Abstract
Biodiversity of ecological communities has been examined widely. However, comparisons of observed species richness are limited because they fail to reveal what part of the differences are caused by natural variation in species pool size and what part is due to dark diversity – the absence of suitable species from a species pool. In other words, conventional biodiversity inventories do not convey information about how complete local plant communities are. We therefore propose the community completeness concept – a new perspective on the species pool framework. In order to ascertain community completeness, we need to estimate the extent of dark diversity, for which several methods are under development. We recommend the Community Completeness Index based on a log-ratio (or logistic) expression: ln(observed richness/dark diversity). This metric offers statistical advantages over other methods (e.g. the proportion of observed richness from the species pool). We discuss how community completeness can be related to long-term and successional community stability, landscape properties and disturbance patterns as well as to a variety of biotic interactions within and among trophic levels. The community completeness concept is related to but distinctive from the alpha-beta-gamma diversity approach and the community saturation phenomenon. The Community Completeness Index is a valuable metric for comparing biodiversity of different ecosystems for nature conservation. It can be used to measure the success of ecological restoration and vulnerability to invasion by alien species. In summary, community completeness is an interface between observed local observed species richness and dark diversity, which can be useful both in theoretical and applied biodiversity research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological communities typically comprise numerous species, and their biological diversity can be remarkably rich. This high biodiversity has always fascinated ecologists and conservationists. The ‘world records’ of plant species richness are held by tropical rainforests at larger spatial scales and by temperate grasslands at smaller spatial scales (Wilson et al. 2012). Differences in species diversity of ecological communities have been attributed to natural processes and anthropogenic impacts (Lepš 2005). However, simple comparisons of raw numbers of observed species offer little insight, since they fail to reveal how many suitable species are absent (Pärtel et al. 2011), or, in other words, how complete communities are.
Each ecological community has its species pool – a set of species that can potentially reach and inhabit it (Eriksson 1993; Zobel 1997). The fraction of the species pool absent at any particular point in time is called dark diversity (Pärtel et al. 2011). Which species constitute the species pool? Firstly, the probability that a species can disperse to a given community must be reasonably high. In this respect, the arrival of species inhabiting other regions or even continents is unlikely. We should therefore include only species already present in the region surrounding a given community. Secondly, only a fraction of species able to disperse to a community can actually tolerate the community’s particular environmental conditions. In other words, membership in a species pool demands a match between habitat requirements of species (beta niche, habitat niche or Grinnellian niche) and environmental conditions in a local community. As a result, species pools are habitat-specific, and different community types within the same region have different species pools. Species pools are formed by speciation, which often occurs in areas beyond the location of our target community. Because of niche conservatism (Wiens et al. 2010; Peterson 2011), however, species inhabit regions with conditions analogous to those of their evolutionary origin. Species have reached new areas through historical migrations between and within continents. How many species from the regional species pool actually form a community depends, in turn, on dispersal limitations within the region and on biotic interactions within the community, which might exclude some species and support others (known as assembly rules, cf. Götzenberger et al. 2012). Biotic interactions are not limited to competition, although competition has been considered historically to be among the most important interactions, but also on interactions between plants and organisms of other tropic levels, which might exhibit a positive effect (such as mycorrhizal fungi or pollinators) or a negative one (such as predators). Last but not least, the presence or absence of particular species in a particular community is also affected by an array of biotic and abiotic stochastic variations (e.g. population dynamics, disturbances through fire or flooding). As summarized by Vellend (2010), community ecology is governed by only a few principal processes: speciation (determining the number of species and the beta niche of species), dispersal (both historical migration and dispersal within a given region), selection (both abiotic filtering and assembly rules) and drift (abiotic and biotic stochastic variation).
Only two numbers, the number of species actually present and the number of species potentially present, determine the completeness of a local community. Dark diversity, though locally absent, constitutes an important characteristic of a local community since these species are both present in the region and can potentially inhabit the site. Analogous ideas have been suggested earlier, but here we introduce and formalize the community completeness concept, introduce the Community Completeness Index and suggest practical strategies of how it can be applied in theoretical ecology and nature conservation.
Community Completeness: The Concept
The idea of community completeness (and even the term itself) originates from early Russian plant community ecology. Ramensky (1924) defined completeness (in Russian полночленность, ‘polnochlennost’) as the situation in which all potential inhabitants that are able to disperse into a community and inhabit it (i.e., pass both abiotic and biotic filters) are actually present in the community. This concept was further developed and discussed by Rabotnov (1984), who mentioned that the degree of community completeness could be assessed with the help of field experiments involving species introductions. However, to the best of our knowledge, no empirical analysis of such experiments exists. In another work, Rabotnov (1987) distinguished between floristic completeness (the degree of presence of potential species) and coenotic completeness (whether abundances of species correspond to typical natural conditions). He also emphasized the need to address community completeness at different trophic levels, not only in plants. The English-language scientific literature contains only a single article containing the term ‘community completeness’ in its title (a study of bird communities, Cam et al. 2000). Community completeness metrics (relative richness, see below) have more often been implicitly used in studies concerning regional species pools (e.g., Zobel and Liira 1997; Ingerpuu et al. 2001; Pärtel et al. 2007a).
The species pool concept originated as a hypothesis suggesting that local richness in communities is related to the historical and regional commonness of a habitat type (Taylor et al. 1990), or in other words, how many suitable species are present in a region (Zobel 1992; Eriksson 1993; Pärtel et al. 1996). We advocate a more general species pool concept, which declares that local communities can be better described with respect to their species pools. Moreover, even community assembly rules governed by local biotic interactions can sometimes be revealed efficiently only after prior consideration of the species pool (de Bello et al. 2012; Lessard et al. 2012). The main purpose of the species pool concept is to estimate which species composing the species pool are absent and therefore form dark diversity.
Community completeness is a new perspective on how to advance the species pool concept. In essence, completeness expresses how much of the species pool is realized within a local community. Expressing community completeness on a relative scale facilitates comparisons among similar communities in different geographical regions, among different communities within a region and among communities of different trophic levels, for example, insects, birds and plants (Pärtel et al. 2011).
The locally absent component of the species pool – dark diversity – shares characteristics with the concept of beta-diversity, yet it is fundamentally distinct (Pärtel et al. 2011). Both dark diversity and beta-diversity are derived from common local richness or alpha-diversity. Beta-diversity is determined by gamma-diversity whereas dark diversity is derived from the species pool. Gamma-diversity is basically just the sum or an extrapolation of the number of species encountered locally, that is, directly related to alpha-diversity. The species pool, by contrast, is determined by regional distribution and habitat requirements of individual species and is principally independent of which species are actually found locally. Moreover, beta-diversity was originally defined as the extent of community composition change across environmental gradients, for example, altitude (Whittaker 1960). This means, however, that species pools for these particular communities differ as well. Species that inhabit low altitude zones, for example, cannot be considered as dark diversity for high altitude communities, yet both constitute beta-diversity. Dark diversity always entails ecologically ‘filtered’ sets of species that can potentially inhabit a particular local community, whereas beta-diversity is defined by all species which occur in the study region but are absent from a particular locality. This difference is also highly relevant for nature conservation: High beta-diversity is typically considered a good indicator of biodiversity at the landscape scale, whereas high dark diversity is rather an indicator of community impoverishment (see below).
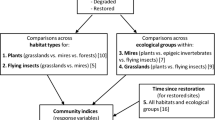
One question related to the species pool concept is whether local communities are unsaturated or saturated with species (Cornell and Lawton 1992). In unsaturated communities, local richness is unaffected by local biotic interactions and increases proportionately with species pool size (Szava-Kovats et al. 2012). In saturated communities, by contrast, biotic interactions cause local richness to gradually reach an upper asymptote limit with increasing species pool size. The concepts of community completeness and community saturation are related yet distinct. Community completeness can be defined for a single local habitat (Fig. 1 ). However, to recognize saturation requires observations across multiple sites. A test for saturation determines whether community completeness is negatively related to species pool size. Community completeness indicates how far a community is from its potential diversity. It does not address how this diversity is regulated, however. Community saturation, by contrast, indicates how biotic interactions regulate the number of coexisting species. It does not reveal how much of the species pool is realized within a local community. Consequently, both measures are useful, but for different purposes.
The species pool concept examines local species richness with respect to the part of the species pool that is absent – dark diversity. Local richness is always a subset of the species pool, so all communities lie below the 1 : 1 line. Community completeness expresses how much of the species pool is realized within a local community, i.e. how close a community is situated to the 1 : 1 line. Community completeness can be assessed for single communities. Community saturation addresses the shape of the relationship between species pool size and local communities and requires several communities. Community saturation examines whether community completeness varies with species pool size
Community Completeness: Methods
In order to evaluate community completeness, we need to estimate the extent of dark diversity, i.e. compile a list of species potentially able to live and reproduce in a given community but which are currently absent. One method to estimate the size of dark diversity is to draw on available biogeographic and ecological information. The first step is to define a region from which species can potentially reach a given community. This region can be a political region that contains an essentially ‘complete’ flora or fauna, for example, a small country, a county or a nature reserve (Pärtel et al. 1996). A more logical approach, however, is to include species from a single relatively uniform biogeographic region (Pärtel et al. 2011). Provided that distribution ranges of individual species are known, circular areas with defined radii can been used (Graves and Gotelli 1983), although more sophisticated approaches, such as dispersion fields (Lessard et al. 2012), might be preferable. The probability of arrival of a species in a community can be further specified by using species’ regional abundance and dispersal potential (Lessard et al. 2012). The second step is to ascertain the ecological requirements of species in the regional flora or fauna. This can be determined by inventorying similar habitats, using habitat suitability models created with GIS (Guisan and Rahbek 2011; Mokany and Paini 2011), and analysing species co-occurrence patterns (Ewald 2002; Münzbergová and Herben 2004). Regional lists of species can be filtered for ecological requirements through (semi)quantitative habitat requirement characteristics (e.g. Ellenberg indicator values, de Bello et al. 2012) or measured traits (Sonnier et al. 2010). If communities can be assigned to predefined habitat types, local expert knowledge can be applied to define species pools for each habitat type (Sádlo et al. 2007; Zobel et al. 2011).
Dark diversity can also be determined experimentally. For example, early Russian works suggested that completeness of plant communities can be estimated experimentally by sowing seeds of a large number of species (Ramensky 1924; Rabotnov 1987). Seed sowing experiments have been a common tool in plant community ecology for many years and have provided valuable insights into the habitat preferences of species as well as into the role of dispersal limitation in determining local diversity (e.g. Myers and Harms 2009; Vítová and Lepš 2011). It is exceedingly difficult, however, to test a very large number of species experimentally. For long-living plant species, germination might also need a proper “window of opportunity” (Eriksson and Fröborg 1996); failure to establish after sowing does not necessarily exclude a species from the species pool. Initial establishment of a species does not guarantee long-term persistence either (Gustafsson et al. 2002). Some species may die out during more extreme years (e.g. because of drought or cold), while other species may become locally extinct due to long-term pressure from predators. Moreover, experimental introduction is technically demanding when animal communities are concerned. Although experimental determination of the size of dark diversity sounds appealing, its practical difficulties may overshadow its advantages.
The estimation of dark diversity is always confounded by whether some species are really absent from the community or whether they simply have evaded detection. Studies of animal communities have suggested that expected richness be used rather than raw inventories (Cam et al. 2000). Sampling sufficiency can be estimated using sampling intensity-richness curves and Jackknife or Chao estimates of richness (Gotelli and Golwell 2011). Some species in a community can be detected only when active (e.g. butterflies, Sang et al. 2010). Some plant species can be dormant and hidden in the soil. Molecular identification of roots can offer a solution for this (e.g. Hiiesalu et al. 2012). Environmental meta-genomics is a developing field, which may detect rare species in ecosystems from DNA samples (Taberlet et al. 2012).
Relative richness has traditionally been expressed in terms of simple proportions, for example, observed richness/species pool, which are constrained to values between 0 and 1. Differences in this ratio reflect absolute differences in observed richness. We argue that relative differences are more meaningful: an increase from 5 to 10 observed species is not equivalent to an increase from 50 to 55 species but rather an increase from 50 to 100 species. Ln(observed richness/species pool), which is constrained to values <0, expresses differences in observed richness on a relative basis, but is mathematically incoherent, inasmuch as the difference between two dissimilar communities depends on whether we compare completeness or incompleteness. Dark diversity is by definition species pool – observed richness, yet differences expressed as ln(observed richness/species pool) are not necessarily equivalent to those expressed as ln(dark diversity/species pool). We therefore introduce the Community Completeness Index based on a log-ratio (or logistic) expression, ln(observed richness/dark diversity) (sensu Szava-Kovats et al. 2012). This expression preserves not only a relative description of a difference but maintains the same magnitude with respect to either observed richness or dark diversity. Moreover, this metric places data on an unbounded line in real space (theoretically from –∞ to + ∞), which is a fundamental assumption in all forms of conventional statistics (Bacon-Shone 2011). Our logistic Community Completeness Index cannot be applied if either local observed richness or dark diversity is totally absent. This, however, is basically just a theoretical hindrance. In nature we typically cannot define a community without species. An empty site might seem like a totally ‘incomplete community’ after a major disturbance such as poisoning or a volcanic eruption, but even then we would expect species to arrive soon thereafter. Similarly, it is not very likely to have a fully complete community with zero dark diversity; even a site at which the entire species pool is represented at one particular moment is likely to exhibit temporal species loss with multiple sampling.
Just as species pools can be approached hierarchically depending on the spatial scale of interest (Zobel 1997), so can community completeness. Species pools are typically defined at the regional scale (regional species pool), but it is possible to define species pools at smaller scales as well. For instance, if we are interested in how communities assemble within landscapes during shorter time periods (say decades), a local species pool can be defined by including species from the surrounding landscape within a radius of a few kilometres. This approach can be useful if our aim is to explore how dynamic landscapes influence species arrival and persistence (e.g. through loss of habitat size and connectivity). Furthermore, if we want to study community assembly processes within a community, we can address local richness in a fixed sample (e.g. 1 × 1 m sample in a grassland) and consider all species actually present in a habitat patch as a community species pool. Small-scale community completeness can indicate a response to biotic interactions, including anthropogenic effects.
Community Completeness: Applications in Ecological Theory
In order to understand local communities, it is important to know why their completeness differs. Various processes can ‘sentence’ potential species to be part of dark diversity: dispersal limitation, biotic interactions or simple stochastic variation.
We can expect that areas that have experienced long-term (evolutionary, geological) stability will be more complete than communities in areas which are comparatively young (e.g. post-glacial) simply because stable sites have had more time to ‘collect’ arriving species from the surrounding region. In Northern Europe many species have not yet reached their climatically suitable areas, probably due to dispersal limitation and the short time since the last Ice Age (Normand et al. 2011). In addition, evolutionary older communities on oceanic islands likely have a greater share of endemic species, which also comprise the regional species pool (Zobel et al. 2011).
We can also expect successionally stable communities to be more complete. Succession entails that species themselves modify environmental conditions through soil formation (e.g. primary succession in areas newly exposed by tectonic uplift) and through decreasing light availability (e.g. old-field succession). Early and late successional stages might therefore be represented by different species pools (Pykälä 2004). Indeed, during succession the species pool associated with early successional stages is replaced by that of later successional stages. Dynamic plant communities may have a transient rich mixture of species from different species pools, but long-term persistence of these species is impossible. Consequently, completeness is low regardless of how the species pool is defined: many species from the early-successional species pool are already lost, but species from the late-successional species pool have not yet arrived.
Community completeness is clearly linked to the landscape patterns surrounding the community. Large and well-connected local communities are expected to be more complete because local extinctions are less likely and the arrival of new species from neighbouring habitat patches is more probable (MacArthur and Wilson 1967). If different habitat types are present within a landscape, community completeness can be compared among them. For example, if a stable landscape pattern has changed recently, community completeness likely reflects extinction debt or colonization credit (Kuussaari et al. 2009). This, however, might be different for plant and animal communities (Brändle et al. 2003; Krauss et al. 2010).
Occasional atypical disturbances within communities can weaken local populations, leading to local extinctions and consequentially to a decrease in community completeness. Disturbed microhabitats are occasionally invaded by ruderal or opportunistic species, thereby increasing local species richness (Moora et al. 2007; Questad and Foster 2007; Schnoor and Olsson 2010). This increase, however, will not increase completeness because these invading species do not belong to the original species pool of the target community. Instead, they belong to the species pool of disturbed habitats. At the same time, a historically constant disturbance regime might be associated with successional stability and thus represent a ‘natural part’ of local habitat conditions (Moles et al. 2012). For instance, semi-natural calcareous grasslands (alvars) on thin calcareous soil experience regular disturbances caused by animal grazing and frost upheaval of the topsoil but still exhibit high vegetation diversity and community completeness (Pärtel et al. 1999; Rosén and van der Maarel 2000). These disturbances are historically relevant and form part of the ‘normal environment’ of this grassland type. Cessation of grazing reduces the level of local disturbance but also leads to overgrowing of open grasslands with scrub and a decline in diversity.
Community completeness depends on negative and positive biotic interactions among members of a community. Local richness in temperate regions is often unimodally related to habitat productivity (Pärtel et al. 2007b). This relationship was initially attributed to competition; that is, weaker species are out-competed when productivity is high (Al Mufti et al. 1977). Subsequent studies have additionally attributed lower species richness in highly productive communities to a smaller species pool (Taylor et al. 1990; Pärtel et al. 2007b). Zobel and Liira (1997) addressed small-scale community completeness (referred to as ‘relative richness’) and still found a unimodal relationship with habitat productivity, although the relationship is more pronounced when raw richness values are used. This finding suggests that both the species pool and competition might play a role in producing the unimodal diversity-productivity relationship.
Community completeness depends on other trophic levels in the ecosystem. For instance, positive and negative microbial feedback mechanisms both affect the number of coexisting plant species (Bever et al. 2010), thereby changing the completeness of communities. The actual numbers of coexisting plant species may also depend of herbivory (Hillebrand et al. 2007), pathogens (Allan et al. 2010) and other biotic interactions. It is, however, too early to ascertain whether general patterns of community completeness depend on the impact of other tropic levels.
Humans can act both as predators/parasites and mutualists in ecological communities, and anthropogenic influence can be a major determinant of community completeness. Small-scale community completeness in semi-natural alvar grasslands in Estonia is unimodally related to current human population density, indicating that extreme human influence (be it too low or too high) is unfavourable (Pärtel et al. 2007a). Community completeness might therefore be a valuable indicator for nature conservation.
Community Completeness: Applications in Nature Conservation
Community completeness allows meaningful comparisons of local diversity across communities from different regions, landscapes, vegetation types, successional stages as well as across different trophic levels. Community completeness has important implications for conservation. No single principle can be applied when selecting areas for conservation (Tuvi et al. 2011), but community completeness provides a useful metric. Although it is prudent to protect all major habitat types within a territory, community completeness allows identification of the most complete locations for each habitat type.
Community completeness might be a vital metric indicating the status of local biodiversity in natural communities (de Bello et al. 2010). It can also quantify the success of ecological restoration for different regions and different trophic levels (Suding 2011). Restoring a lake, for example, can entail reintroduction of fish species and macrophytes, while invertebrates and algae are expected to disperse spontaneously. The community completeness of all these trophic levels can be compared in order to assess restoration success at the ecosystem level.
For conservation purposes, it is valid to distinguish between native and alien species since recent human-induced invasions pose an increasing threat to local biodiversity. A long-standing debate revolves around whether diverse plant communities are more resistant to biological invasions than less diverse ones (Fridley et al. 2007). The use of relative measures will provide a dimensionless approach to addressing this question. Akatov and Akatova (2010) showed that higher completeness in plant communities is associated with lower invasibility of alien species. Recently, Catford et al. (2012) suggested two general relative measures of ecosystem invasibility, relative alien species richness and relative alien species abundance. Future studies need to examine whether relative alien richness and abundance are significantly dependent on community completeness.
Conclusions
Species diversity and coexistence have been major topics in both basic and applied community ecology (Lepš 2005). Community completeness explores how many species from the species pool are actually present locally and how many are absent. The absent part of the species pool – dark diversity – can be estimated by means of various ecoinformatics and experimental methods. We introduce the Community Completeness Index as a statistically sound log-ratio expression. Community completeness can be linked to the theory of ecological communities and can be utilized in conservation biology, where it can serve as a suitable indicator for nature conservation prioritization and for ecosystem restoration monitoring or as a measure of how biodiversity responds to different threats.
References
Akatov V, Akatova T (2010) Saturation and invasion resistance of non-interactive plant communities. Russian J Ecol 41:229–236
Allan E, van Ruijven J, Crawley MJ (2010) Foliar fungal pathogens and grassland biodiversity. Ecology 91:2572–2582
Al Mufti MM, Sydes CL, Furness SB, Grime JP, Band SR (1977) A quantitative analysis of shoot phenology and dominance in herbaceous vegetation. J Ecol 65:759–791
Bacon-Shone J (2011) A short history of compositional data analysis: Theory and Applications. In Pawlowsky-Glahn V, Buccianti A (eds) Compositional data analysis. John Wiley & Sons, West Sussex, pp 3–11
Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25:468–478
Brändle M, Durka W, Krug H, Brandl R (2003) The assembly of local communities: plants and birds in non-reclaimed mining sites. Ecography 26:652–660
Cam E, Nichols JD, Sauer JR, Hines JE, Flather CH (2000) Relative species richness and community completeness: bird and urbanization in the Mid-Atlantic states. Ecol Appl 10:1196–1210
Catford JA, Vesk PA, Richardson DM, Pyšek P (2012) Quantifying levels of biological invasion: towards the objective classification of invaded and invasible ecosystems. Global Change Biol 18:44–62
Cornell HV, Lawton JH (1992) Species interactions, local and regional processes, and limits to the richness of ecological communities: a theoretical perspective. J Anim Ecol 61:1–12
de Bello F, Lavorel S, Gerhold P, Reier Ü, Pärtel M (2010) A biodiversity monitoring framework for practical conservation of grasslands and shrublands. Biol Conservation 143:9–17
de Bello F, Price JN, Münkemüller T, Liira J, Zobel M, Thuiller W, Gerhold P, Götzenberger L, Lavergne S, Lepš J, Zobel K, Pärtel M (2012) Functional species pool framework to test for biotic effects on community assembly. Ecology 93:2263–2273
Eriksson O (1993) The species-pool hypothesis and plant community diversity. Oikos 68:371–374
Eriksson O, Fröborg H (1996) “Windows of opportunity” for recruitment in long-lived clonal plants: experimental studies of seedling establishment in Vaccinum shrubs. Canad J Bot 74:1369–1374
Ewald J (2002) A probabilistic approach to estimating species pools from large compositional matrices. J Veg Sci 13:191–198
Fridley J, Stachowicz J, Naeem S, Sax D, Seabloom E, Smith M, Stohlgren T, Tilman D, Von Holle B (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17
Gotelli NJ, Golwell RK (2011) Estimating species richness. In Magurran AE, McGill BJ (eds) Biological diversity; frontiers in management and assessment. Oxford University Press, Oxford, pp 39–54
Götzenberger L, de Bello F, Bråthen KA, Davison J, Dubuis A, Guisan A, Lepš J, Lindborg R, Moora M, Pärtel M, Pellissier L, Pottier J, Vittoz P, Zobel K, Zobel M (2012) Ecological assembly rules in plant communities – approaches, patterns and prospects. Biol Rev 87:111–127
Graves GR, Gotelli NJ (1983) Neotropical land-bridge avifaunas: new approaches to null hypotheses in biogegoraphy. Oikos 41:322–333
Guisan A, Rahbek C (2011) SESAM – a new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J Biogeogr 38:1433–1444
Gustafsson C, Ehrlén J, Eriksson O (2002) Recruitment in Dentaria bulbifera; the roles of dispersal, habitat quality and mollusc herbivory. J Veg Sci 13:719–724
Hiiesalu I, Öpik M, Metsis M, Lilje L, Davidson J, Vasar M, Moora M, Zobel M, Wilson SD, Pärtel M (2012) Plant species richness belowground: higher richness and new patterns revealed by next generation sequencing. Molec Ecol 21:2004–2016
Hillebrand H, Gruner DS, Borer ET, Bracken MES, Cleland EE, Elser JJ, Harpole WS, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc Natl Acad Sci USA 104:10904–10909
Ingerpuu N, Vellak K, Kukk T, Pärtel M (2001) Bryophyte and vascular plant species richness in boreo-nemoral moist forests and mires. Biodivers & Conservation 10:2153–2166
Krauss J, Bommarco R, Guardiola M, Heikkinen RK, Helm A, Kuussaari M, Lindborg R, Öckinger E, Pärtel M, Pino J, Pöyry J, Raatikainen KM, Sang A, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I (2010) Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol Lett 13:597–605
Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R, Öckinger E, Pärtel M, Pino J, Roda F, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I (2009) Extinction debt: a challenge for biodiversity conservation. Trends Ecol Evol 24:564–571
Lepš J (2005) Diversity and ecosystem function. In van der Maarel E (ed) Vegetation ecology. Blackwell Science, Malden, MA, pp 199–237
Lessard J-P, Belmaker J, Myers JA, Chase JM, Rahbek C (2012) Inferring local ecological processes amid species pool influences. Trends Ecol Evol 27:600–607
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Mokany K, Paini DR (2011) Dark diversity: adding the grey. Trends Ecol Evol 26:264–265
Moles AT, Flores-Moreno H, Bonser SP, Warton DI, Helm A, Warman L, Eldridge DJ, Jurado E, Hemmings FA, Reich PB, Cavender-Bares J, Seabloom EW, Mayfield MM, Sheil D, Djietror JC, Peri PL, Enrico L, Cabido MR, Setterfield SA, Lehmann CER, Thomson FJ (2012) Invasions: the trail behind, the path ahead, and a test of a disturbing idea. J Ecol 100:116–127
Moora M, Daniell T, Kalle H, Liira J, Püssa K, Roosaluste E, Öpik M, Wheatley R, Zobel M (2007) Spatial pattern and species richness of boreonemoral forest understorey and its determinants – A comparison of diffirently managed forests. Forest Ecol Managem 250:64–70
Münzbergová Z, Herben T (2004) Identification of suitable unoccupied habitats in metapopulation studies using co-occurrence of species. Oikos 105:408–414
Myers JA, Harms KE (2009) Seed arrival, ecological filters, and plant species richness: a meta-analysis. Ecol Lett 12:1250–1260
Normand S, Ricklefs RE, Skov F, Bladt J, Tackenberg O, Svenning J-C (2011) Postglacial migration supplements climate in determining plant species ranges in Europe. Proc Roy Soc B 278:3644–3653
Pärtel M, Laanisto L, Zobel M (2007b) Contrasting plant productivity-diversity relationships across latitude: the role of evolutionary history. Ecology 88:1091–1097
Pärtel M, Szava-Kovats R, Zobel M (2011) Dark diversity: shedding light on absent species. Trends Ecol Evol 26:124–128
Pärtel M, Kalamees R, Zobel M, Rosén E (1999) Alvar grasslands in Estonia: variation in species composition and community structure. J Veg Sci 10:561–570
Pärtel M, Zobel M, Zobel K, van der Maarel E (1996) The species pool and its relation to species richness: evidence from Estonian plant communities. Oikos 75:111–117
Pärtel M, Helm A, Reitalu T, Liira J, Zobel M (2007a) Grassland diversity related to the Late Iron Age human population density. J Ecol 95:574–582
Peterson AT (2011) Ecological niche conservatism: a time-structured review of evidence. J Biogeogr 38:817–827
Pykälä J (2004) Immediate increase in plant species richness after clear cutting of boreal herb-rich forests. Appl Veg Sci 7:29–34
Questad EJ, Foster BL (2007) Vole disturbances and plant diversity in a grassland metacommunity. Oecologia 153:341–351
Rabotnov TA (1984) Phytocoenology. Moscow State University, Moscow
Rabotnov TA (1987) Experimental phytocoenology. Moscow State University, Moscow
Ramenskii LG (1924) Osnovnye zakonomernosti rastitel'nogo pokrova i metody ikh izucheniya (na osnovanii geobotanicheskikh issledovanii v Voronezhskoi guberinii) (Basic regularities of vegetation cover and their study (on the basis of geobotanic researches in Voronezh province)). Vestnik opytnogo dela Sredne-chernozemnoi oblasti 1924(Jan–Feb):37–73
Rosén E, van der Maarel E (2000) Restoration of alvar vegetation on Öland, Sweden. Appl Veg Sci 3:65–72
Sádlo J, Chytrý M, Pyšek P (2007) Regional species pools of vascular plants in habitats of the Czech Republic. Preslia 79:303–321
Sang A, Teder T, Helm A, Pärtel M (2010) Indirect evidence for an extinction debt of grassland butterflies half century after habitat loss. Biol Conservation 143:1405–1413
Schnoor TK, Olsson PA (2010) Effects of soil disturbance on plant diversity of calcareous grasslands. Agric Ecosyst Environm 139:714–719
Sonnier G, Shipley B, Navas M (2010) Plant traits, species pools and the prediction of relative abundance in plant communities: a maximum entropy approach. J Veg Sci 21:318–331
Suding KN (2011) Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annual Rev Ecol Evol Syst 42:465–487
Szava-Kovats R, Zobel M, Pärtel M (2012) The local–regional species richness relationship: new perspectives on the null-hypothesis. Oikos 121:321–326
Taberlet P, Coissac E, Hajibabaei M, Rieseberg LH (2012) Environmental DNA. Molec Ecol 21:1789–1793
Taylor DR, Aarssen LW, Loehle C (1990) On the relationship between r/K selection and environmental carrying capacity: a new habitat templet for plant life history strategies. Oikos 58:239–250
Tuvi EL, Reier Ü, Vellak A, Szava-Kovats R, Pärtel M (2011) Establishment of protected areas in different ecoregions, ecosystems, and diversity hotspots under successive political systems. Biol Conservation 144:1726–1732
Vellend M (2010) Conceptual synthesis in community ecology. Quart Rev Biol 85:183–206
Vítová A, Lepš J (2011) Experimental assessment of dispersal and habitat limitation in an oligotrophic wet meadow. Pl Ecol 212:1231–1242
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338
Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Jonathan Davies T, Grytnes JA, Harrison SP, Hawkins BA, Holt RD, McCain CM, Stephens PR (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324
Wilson JB, Peet RK, Dengler J, Pärtel M (2012) Plant species richness: the world records. J Veg Sci 23:796–802
Zobel M (1992) Plant species coexistence: The role of historical, evolutionary and ecological factors. Oikos 65:314–320
Zobel M (1997) The relative role of species pools in determining plant species richness: an alternative explanation of species coexistence? Trends Ecol Evol 12:266–269
Zobel K, Liira J (1997) A scale-independent approach to the richness vs biomass relationship in ground-layer plant communities. Oikos 80:325–332
Zobel M, Otto R, Laanisto L, Naranjo-Cigala A, Pärtel M, Fernandez-Palacios JM (2011) The formation of species pools: historical habitat abundance affects current local diversity. Global Ecol Biogeogr 20:251–259
Acknowledgments
This study was supported by the European Union 7th framework project SCALES (FP7-226852), European Regional Development Fund (Center of Excellence FIBIR) and the University of Tartu (SF0180095s08, SF0180098s08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pärtel, M., Szava-Kovats, R. & Zobel, M. Community Completeness: Linking Local and Dark Diversity within the Species Pool Concept. Folia Geobot 48, 307–317 (2013). https://doi.org/10.1007/s12224-013-9169-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-013-9169-x