997. Peroxidase-mediated conjugation of glutathione to unsaturated phenylpropanoids: evidence against glutathione S-transferase involvement. Physiol. Plant 99: 271–278.
DeRidder, B. P., D. P. Dixon, D. J. Beussman, R. Edwards & P. B. Goldsbrough. 2002. Induction of glutathione S-transferases in Arabidopsis by herbicide safeners. Plant Physiol. 130: 1497–1505.
Diesperger, H. & H. Sandermann. 1979. Soluble and microsomal glutathione S-transferase activities in pea seedlings (Pisum sativum L.). Planta 146: 643–648.
Dirr, H., P. Reinemer & R. Huber. 1994. X-ray crystal structures of cytosolic glutathione S-transferases-implications for protein architecture, substrate recognition and catalytic function. Eur. J. Biochem. 220: 645–661.
Dixon, D. P., D. J. Cole & R. Edwards. 1997. Characteristics of multiple GSTs containing the GST I subunit with activities toward herbicide substrates in maize (Zea mays). Pesticide Sci. 50: 72–82.
———, D. J. Cole & R. Edwards. 1998a. Purification, regulation and cloning of a GST from maize resembling the auxin-inducible type-III GSTs. Plant Mol. Biol. 36: 75–87.
———, L. Cummins, D. J. Cole & R. Edwards. 1998b. Glutathione-mediated detoxification systems in plants. Curr. Opin. in Plant Biol. 1: 258–266.
———, D. J. Cole & R. Edwards. 1999. Dimerization of maize GSTs in recombinant bacteria. Plant Mol. Biol. 40: 997–1008.
———, D. J. Cole & R. Edwards. 2000. Characteristics of a zeta class GSTs from Arabidopsis thaliana with a putative role in tyrosine catabolism. Arch. Biochem. Biophys. 384: 407–412.
———, B. G. Davis & R. Edwards. 2002a. Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in A. thaliana. The Journal of Biol. Chem. 277: 30859–30869
———, A. Lapthorn & R. Edwards. 2002b. Plant glutathione transferases. Genome Biology. 3: 3004.1–3004.10.
Droog, F. 1997. Plant glutathione S-transferase, a tale of theta and tau. J. Plant Growth Regul. 16: 95–107.
Eaton, D. L. & T. K. Bammler. 1999. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol. Sci. 49: 156–164.
Edwards, R. 1996. Characterization of glutathione transferases and glutathione peroxidases in pea (Pisum sativum). Physiol Plant. 98: 594–604.
——— & D. P. Dixon. 2005. Plant glutathione transferases. Methods in Enzymology. 401: 169–186.
———, J. W. Blount & R. Dixon. 1991. Glutathione and elicitation of the phytoalexin response in legume cell cultures. Planta. 184: 403–409.
———, P. D. Dixon & V. Walbot. 2000. Plant glutathione S-tranferases: Enzymes with multiple functions in sickness and health. Trends in Plant Sci. 5: 193–198.
Erhardt, J. & H. W. Dir. 1995. Native dimer stabilizes the subunit tertiary structure of porcine class Pi glutathione S-transferase. Eur. J. Biochem. 230: 614–620.
Eshdat, Y., D. Holland, Z. Faltin, & G. Ben-Hayyim. 1997. Plant glutathione peroxidases. Physiol Plant. 100: 234–240.
Fernandez-Canon, J. M., & M. A. Penalva. 1998. Characterization of a fungal maleylacetoacetate isomerase gene and identification of its human homologue. J. Biol. Chem. 273: 329–337.
Fernandez-Canon, J. M., M. W. Baetscher, M. Finegold, T. Burlingame, K. M. Gibson, & M. Grompe. 2002. Maleylacetoacetate isomerase (MAAI/GSTZ)-deficient mice reveal a glutathione-dependent nonenzymatic bypass in tyrosine catabolism. Mol Cell Biol 22: 4943–4951.
Flury, T., E. Wagner & K. Kreuz. 1996. An inducible glutathione S-transferase in soybean hypocotyls is localized in the apoplast. Plant Physiol. 112: 1185–1190.
Frear, D. S., & H. R. Swanson. 1970. Biosynthesis of S-(4-ethylamino-6-isopropylamino-2-s-triazine) glutathione: Partial purification and properties of glutathione S-transferase from corn. Phytochemistry. 9: 2123–2132.
Frova, C. 2006. Glutathione transferases in the genomic era: New insights and perspectives. Biomolecular Engineering. 23: 149–169.
Gonneau, M., R. Mornet & M. Laloue. 1998. A Nicotiana plumbaginifolia protein labelled with an azido cytokinin agonist is a glutathione S-transferase. Physiol Plant. 103: 114–124.
Graminski, G. F., P. Zhang, M. A. Sesay, H. L. Ammon & R. N. Armstrong. 1989. Formation of 1-(S-glutathionyl)-2,4,6-trinitrocyclohexadienate anion at the active site of glutathione S-transferase: Evidence for enzymic stabilization of s-complex intermediates in nucleophilic aromatic substitution reactions. Biochemistry. 28: 6252–6258.
Gronwald, J. W. & K. L. Plaisance. 1998. Isolation and characterisation of glutathione S-transferase isozymes from sorghum. Plant Physiol. 117: 877–892.
Habig, W. H., M. J. Pabst & W. B. Jakoby. 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130–7139.
Harris, J. M., D. J. Meyer, B. Coles & B. Ketterer. 1991. A novel glutathione transferase (13–13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem. J. 278: 137–141.
Hayes, J. D., & D. J. Pulford. 1995. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. CRC CritRev. Biochem. Mol. Biol. 30: 445–600.
——— & L. I. Mc Lennan. 1999. Glutathione and glutathione-dependent enzymes represent a coordinately regulated defense against oxidative stress. Free Radic. Res. 31: 273–300
Hegazy, U. M., B. Mannervik & G. Stenberg. 2004. Functional role of the lock and key motif at the subunit interface of glutathione transferase P1–1. J. Biol. Chem. 279: 9586–9596.
Henderson, C. J., A. G. Smith, J. Ure, K. Brown, E. J. Bacon & C. R. Wolf. 1998. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc. Natl. Acad. Sci. USA. 95: 5275–5280.
Hussey, A. J. & J. D. Hayes. 1992. Characterization of a human class theta glutathione S-transferase with activity towards 1-menaphthyl sulphate. Biochem. J. 286: 929–935.
Ishikawa, T., C. D. Wright & H. Ishizuka. 1994. GS-X pump is functionally overexpressed in cis-diamminedichloroplatinum (II)-resistant human leukemia HL-60 cells and down-regulated by cell differentiation. J. Biol. Chem. 269: 29085–29093.
Ji, X., P. Zhang, R. N. Armstrong & G. L. Gilliland. 1992. The three-dimensional structure of a glutathione S-transferase from the Mu gene class. Structural analysis of the binary complex of isoenzyme 3–3 and glutathione at 2.2 A resolution. Biochemistry. 31: 10169–10184.
Ji, X., E. C. von Rosenvinge, W. W. Johnson, R. N. Armstrong & G. L. Gilliland. 1996. Location of a potential transport binding site in a sigma class glutathione transferase by x-ray crystallography. Proc. Natl. Acad. Sci. USA. 93: 8208–8213.
Jones, A. M. 1994. Auxin-binding proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 393–420.
Kampranis, S. C., R. Damianova, M. Atallah, G. Toby, G. Kondi, P. N. Tsichlis & A. M. Makris. 2000. A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. J. Biol. Chem. 275: 29207–29216.
Karam, D. 1998. Glutathione S-transferase: an enzyme for chemical defense in plants. http://www.colostate.edu/Depts/Entomology/courses/en570/papers_1998/karam.htm. Cited 24 April 2007
Katti, S. K., D. M. LeMaster & H. Eklund. 1990. Crystal structure of thioredoxin from Escherichia coli at 1.68 A resolution. J. Mol. Biol. 212: 167–184.
Ketley, J. N., W. H. Habig & W. B. Jacoby. 1975. Binding of nonsubstrate ligands to the glutathione S-transferases. J. Biol. Chem. 250: 8670–8673.
Labrau, N. E., L. V. Mello & Y. D. Clonis. 2001. Functional and structural roles of the glutathione-binding residues in maize (Zea mays) glutathione S-transferase I. Biochem. J. 358: 101–110.
Lamoureux, G. L. & D. G. Rusness. 1986. Xenobiotic conjugation in higher plants. In: Paulson, G. D., Caldwell, J., Hutson, D. H., and Menn, J. J. (eds). Xenobiotic conjugation chemistry. Am. Chem. Soc. Washington DC. 299: 62–105.
——— & D. G. Rusness. 1993. Glutathione in the metabolism and detoxification of xenobiotics in plants. In: Sulfur nutrition and assimilation in higher plants. De Kok et al (eds), The Hague, SPB Acad. Publ. 221–237.
Li, Z.-S., M. Alfenito, P. A. Rea, V. Walbot & R. A. Dixon. 1997. Vacuolar uptake of the phytoalexin medicarpin by the glutathione conjugate pump. Phytochemistry. 45: 689–693.
Lim, C. E., K. I. Matthaei, A. C. Blackburn, R. P. Davis, J. E. Dahlstrom, M. E. Koina, M. W. Anders, & P. G. Board. 2004. Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu and pi class glutathione transferases. Am. J. Pathol. 165: 679–693.
Listowsky, I., M. Abramovitz, H. Homma & Y. Niitsu. 1988. Intracellular binding and transport of hormones and xenobiotics by glutathione S-transferases. Drug Met. Rev. 19: 305–318.
Litwack, G., B. Ketterer & I. M. Arias. 1971. Ligandin; a hepatic protein which binds steroids, bilirubin, carcinogens, and a number of exogenous organic anions. Nature. 234: 466–467.
Loyall, L., K. Uchida, S. Braun, M. Furuya & H. Frohnmeyer. 2000. Glutathione and a UV light induced GST are involved in signalling to chalcone synthase in cell cultures. Plant Cell. 12: 1939–1950.
Mannervik, B. & U. H. Danielson. 1988. Glutathione transferases- Structure and catalytic activity. CRC Crit. Rev. Biochem. Mol. Biol. 23: 283–337.
Marrs, K. A. 1996. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 127–158.
Marrs, K. A., M. R. Alfenito, A. M. Lloyd & V. Walbot. 1995. Glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 375: 397–400.
Martin, J. L. 1995. Thioredoxin—a fold for all reasons. Structure 3: 245–250.
Matern, U., C. Reichenbach & W. Heller. 1986. Efficient uptake of flavonoids into parsley (Petroselinum hortense) vacuoles requires acylated glycosides. Planta. 167: 183–189.
Maxon, J. M. & W. R. Woodson. 1998. Ethylene-responsive gene expression during carnation flower senescenze. Acta Horticulture. 464: 135–140.
McGonigle, B., S. J. Keeler, S.-M. C. Lau, M. K. Koeppe, D. P. O’Keefe. 2000. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 124: 1105–1120.
McTigue, M. A., D. R. Williams & J. A. Tainer. 1995. Crystal structures of a schistosomal drug and vaccine target: glutathione S-transferase from Schistosoma japonica and its complex with the leading antischistosomal drug praziquantel. J. Mol. Biol. 246: 21–27.
Meyer, D. J., B. Coles, S. E. Pemble, K. S. Gilmore, G. M. Fraser & B. Ketterer. 1991b. Theta, a new class of glutathione transferases purified from rat and man. Biochem. J. 274: 409–414.
Morel, F., C. Rauch, B. Coles, E. Le Ferrac & A. Guillouzo. 2002. The human glutathione transferase alpha locus: genomic organisation of gene cluster and functional characterisation of the genetic polymorphysm in the hGSTA1 promoter. Pharmacogenetics. 12: 277–286.
Mueller, L. A., C. D. Goodman, R. A. Silady & V. Walbot. 2000. AN9, a petunia GST required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 123: 1561–1570.
Nebert, D. W. & M. Z. Dieter. 2000. The evolution of drug metabolism. Pharmacology. 61: 124–135.
Neuefeind, T., R. Huber, H. Dasenbrock, L. Prade & B. Bieseler. 1997a. Crystal structure of herbicide-detoxifiying maize glutathione S-transferase-I in complex with lactolglutathione: evidence for an induced-fit mechanism. J. Mol. Biol. 274: 446–453.
———, R. Huber, P. Reinemer, J. Knablein, L. Prade, K. Mann & B. Bieseler. 1997b. Cloning, sequencing, crystallization and X-ray structure of glutathione S-transferase-III from Zea mays var. mutin: a leading enzyme in detoxification of maize herbicides. J. Mol. Biol. 274: 577–587.
Pearson, W. R. 2005. Phylogenies of glutathione transferase families. Methods in Enzymology. 401: 186–204.
Pemble, S. E. & J. B. Taylor. 1992. An evolutionary perspective on glutathione transferases inferred from class theta glutathione transferase cDNA. Biochem. J. 287: 957–963.
Pemble, S. E., A. F. Wordle & J. B. Taylor. 1996. Glutathione S-transferase Kappa: characterization by the cloning of rat mitochondrial GST and identification of a human homologue. Biochem. J. 319: 349–754.
Prade, L., P. Hof, & B. Bieseler. 1997. Dimer interface of glutathione S-transferase from A.thaliana: influence of the G-site architecture on the dimmer interface and implications for classification. Biol. Chem. 378: 312–320.
Ranson, H., F. Collins & J. Hemingway. 1998. The role of alternative mRNA splicing in generating heterogeneity within the Anopheles gambiae class I glutathione S-transferase family. Proc. Natl. Acad. Sci. USA. 95: 14284–14289.
Rea, P. A. 1999. MRP subfamily ABC transporters from plants and yeast. J Exp. Bot. 50: 895–913.
Rossjohn, J., P. G. Board, M. W. Parker & M. C. J. Wilce. 1996. A structurally-derived consensus pattern for theta class GSTs. Protein Eng. 9: 327–332.
———, W. J. Mc Kinstry, A. J. Oakley, D. Verger, J. Flanagan, G. Chelvanayagam, K. L. Tan, P. G. Board & M. W. Parker. 1998. Human theta class glutathione transferase: the crystal structure reveals a sulfate-binding pocket within a buried active site. Structure 6: 309–322.
Roxas, V. P., R. K. Smith, E. R. Allen & R. D. Allen. 1997. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat. Biotechnol. 15: 988–991.
Reinemer, P., H. W. Dirr, R. Ladenstein, J. Schaffer, O. Gallay & R. Huber. 1991. The three-dimensional structure of class p glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 10: 1997–2005.
———, L. Prade, P. Hof, T. Neuefiend, R. Huber, R. Zettl, K. Palme, J. Schell, I. Koelln, H. D. Bartunik & B. Bieseler. 1996. Three-dimensional structure of GST from A.thaliana at 2.2 A resolution: Structural characterization of herbicide-conjugating plant GSTs and a novel active site architecture. J. Mol. Biol. 255: 289–309.
Sandermann, H. 1992. Plant metabolism of xenobiotics. TIBS. 17: 82–84.
Sandermann, H. 1994. Higher plant metabolism of xenobiotics: the ‘green liver’ concept. Pharmacogenetics. 4: 225–241.
Sari-Gorla, M., S. Ferrario, L. Rossini, C. Frova &M. Villa. 1993. Developmental expression of glutathione S-transferase in maize and it is possible connection with herbicide tolerance. Euphytica. 67: 221–230.
Scalla, R. & A. Roulet. 2002. Cloning and characterisation of a glutathione S-transferase induced by a herbicide safener in barley (Hordeum vulgare). Physiol Plant. 116: 336–344.
Schuphan, I., D. Westpal, A. Hague & W. Ebing. 1981. Biological and chemical behavior of perhalogenmethylmercapto fungicides: Metabolism and in vitro reactions of dichlofluanid in comparison with captan sulfur. In: Rosen, J.D., Magee, P.S., and Casida, J.E., (eds), Pesticide Action and Metabolism. Am. Chem. Soc. Washington DC. 158: 65–85.
Sheehan, D., G. Meade, V. M. Foley,& C. A. Dowd. 2001. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of ancient enzyme superfamily. Biochem. J. 360: 1–16.
Sinning, I., G. J. Kleywegt, S. W. Cowan, P. Reinemer, H. W. Dirr, R. Huber, G. L. Gilliland, R. N. Armstrong, X. Ji, P. G. Board, B. Olin, B. Mannervik & T. A. Jones. 1993. Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the mu and pi class enzymes. J. Mol. Biol. 232: 192–212.
Skipsey, M., C. J. Andrews, J. K. Townson, I. Jepson & R. Edwards. 1997. Substrate and thiol specificity of a stress-inducible GST from soybean. FEBS Lett. 409: 370–374.
Soranzo, N., M. Sari Gorla, L. Mizzi, G. De Toma, C. Frova. 2004. Organisation and structural evolution of the rice glutathione S-transferase gene family. Mol. Gen. Genomics. 271: 511–521.
Tan, K. L., G. Chelvanayagam, M. W. Parker & P. G. Board. 1996. Mutagenesis of the active site of the human Theta-class glutathione transferase GSTT2–2: catalysis with different substrates involves different residues. Biochem. J. 319: 315–321.
Thom, R., D. P. Dixon, R. Edwards, D. J. Cole & A. Lapthorn. 2001. The structure of a zeta class glutathione S-transferase from A. thaliana: characterization of a GST with novel active site architecture and a putative role in tyrosine catabolism. J. Mol. Biol. 308: 949–962.
Thom, R., I. Cummins, D. P. Dixon, R. Edwards, D. J. Cole & A. J. Lapthorn. 2002. Structure of a tau class glutathione S-transferase from wheat active in herbicide detoxification. Biochemistry. 41: 7008–7020.
Ulmasov, T., G. Hagen & T. Guilfoyle. 1994. The ocs element in the soybean GH2/4 promoter is activated by both active and inactive auxin and salicylic acid analogues. Plant Mol. Biol. 26: 1055–1064.
Urano, J., T. Nakagawa, Y. Maki, T. Masumara, K. Tanaka, N. Murata, T. Ushimaru. 2000. Molecular cloning and characterization of a rice dehydroascorbate reductase. FEBS Lett. 466: 107–111.
Wagner, U., R. Edwards, D. P. Dixon & F. Mauch. 2002. Probing the diversity of the Arabidopsis GST gene family. Plant Mol. Biol. 49: 515–532.
Wilce, M. C. J. & M. W. Parker. 1994. Structure and function of glutathione S-transferases. Biochim. Biophys. Acta. 1205: 1–18.
Wolf, A. E., K. J. Dietz & P. Schröder. 1996. Degredation of GST conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett. 384: 31–34.
Wolfe, K. H., M. Gouy, Y.-W. Yang, P. M. Sharp & W.-H. Li. 1989. Date of monocot–dicot divergence estimated from chloroplast DNA sequence data. Proc. Natl. Acad. Sci. USA. 86: 6201–6205.
Zhang, B., & K. B. Singh. 1994. Ocs element promoter sequences are activated by auxin and salicylic acid in Arabidopsis. Proc. Natl. Acad. Sci. USA. 91: 2507–2511.
Zeng, Q.-Y., H. Lu & X.-R. Wang. 2005. Molecular characterization of a glutathione transferase from Pinus tabulaeformis (Pinaceae). Biochimie 87: 445–455.
Zettl, R., J. Schell & K. Plame. 1994. Photoaffinity labeling of Arabidopsis plasma membrane vesicles by 5-azido[7–3H]indole-3-acetic acid: Identification of a glutathione S-transferase. Proc. Natl. Acad. Sci. USA. 91: 689–693
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öztetik, E. A Tale of Plant Glutathione S-Transferases: Since 1970. Bot. Rev 74, 419–437 (2008). https://doi.org/10.1007/s12229-008-9013-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-008-9013-9