Summary
The description and illustration of a new species of Eremanthus (Asteraceae: Vernonieae), E. brevifolius, endemic to Minas Gerais State, is presented and its affinities assessed. E. graciellae and E. pohlii are newly synonymised under E. capitatus, and E. seidelii under E. elaeagnus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eremanthus Less. is a member of the Vernonieae subtribe Lychnophorinae Benth. Its species are almost all endemic to cerrado of the arid Central Plateau of Brazil. They are characteristically small trees or shrubs with compound cymes of capitula arranged in glomerules or sometimes syncephalia, the capitula are few-flowered and the cypselae possess persistent, deciduous or caducous pappus setae in 2 – 5 intergrading series. Generic limits between Eremanthus, Lychnophora Mart., and Paralychnophora MacLeish are highly problematic (see Hind 2000 for a recent review). Molecular and morphological phylogenies of the subtribe and of Eremanthus are underway by the first author and should help to resolve this problem. The narrower concept of Eremanthus, i.e. including Vanillosmopsis Sch. Bip. but not Paralychnophora (MacLeish 1987; Hind 2000), is here followed (for a different point of view, see Robinson 1997, 1999, 2006). The last review of Eremanthus by MacLeish (1987) included 18 species and only one species has since been described by Robinson (1995) that fits the narrower concept (E. hatschbachii H. Rob.). Additionally, preliminary data from the molecular phylogeny has indicated that Vernonia veadeiroensis (H. Rob.) MacLeish (MacLeish 1984a) is better placed into Eremanthus as originally described (Robinson 1980), giving a current total of 20 species in the genus. The present study provides preliminary results of an ongoing taxonomic review of Eremanthus and intensive fieldwork in the Brazilian cerrado between 2006 and 2010. A new species is described and new synonyms, two for E. capitatus and one for E. elaeagnus, are provided as a result of this investigation.
Recircumscription of Eremanthus capitatus, with two new synonyms
Eremanthus capitatus (Spreng.) MacLeish, E. graciellae MacLeish & H. Schumach. and E. pohlii (Baker) MacLeish belong to the subgenus Vanillosmopsis (Sch. Bip.) MacLeish, distinguished by its cylindrical glabrate cypselae and its caducous filiform pappus (MacLeish 1987). Two groups have been recognised in this subgenus (Schultz-Bipontinus 1863; MacLeish 1987): section Nectaridium (Sch. Bip.) MacLeish characterised by ‘Heads cylindric, solitary or in pairs, slightly appressed basally. Pappus never twisted’ (MacLeish 1987: 281), containing E. brasiliensis (Gardner) MacLeish, E. graciellae and E. pohlii, while section Vanillosmopsis (Sch. Bip.) MacLeish has ‘Heads cylindric or obconic. Heads per glomerule (2 –) 5 – 12. Pappus often twisted’ (MacLeish 1987: 283), comprising E. arboreus (Gardner) MacLeish, E. capitatus, E. erythropappus (DC.) MacLeish, E. polycephalus (DC.) MacLeish and E. uniflorus MacLeish & H. Schumach. Alternatively, Baker (1873) defined three informal groups within Eremanthus, based on the degree of fusion of the capitula and the nature of its attachment. To separate E. graciellae from E. pohlii, MacLeish (1987: 281) used the number of florets per capitula (two vs three) and leaf length (4.5 – 6.5 cm vs 6.5 – 7.5 cm).
Though Schultz-Bipontinus (1861) considered Polypappus discolor DC. to be conspecific with Vanillosmopsis capitata (Spreng.) Sch. Bip. (= E. capitatus), Baker (1873) excluded it from synonymy and provided the new combination V. discolor (DC.) Baker. Baker distinguished this latter species from E. capitatus by its appressed capitula or frequently distinctly pedunculate capitula (vs appressed capitula that are never pedunculate), with the base of the capitula never connate (vs slightly connate) and always with three florets (vs two – three). Interestingly, Baker placed this species in the same group as V. pohlii Baker (= E. pohlii), differentiating from V. discolor by the involucre shape (turbinate vs cylindric-turbinate) and the indumentum of the outer phyllaries (densely vs scarcely hoary). MacLeish (1987) did not share Baker’s concept of E. capitatus, considered V. discolor a synonym and disagreed on the number of florets per capitulum (two to three vs three to four in Baker’s description of V. capitata). She commented on this synonymy in her thesis by saying ‘in addition, specimens which exhibit smaller and more obovate leaves are frequently identified as ‘discolor’, a distinction which is not considered to be specifically unique in the present study’ (MacLeish 1984b: 144 – 145). In the herbaria of Brazil, most collections of V. discolor have been identified using Baker’s key that does not consider leaf shape. Accepting this synonymy, the close relationship between E. pohlii and E. capitatus is therefore obvious.
In addition to morphological characters, MacLeish & Schumacher (1984) took the geographic distribution of these species into account to distinguish them. Eremanthus capitatus is restricted to the northeastern arm of the Central Plateau (the states of Bahia, northeastern Minas Gerais and Sergipe), E. graciellae to the Serra Geral de Goiás (Bahia and Goiás) and E. pohlii to the Chapada dos Veadeiros (Goiás) and northwestern Minas Gerais. However, the distributions of E. graciellae and E. pohlii are separated geographically only by the very narrow basin of the Rio Paranã (Vão do Paranã region) in extreme northeastern Goiás, and some populations of E. pohlii cited by MacLeish (1987: 281) from northeastern Minas Gerais (Heringer 11531) are separated from populations of Chapada dos Veadeiros (Goiás) by the same basin and several minor basins of Rio São Francisco’s tributaries. Thus, populations of both species cannot be considered geographically isolated from each other. By contrast, the large basin of the Rio São Francisco in western Bahia apparently separates populations of E. capitatus from the two others. This geographic distribution pattern might be explained by habitat preference: the three species occur in cerrado and/or campos rupestres at high elevations (above c. 800 m) except for some populations of E. capitatus found in restinga (Atlantic coastal strand vegetation on sandy soils at low altitude). Since low altitude cerrado occupies the basin of the Rio São Francisco, these Eremanthus species do not occur there. This gap in the distribution found in other plant species, for example Gaylussacia brasiliensis (Spreng.) Meisn. is reported from campos rupestres in mountain ranges of Goiás, the Distrito Federal, and the Espinhaço mountain range (Bahia, Minas Gerais), campos de altitude of the Serra do Mar (Paraná and São Paulo) and restinga, with no documented presence in the Rio São Francisco basin (Alves et al. 2007; Rizzini 1997).
In the last two decades, a tremendous collecting effort in the distribution range of these species (especially in Chapada Diamantina) has made available a large number of specimens. Analysis of this material has revealed that the twisted pappus is highly variable even at specific level and is useless for taxonomic purposes. Table 1 shows the main characters that have been used to separate the three species. The other two characters used at subgeneric level (involucre shape and number of capitula per glomerule), overlap (Table 1). The number of florets per capitulum has been used to distinguish these three species: E. graciellae has two florets per capitulum, E. pohlii three and E. capitatus three to four (MacLeish 1987). However, a careful analysis of specimens indicates that this character also varies (Table 1); most capitula on the holotype of E. graciellae have two florets, but some have three. None of the remaining minor characters cited in Table 1 could satisfactorily be employed for taxonomic purpose in this species group. Our conclusion, therefore, is that there are no consistent morphological characters that separate the three species, despite the disjunct distribution pattern, and thus, E. graciellae and E. pohlii are synonymised under E. capitatus, as follows:
Eremanthus capitatus (Spreng.) MacLeish (1987: 285). Type: Brazil, Bahia: inter Victoria et Bahia (now Salvador), Sellow s.n. (lectotype K [scan seen], selected by MacLeish, 1987: 285; isolectotype GH!).
Conyza capitata Spreng. (Sprengel 1826: 507).
Vernonia capitata (Spreng.) Less. (Lessing 1829: 270).
Albertinia capitata (Spreng.) DC. (Candolle 1836: 82).
Polypappus discolor DC. (Candolle 1838: 281). Type: Brazil, Bahia: ‘in montibus Jacobinae propè Bahiam’, Blanchet 2591 (holotype G; isotypes BM, BR, C (photo of C: F), G, GH!, K [scan seen], MO, NY![×3], P!, US!).
Vanillosmopsis capitata (Spreng.) Sch. Bip. (Schultz-Bipontinus 1861: 167).
Vanillosmopsis albertinioides Sch. Bip. (Schultz-Bipontinus 1861: 168). Type: Brazil, Sellow s.n. (holotype B†).
Vanillosmopsis discolor (DC.) Baker (1873: 17).
Vanillosmopsis pohlii Baker (1873: 18). Type: Brazil, ‘in Brasilia centrali, loco speciali non addicto’ [Foz do Viera], Pohl 556 (holotype K [scan seen]; isotypes B†, F, GH! [fragment], NY!, W, photo of B: GH, F, US!), synon. nov.
Eremanthus graciellae MacLeish & H. Schumach. (MacLeish & Schumacher 1984: 87). Type: Brazil, Bahia: BR 020 Brasília richtung Barreiras, 15 km weiter in richtung Barreiras von Fazenda Prainha, km 374, Schumacher 1048 (holotype RB!; isotypes GA!, K [scan seen], M, MB), synon. nov.
Eremanthus pohlii (Baker) MacLeish (1987: 281). Type: as for V. pohlii, syn. nov.
distribution. Brazil (Bahia, Goiás, Minas Gerais, Pernambuco and Sergipe) (Map 1).
Geographical distribution of Eremanthus capitatus, depicting variation of glomerules and involucres (A Mendonça et al. 2329; B Loeuille et al. 345; C Coradin et al. 8694; D Vandely 106; E Ganev 2009; F Macleish & Soares Nunes 758; G Schumacher 1080; H & J Hatschbach et al. 67936; K Loeuille et al. 287; L Schumacher 1032; M Aparecida da Silva 1527).
selected collections examined. brazil . Bahia State: Mun. de Abaíra, Catolés Distr., Campo do Bicota, 13°20'22"S, 41°50'01"S, 1491 m, 19 Sept. 2007, fl. fr., Loeuille et al. 345 (HUEFS, K, SPF); Mun. de Barreiras, Brasília richtung Barreiras, bei Km 323, kurz nach grenze GO/BA auf der hochebene, 5 July 1983, fl. fr., Schumacher 3048 (MB,UEC); Mun. de Canavieiras, rodovia BR 101 – Canavieiras, a 33 km da BR 101, 23 Oct. 1980, fr., MacLeish & Soares Nunes 758 (GH, NY, RB, US); Mun. de Conde, Fazendo do Bu, 12°00'54"S, 37°41'16"W, 11 Nov. 1996, fr., Ferreira & Jost 1056 (HRB); Mun. de Correntina, Fazenda Jatobá, grameal, parcela 41, 13°00'S, 46°45'W, 19 July 1992, fl. fr., Aparecida da Silva et al. 1527 (UB); Velha da Galinha, trecho entre o aeroporto e a entrada para o bar Estrela Galdina, 24 Aug. 1995, fr., Mendonça et al. 2329 (IBGE, US); Mun. de Morro do Chapéu, estrada Morro do Chapéu – Utinga, Km 6, 12°06'S, 41°04'S, 1100 m, 22 Sept. 1992, fl. fr., Coradin et al. 8694 (CEN, SPF); Mun. de Rio de Contas, 5 km da cidade na estrada para Livramento do Brumado, 13°37'S, 41°49'W, 600 – 800 m, 25 Oct. 1988, fl., Harley et al. 25396 (ALCB, HUEFS, K, SPF); Mun. de Rio de Pires, capão da Mata de Zé do Amabica (Marques), caminho Outeiro-Marques, 13°46'S, 42°22'W, 1200 m, 5 Aug. 1993, fl. fr., Ganev 2009 (ALCB, HUEFS, K, SPF, US); Mun. de Salvador, Parque Metropolitano de Pituaçu, 4 Sept. 2001, fl. fr., Teles & Faustino 35 (HRB, SPF); Mun. de Santa Luzia, rodovia BA 270 que liga Santa Luzia, Canavieiras e Uma, Km 15, 15°30'05"S, 39°13'32"S, 7 Oct. 2000, fl. fr., Mattos Silva et al. 4274 (ALCB, HUEFS, NY, SPF); Goiás State: Mun. de Alto Paraíso: Chapada dos Veadeiros, 5 – 10 km N of Veadeiros, valley of Rio Paranã, 19 July 1964, fl. fr., Prance & Silva 58251 (NY, UB, US); Brasília richtung Campos Belos, 15 km nach Alto Paraíso, 28 Aug. 1981, fr., Schumacher 1032 (MB, UEC); estrada GO 118 para Cavalcante, 15 km depois de Alto Paraíso, 20 July 2007, fl. fr., Loeuille et al. 287 (K, MBM, SPF, UFG, UB, US); id., fl. fr., Loeuille et al. 288 (K, SPF, UFG); Minas Gerais State: Mun. de Águas Vermelhas, BR 116, a 4 km da fronteira MG/BA, 975 m, 17 Sept. 1998, fl. fr., Bautista & Ortiz 2656 (HRB); Mun. de Burutizeiro, rodovia BR 365, próximo do trevo para Vereda da Onça, 2 July 2003, fr., Hatschbach et al. 75998 (MBM, US); Mun. de João Pinheiro, 15 Aug. 1967, fr., Heringer 11531 (NY, RB, UB); rodovia BR 040, 24 June 1983, fl. fr., Hatschbach & Kummrow 46632 (MBM, US); Mun. de Pedra Azul, ligação rodovia BR 116 Divisopolis, 13 Sept. 1984, fl. fr., Hatschbach 48178 (MBM, NY, SPF, US); Mun. de São Gonçalo do Abaeté, 16 July 1998, fl. fr., Hatschbach et al. 67936 (BHCB, MBM, US); Mun. de Teófilo Otoni, BR 116, Teófilo Otoni richtung Bahia, 65 km nach Teófilo Otoni, 6 Sept. 1981, fl. fr., Schumacher 1080 (MB, UEC, US); Pernambuco State: Mun. de Buíque, Serra de Catimbal, 19 Oct. 1995, fl. fr., Félix et al. 7457 (IPA); Sergipe State: Mun. de Japaratuba, a margem da rodovia, 2 Oct. 1974, fr., Vandely 106 (RB). [246 collections studied.]
habitat. Campo rupestre, cerrado, restinga and margins of secondary forests; 50 – 1700 m.
An expanded synonymy for Eremanthus elaeagnus
Eremanthus elaeagnus (Mart. ex DC.) Sch. Bip. and E. seidelii MacLeish & H. Schumach. belong to the subgenus Pseuderemanthus Sch. Bip., characterised by its hemispherical glomerule, capitula slightly appressed and free, with 3 – 4 florets per capitulum, setuliferous cylindric cypselas and persistent subpaleaceous pappus setae (MacLeish 1987). MacLeish & Schumacher (1984) set apart E. seidelii from E. elaeagnus using differences in the number of capitula per glomerule, leaf shape, pappus colour, flowering period as well as a disjunct distribution pattern (see map in MacLeish 1987: 278).
Fieldwork undertaken in the Espinhaço mountain range during the last two decades by personnel from IB-USP (Instituto de Biociências de Universidade de São Paulo) and in Serra da Canastra by Jimi Nakajima (Nakajima 2000; Nakajima & Semir 2001) has provided nearly one hundred collections of Eremanthus elaeagnus and E. seidelii. Attempts to identify these collections brought to light the necessity to re-evaluate the characters used to distinguish both species.
The main character used by MacLeish (in the identification key, MacLeish 1987: 270) to differentiate both species was the number of capitula per glomerule: 1 – 7 vs 9 – 20, for Eremanthus seidelii and E. elaeagnus respectively. Nakajima (2000) obtained similar values 3 – 7 vs 10 – 20 for plants from Serra da Canastra. In the present study, we have found different values: 3 – 11 vs 4 – 11 for the putative species; interestingly, Baker (1873: 165) obtained a similar value for E. elaeagnus: 3 – 9. These discrepancies are probably related to different concepts of the glomerule: here, we follow Hind (2009): ‘strictly an indeterminate dense cluster of sessile or subsessile flowers, but in the Compositae it refers to a condensed fascicle of capitula, typically on the terminal branches of an inflorescence’. However we recognise only sessile capitula, because ‘subsessile’ is a subjective notion that confuses limits between glomerules. In any case, our results clearly show that there are no differences in the number of capitula per glomerule between the two species.
MacLeish & Schumacher (1984) also distinguished both species by the leaf shape: elliptic for Eremanthus elaeagnus and narrowly elliptic for E. seidelii. This character is highly inconsistent because most specimens exhibit narrow elliptic and elliptic leaves on the same shoot. The same authors affirmed that the pappus of E. elaeagnus is mostly purple, whilst straw-coloured in E. seidelii. This is the only mention in the literature of a purple pappus in E. elaeagnus: all specimens examined in this study (including the holotype) display a stramineous pappus. Candolle (1836: 81) described it as ‘rufescens’, Schultz-Bipontinus (1863: 395) as ‘brunneus’ and Baker (1873: 165) as ‘rufescens’. Phenological data do not set apart the species either: E. elaeagnus blooms between May and September and E. seidelii between May and July. The last criterion used by the authors is not stated in the text but is obvious on the map provided in the generic revision (MacLeish 1987: 278), which restricts E. seidelii to the Serra da Canastra and Furnas reservoir in southwestern Minas Gerais and E. elaeagnus to the Espinhaço mountain range. Nevertheless, extensive fieldwork carried out by Jimi Nakajima in the Serra da Canastra revealed the presence of E. elaeagnus (Nakajima 2000; Nakajima & Semir 2001) (Map 2).
In summary, morphological, phenological and geographical data clearly indicate that Eremanthus seidelii should be treated as a synonym of E. elaeagnus. The revised synonymy is as follows:
Eremanthus elaeagnus (Mart. ex. DC.) Sch. Bip. (Schultz-Bipontinus 1863: 395). Type: Brazil, Minas Gerais, altis lapidosis Serro Frio prope Tejuco (now Diamantina), Martius s.n. (holotype M [scan seen]).
Albertinia elaeagnus Mart. ex DC. (de Candolle 1836: 81). Type: as above.
Vernonia elaeagnus (Mart. ex DC.) Sch. Bip. (Schultz-Bipontinus 1861: 166).
Eremanthus seidelii MacLeish & Schumach. (MacLeish & Schumacher 1984: 89). Type: Brazil, Minas Gerais: Furnas richtung Piuí, kurz von Staumauer, Schumacher 1006 (holotype RB!; isotypes GA!, K[scan seen], M, MB, US!), synon. nov.
selected collections examined. brazil . Minas Gerais state: Mun. de Capitolió, Represa de Furnas, 2 July 1987, fl. fr., Vichnewski & Lopes s.n. (UEC); estrada depois do Paraíso Perdido, 25 Oct. 2006, fr., Loeuille et al. 41 (HUFU, SPF); Mun. de Diamantina, 20 – 26 km WSW, caminho a Conselheiro Mata, MG 220, 18°17'S, 43°49"S, 1270 – 1300 m, 18 May 1990, fl. fr., Arbo et al. 4385 (CTES, SI, SPF); estrada para Mendanha, Km 571.5, 14 July 1996, fl. fr., Roque et al. 206 (NY, SPF); Mun. de Formiga, Formiga richtung Passos, bei Km 288, 4 June 1983, fl. fr., Schumacher 3027 (MB, UEC); Mun. de Furnas, Represa de Furnas, área em torno das eclusas da represa, 1 July 1994, fr., Lombardi 556 (BHCB, UEC); Mun. de Grão Mogol, Córrego Escurona, 16°35'S, 42°58'S, 750 m, 16 July 1990, fl. fr., Simão Bianchini et al. CFCR 13174 (K, SPF, UEC); Mun. de Joaquim Felício, Serra do Cabral, subida, 5 June 2004, fl. fr., Hatschbach et al. 77414 (MBM, US); estrada Joaquim Felício – Várzea da Palma, 17°43'38"S, 44°11'02"S, 950 m, 3 June 2008, fl. fr., Loeuille et al. 430 (HAW, K, SPF); Mun. de Patrocínio, Fazendas Daterra, Boa Vista, 14 July 1998, fr., Farah & Freitas 372 (ESA); Mun. de Sacramento, 16 km da divisa MG/SP, 7 km do ribeirão Canabrava, em direção a Araxá, 6 July 1996, fl. fr., Souza et al. 12060 (ESA, HUFU, K); Mun. de São Roque de Minas, Parque Nacional da Serra da Canastra, próximo à portaria de Sacramento, 19 Aug. 1983, fr., Ramalho & Mota 2601 (HUFU); cachoeira da Casca d’Anta, 12 May 1995, fl. fr., Nakajima et al. 1057 (HUFU, SPF); próximo ao Centro de Visitantes, 15 May 2007, fl. fr., Loeuille et al. 258 (K, SPF, US); São Paulo state: Mun. de Estreito, perto da Fazenda 3 Irmãos, 12 July 1995, fr., Marcondes Ferreira et al. 1215 (SPF, UEC). [126 collections studied.]
A new species of Eremanthus
Eremanthus brevifolius Loeuille sp. nov. affinis E. hatschbachii foliis parvis et pappo biseriali sine serie exteriore reducto sed foliis anguste oblongis ad elliptica, raro oblanceolatis vel lanceolatis (non ovatis), supra dense nigra glanduloso-punctata et tomentosa (non glabra), floribus 4 – 7 (non 2 – 3) differt. Typus: Brazil, Minas Gerais: Mun. de Congonhas do Norte: Serra Talhada, Fazenda Imbaúbas, 20 Jan. 2007, Loeuille et al. 71 (holotypus SPF!; isotypi K!, US!).
http://www.ipni.org/urn:lsid:ipni.org:names:77118095-1
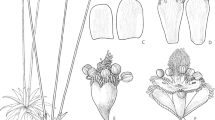
Treelet to 1 m; bark ± fissured longitudinally. Stems well-branched in upper part, leafy at first becoming leafless, greyish tomentose with triangular leaf-scars following leaf falls. Leaves alternate, simple, petiole 25 – 45 mm long, blade narrowly oblong to elliptic, rarely oblanceolate or lanceolate, 2 – 2.5 × 0.6 – 0.8 cm, venation brochidodromous to weakly eucamptodromous in upper part, midrib furrowed, adaxially densely black glandular dotted, grey tomentose, abaxially dirty white yellowish, densely tomentose, tomentum of simple, uniseriate hairs, margins entire, usually flat to slightly revolute on lower part, apex obtuse to acute, base decurrent to cuneate. Glomerulescence a cyme of 2 – 4 glomerules. Capitula homogamous, discoid, sessile, 5 – 10 per glomerule, interspersed with leaf-like bracts, slightly appressed at base and free; involucre 4 – 5-seriate, imbricate, cylindrical to slightly urceolate, rarely obconical; outer phyllaries ovate to lanceolate, 2.5 – 3.6 × 0.75 – 1.1 mm, margins scarious, apex obtuse to slightly acute, lanate to tomentose, purple apex, inner phyllaries linear to lanceolate, 4.8 – 6.5 × 1 – 1.4 mm, margins subscarious, apex obtuse to slightly acute, tomentulose to glabrescent, apex purple; receptacle flat, foveolate with some isolated fimbriae to c. 0.15 mm. Florets 4 – 7 per capitulum, bisexual, fertile; corollas actinomorphic, deeply 5-lobed, lilac becoming whitish, corolla tube 2.8 – 4.9 × 0.5 – 0.8 mm, glabrous, corolla lobes 2.5 – 3.2 × 0.5 – 0.6 mm, glandular-punctate at base of lobes, apex acute; apical anther appendages narrowly trullate, acute, anther base sagittate, acute; style shaft 4.3 – 7.6 mm long, lilac to pale pink, glabrous throughout except for pubescent upper 0.9 – 1.1 mm beneath style-arms, style-arms 2.6 – 2.8 mm long, apex acute, pubescent outside, hairs acute, style-base glabrous, lacking basal node. Cypsela turbinate, 0.8 – 1.5 × 0.4 – 0.8 mm, strongly 10-ribbed, glabrous, densely glandular-punctate; carpopodium minute; pappus setae biseriate, equal length, stramineous, persistent or deciduous, 5 – 6 × 0.1 – 0.2 mm, ± barbellate, twisted throughout length or limited to proximal half. Fig. 1.
distribution. South America: endemic to Minas Gerais State, Brazil (Map 2).
material examined. brazil. Minas Gerais State: Mun. de Congonhas do Norte, Serra Talhada (setor nordeste da Serra do Cipó), 9 km S de Congonhas do Norte na estrada para Conceição do Mato Dentro, entrada para Extrema seguindo 11 km – Fazenda Imbaúbas (propriedade do Sr. Helvécio Lacerda de Queiroz), 18°55'48"S, 43°40'17"W, 1130 m, 20 Jan. 2007, Loeuille et al. 71 (holotype SPF, isotypes K, US).
habitat. In campos rupestres, among rocks.
conservation assessment. According to available information about Eremanthus brevifolius, it can be scored using IUCN conservation criteria (IUCN 2001) as Vulnerable (VU), since its area of occupancy is very restricted VU (criteria D2). The single locality known is outside of a protected area and thus, prone to the effects of human activities.
notes. Preliminary data from the molecular phylogeny has conflicted with the infrageneric classification proposed by MacLeish (1987), thus Eremanthus brevifolius is not being assigned to any infrageneric categories at this time. It is related to E. hatschbachii H. Rob., by the small size of its leaves and a biseriate pappus without an outer reduced series, but this new species differs by the leaf shape (narrowly oblong to elliptic, rarely oblanceolate or lanceolate vs ovate), the indument of the adaxial face (densely black glandular dotted, tomentose vs glabrous) and the number of florets (4 – 7 vs 2 – 3). E. brevifolius is also similar superficially to E. elaeagnus in habit, indumentum of abaxial leaves and capitula sessile slightly appressed and free, but differs in leaf size (2 – 2.5 × 0.6 – 0.8 cm vs 5 – 12 × 1.5 – 3 cm), the indumentum of the adaxial surface (densely black glandular dotted, tomentose vs sparsely lepidote), the number of florets per capitulum (4 – 7 vs 3 – 4) and the number of series of pappus setae (2 vs 3 – 5).
In terms of distribution, Eremanthus hatschbachii is presently known only from Serra do Cabeludo (Mun. Mucugê) in the state of Bahia, while E. elaeagnus is a common species in Minas Gerais especially in the Espinhaço moutain range as well as in Serra da Canastra. E. brevifolius is currently known only from the type collection in Serra Talhada, a northeastern extension of Serra do Cipó, a region well known for its many endemics in the Compositae. Two other species of Eremanthus (E. elaeagnus and E. erythropappus) have been recorded, so far, in Serra Talhada. E. brevifolius is probably a microendemic to this region, a distribution pattern common in the Lychnophorinae. The type locality has been very poorly collected until now which probably explains why we have found only a single collection even after visiting most of the Brazilian herbaria. Even if it might be considered challenging to describe a new species based on a single collection, such characteristics as the number of series of pappus setae and of florets per capitulum clearly prevent us from considering it conspecific with E. elaeagnus.
References
Alves, R. J. V., Cardin, L. & Kropf, M. S. (2007). Angiosperm disjunction ‘Campos rupestres – restingas’: a re-evaluation. Acta Bot. Bras. 21: 675 – 685.
Baker, J. G. (1873). Compositae I. Vernoniaceae. In: C. F. P. von Martius† & A. W. Eichler (eds), Flora Brasiliensis 6(2): 5 – 180. Fried. Fleischer, Münich, Vienna, Leipzig.
Candolle, A. P. de (1836). Vernoniaceae. Prodromus Systematis Naturalis Regni Vegetabilis, … v. 5, pp. 9 – 103. Treutel et Würtz, Paris.
____ (1838). Mantissa Compositarum. pp. 263 – 287 [Trib. III. Asteroideae: 271 – 287]. Prodromus Systematis Naturalis Regni Vegetabilis, … vol. 7,. Treutel et Würtz, Paris.
Hind, D. J. N. (2000). Two new species of Paralychnophora (Compositae: Vernonieae) from Bahia. Kew Bull. 55: 367 – 379.
____ (2009). Glossary. In: An annotated preliminary checklist of the Compositae of Bolivia. Published on the internet http://www.kew.org/science/tropamerica/boliviacompositae/index.html [accessed August 2009].
IUCN (2001). IUCN Red List Categories: version 3.1. Prepared by the IUCN Species Survival Commission. IUCN, Gland and Cambridge. http://www.iucnredlist.org/technical-documents/categories-and-criteria/2001-categories-criteria
Lessing, C. F. (1829). De synantheris herbarii regii berolinensis dissertatio prima. Vernonieae. Linnaea 4: 240 – 356.
MacLeish, N. F. F. (1984a). Eight new combinations in Vernonia (Compositae: Vernonieae). Syst. Bot. 9: 133 – 136.
____ (1984b). Revision of Eremanthus Less. (Compositae: Vernonieae). PhD thesis, unpublished. University of Georgia.
____ (1987). Revision of Eremanthus (Compositae: Vernonieae). Ann. Missouri Bot. Gard. 74: 265 – 290.
____ & Schumacher, H. (1984). Six new species of Eremanthus (Vernonieae: Compositae) from Brazil. Syst. Bot. 9: 85 – 95.
Nakajima, J. N. (2000). A família Asteraceae no Parque Nacional da Serra da Canastra, MG. PhD thesis, unpublished. Universidade Estadual de Campinas.
____ & Semir, J. (2001). Asteraceae do Parque Nacional da Serra da Canastra, Minas Gerais, Brasil. Revista Brasil. Bot. 24: 471 – 478.
Rizzini, C. T. (1997). Tratado de Fitogeografia do Brasil. 2 ed. Rio de Janeiro, Âmbito Cultural Edições, Ltd.
Robinson, H. (1980). Notes on the Lychnophorine genera Chresta and Eremanthus (Vernonieae: Asteraceae). Phytologia 45: 89 – 100.
____ (1995). New combinations and new species in American Vernonieae (Asteraceae). Phytologia 78: 384 – 399.
____ (1997). The Paralychnophora group of Eremanthus (Vernonieae: Asteraceae). Rhodora 98(no. 893): 85 – 93.
____ (1999). Generic and subtribal classification of American Vernonieae. Smithsonian Contr. Bot. 89: 1 – 116.
____ (2006) [2007]. Vernonieae. In: J. Kadereit & C. Jeffrey (vol. eds), Vol. 8: Asterales. The Families and Genera of Vascular Plants (K. Kubitzki, series ed.): 149 – 174. Springer. Berlin, Heidelberg, New York.
Schultz-Bipontinus, C. H. (1861). Cassiniaceae uniflorae, oder Verzeichniss der Cassiniaceen mit 1-blüthigen Köpfchen. Jahresber. Pollichia 18/19: 157 – 190.
____ (1863) [1864]. Lychnophora Martius! und einige benachbarte Gattungen. Jahresber. Pollichia 20/21: 321 – 439.
Sprengel, C. (1826). Systema vegetabilium, ed. 16, pp. 1 – 936. Göttingae [Göttingen].
Acknowledgements
The drawings were prepared by Leonardo M. Borges of the Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo. The authors would like to thank the curators of all the herbaria listed in the text for loaning the specimens as well as Nicholas Hind and John Pruski for valuable suggestions on the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loeuille, B., Lopes, J.C. & Pirani, J.R. Taxonomic novelties in Eremanthus (Compositae: Vernonieae) from Brazil. Kew Bull 67, 1–9 (2012). https://doi.org/10.1007/s12225-012-9328-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-012-9328-x